Perfluoroalkyl Mixture Exposure in Relation to Fetal Growth: Potential Roles of Maternal Characteristics and Associations with Birth Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population, Birth Outcome Ascertainment and Sample Collection
2.2. Sample Extraction and Instrument Analysis
2.3. Quality Assurance and Quality Control
2.4. Statistical Analysis
3. Results and Discussions
3.1. Demographic Characteristics of the Studied Population
3.2. PFASs Distributions in Maternal Serum
3.3. Potential Roles of Maternal Determinants
3.4. Associations between PFASs Exposure and Birth Weight and Apgar Scores
3.5. PFASs Exposure in Relation to Preterm Birth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banerjee, A.; Liu, Y. Essential Factor of Perfluoroalkyl Surfactants Contributing to Efficacy in Firefighting Foams. Langmuir 2021, 37, 8937–8944. [Google Scholar] [CrossRef] [PubMed]
- Graber, J.M.; Black, T.M.; Shah, N.N.; Caban-Martinez, A.J.; Lu, S.E.; Brancard, T.; Yu, C.H.; Turyk, M.E.; Black, K.; Steinberg, M.B.; et al. Prevalence and Predictors of Per- and Polyfluoroalkyl Substances (PFAS) Serum Levels among Members of a Suburban US Volunteer Fire Department. Int. J. Environ. Res. Public Health 2021, 18, 3730. [Google Scholar] [CrossRef] [PubMed]
- Narizzano, A.M.; Bohannon, M.E.; East, A.G.; McDonough, C.; Choyke, S.; Higgins, C.P.; Quinn, M.J., Jr. Patterns in Serum Toxicokinetics in Peromyscus Exposed to Per- and Polyfluoroalkyl Substances. Environ. Toxicol. Chem. 2021, 40, 2886–2898. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Baumann, K.; Mead, R.N.; Skrabal, S.A.; Kieber, R.J.; Avery, G.B.; Shimizu, M.; DeWitt, J.C.; Sun, M.; Vance, S.A.; et al. PFOS dominates PFAS composition in ambient fine particulate matter (PM2.5) collected across North Carolina nearly 20 years after the end of its US production. Environ. Sci. Process. Impacts 2021, 23, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Brix, N.; Lauridsen, L.L.B.; Olsen, J.; Parner, E.T.; Liew, Z.; Olsen, L.H.; Ramlau-Hansen, C.H. Exposure to Perfluoroalkyl Substances during Fetal Life and Pubertal Development in Boys and Girls from the Danish National Birth Cohort. Environ. Health Perspect. 2019, 127, 17004. [Google Scholar] [CrossRef]
- Gyllenhammar, I.; Diderholm, B.; Gustafsson, J.; Berger, U.; Ridefelt, P.; Benskin, J.P.; Lignell, S.; Lampa, E.; Glynn, A. Perfluoroalkyl acid levels in first-time mothers in relation to offspring weight gain and growth. Environ. Int. 2018, 111, 191–199. [Google Scholar] [CrossRef]
- Li, J.; Cai, D.; Chu, C.; Li, Q.; Zhou, Y.; Hu, L.; Yang, B.; Dong, G.; Zeng, X.; Chen, D. Transplacental Transfer of Per- and Polyfluoroalkyl Substances (PFASs): Differences between Preterm and Full-Term Deliveries and Associations with Placental Transporter mRNA Expression. Environ. Sci. Technol. 2020, 54, 5062–5070. [Google Scholar] [CrossRef]
- Chen, F.F.; Yin, S.S.; Kelly, B.C.; Liu, W.P. Isomer-Specific Transplacental Transfer of Perfluoroalkyl Acids: Results from a Survey of Paired Maternal, Cord Sera, and Placentas. Environ. Sci. Technol. 2017, 51, 5756–5763. [Google Scholar] [CrossRef]
- Schoeters, G.E.R.; Den Hond, E.; Koppen, G.; Smolders, R.; Bloemen, K.; De Boever, P.; Govarts, E. Biomonitoring and biomarkers to unravel the risks from prenatal environmental exposures for later health outcomes. Am. J. Clin. Nutr. 2011, 94, 1964S–1969S. [Google Scholar] [CrossRef]
- Xu, C.; Yin, S.; Liu, Y.; Chen, F.; Zhong, Z.; Li, F.; Liu, K.; Liu, W. Prenatal exposure to chlorinated polyfluoroalkyl ether sulfonic acids and perfluoroalkyl acids: Potential role of maternal determinants and associations with birth outcomes. J. Hazard. Mater. 2019, 380, 120867. [Google Scholar] [CrossRef]
- Liew, Z.; Goudarzi, H.; Oulhote, Y. Developmental Exposures to Perfluoroalkyl Substances (PFASs): An Update of Associated Health Outcomes. Curr. Environ. Health Rep. 2018, 5, 1–19. [Google Scholar] [CrossRef]
- Bamai, Y.A.; Goudarzi, H.; Araki, A.; Okada, E.; Kashino, I.; Miyashita, C.; Kishi, R. Effect of prenatal exposure to per- and polyfluoroalkyl substances on childhood allergies and common infectious diseases in children up to age 7 years: The Hokkaido study on environment and children’s health. Environ. Int. 2020, 143, 105979. [Google Scholar] [CrossRef]
- Bommarito, P.A.; Ferguson, K.K.; Meeker, J.D.; McElrath, T.F.; Cantonwine, D.E. Maternal Levels of Perfluoroalkyl Substances (PFAS) during Early Pregnancy in Relation to Preeclampsia Subtypes and Biomarkers of Preeclampsia Risk. Environ. Health Perspect. 2021, 129, 107004. [Google Scholar] [CrossRef]
- Costa, O.; Iniguez, C.; Manzano-Salgado, C.B.; Amiano, P.; Murcia, M.; Casas, M.; Irizar, A.; Basterrechea, M.; Beneito, A.; Schettgen, T.; et al. First-trimester maternal concentrations of polyfluoroalkyl substances and fetal growth throughout pregnancy. Environ. Int. 2019, 130, 104830. [Google Scholar] [CrossRef]
- Inoue, K.; Ritz, B.; Andersen, S.L.; Ramlau-Hansen, C.H.; Hoyer, B.B.; Bech, B.H.; Henriksen, T.B.; Bonefeld-Jorgensen, E.C.; Olsen, J.; Liew, Z. Perfluoroalkyl Substances and Maternal Thyroid Hormones in Early Pregnancy; Findings in the Danish National Birth Cohort. Environ. Health Perspect. 2019, 127, 117002. [Google Scholar] [CrossRef]
- Kingsley, S.L.; Eliot, M.N.; Kelsey, K.T.; Calafat, A.M.; Ehrlich, S.; Lanphear, B.P.; Chen, A.M.; Braun, J.M. Variability and predictors of serum perfluoroalkyl substance concentrations during pregnancy and early childhood. Environ. Res. 2018, 165, 247–257. [Google Scholar] [CrossRef]
- Liew, Z.; Luo, J.; Nohr, E.A.; Bech, B.H.; Bossi, R.; Arah, O.A.; Olsen, J. Maternal Plasma Perfluoroalkyl Substances and Miscarriage: A Nested Case-Control Study in the Danish National Birth Cohort. Environ. Health Perspect. 2020, 128, 047007. [Google Scholar] [CrossRef]
- Santos, A.D.E.; Meyer, A.; Dabkiewicz, V.E.; Camara, V.D.; Asmus, C. Serum levels of perfluorooctanoic acid and perfluorooctane sulfonic acid in pregnant women: Maternal predictors and associations with birth outcomes in the PIPA Project. J. Obstet. Gynaecol. Res. 2021, 47, 3107–3118. [Google Scholar] [CrossRef]
- Skogheim, T.S.; Weyde, K.V.F.; Aase, H.; Engel, S.M.; Suren, P.; Oie, M.G.; Biele, G.; Reichborn-Kjennerud, T.; Brantsaeter, A.L.; Haug, L.S.; et al. Prenatal exposure to per- and polyfluoroalkyl substances (PFAS) and associations with attention-deficit/hyperactivity disorder and autism spectrum disorder in children. Environ. Res. 2021, 202, 111692. [Google Scholar] [CrossRef]
- Starling, A.P.; Adgate, J.L.; Hamman, R.F.; Kechris, K.; Calafat, A.M.; Ye, X.Y.; Dabelea, D. Perfluoroalkyl Substances during Pregnancy and Offspring Weight and Adiposity at Birth: Examining Mediation by Maternal Fasting Glucose in the Healthy Start Study. Environ. Health Perspect. 2017, 125, 067016. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, Y.; Miao, M.; Wang, Z.; Yuan, W.; Liu, X.; Wang, X.; Wang, Z.; Wen, S.; Liang, H. Determinants of plasma concentrations of perfluoroalkyl and polyfluoroalkyl substances in pregnant women from a birth cohort in Shanghai, China. Environ. Int. 2018, 119, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Grandjean, P.; Valvi, D.; Nielsen, F.; Jensen, T.K.; Weihe, P.; Oulhote, Y. Associations of Exposure to Perfluoroalkyl Substances With Thyroid Hormone Concentrations and Birth Size. J. Clin. Endocrinol. Metab. 2020, 105, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Wong, L.Y.; Chen, A.M.; Dunbar, C.; Webster, G.M.; Lanphear, B.P.; Calafat, A.M. Changes in Serum Concentrations of Maternal Poly- and Perfluoroalkyl Substances over the Course of Pregnancy and Predictors of Exposure in a Multiethnic Cohort of Cincinnati, Ohio Pregnant Women during 2003–2006. Environ. Sci. Technol. 2014, 48, 9600–9608. [Google Scholar] [CrossRef] [PubMed]
- Deji, Z.M.; Liu, P.; Wang, X.; Zhang, X.; Luo, Y.H.; Huang, Z.Z. Association between maternal exposure to perfluoroalkyl and polyfluoroalkyl substances and risks of adverse pregnancy outcomes: A systematic review and meta-analysis. Sci. Total Environ. 2021, 783, 146984. [Google Scholar] [CrossRef] [PubMed]
- Miura, R.; Araki, A.; Miyashita, C.; Kobayashi, S.; Kobayashi, S.; Wang, S.L.; Chen, C.H.; Miyake, K.; Ishizuka, M.; Iwasaki, Y.; et al. An epigenome-wide study of cord blood DNA methylations in relation to prenatal perfluoroalkyl substance exposure: The Hokkaido study. Environ. Int. 2018, 115, 21–28. [Google Scholar] [CrossRef]
- Yin, S.; Tang, M.; Chen, F.; Li, T.; Liu, W. Environmental exposure to polycyclic aromatic hydrocarbons (PAHs): The correlation with and impact on reproductive hormones in umbilical cord serum. Environ. Pollut. 2017, 220 Pt B, 1429–1437. [Google Scholar] [CrossRef]
- Chu, C.; Zhou, Y.; Li, Q.Q.; Bloom, M.S.; Lin, S.; Yu, Y.J.; Chen, D.; Yu, H.Y.; Hu, L.W.; Yang, B.Y.; et al. Are perfluorooctane sulfonate alternatives safer? New insights from a birth cohort study. Environ. Int. 2020, 135, 105365. [Google Scholar] [CrossRef]
- Hall, S.M.; Zhang, S.; Hoffman, K.; Miranda, M.L.; Stapleton, H.M. Concentrations of per- and polyfluoroalkyl substances (PFAS) in human placental tissues and associations with birth outcomes. Chemosphere 2022, 295, 133873. [Google Scholar] [CrossRef]
- Nielsen, C.; Hall, U.A.; Lindh, C.; Ekstrom, U.; Xu, Y.Y.; Li, Y.; Holmang, A.; Jakobsson, K. Pregnancy-induced changes in serum concentrations of perfluoroalkyl substances and the influence of kidney function. Environ. Health 2020, 19, 80. [Google Scholar] [CrossRef]
- Starling, A.P.; Adgate, J.L.; Hamman, R.F.; Kechris, K.; Calafat, A.M.; Dabelea, D. Prenatal exposure to per- and polyfluoroalkyl substances and infant growth and adiposity: The Healthy Start Study. Environ. Int. 2019, 131, 111692. [Google Scholar] [CrossRef]
- Gui, S.Y.; Chen, Y.N.; Wu, K.J.; Liu, W.; Wang, W.J.; Liang, H.R.; Jiang, Z.X.; Li, Z.L.; Hu, C.Y. Association Between Exposure to Per- and Polyfluoroalkyl Substances and Birth Outcomes: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 855348. [Google Scholar] [CrossRef]
- Manzano-Salgado, C.B.; Casas, M.; Lopez-Espinosa, M.J.; Ballester, F.; Iniguez, C.; Martinez, D.; Costa, O.; Santa-Marina, L.; Pereda-Pereda, E.; Schettgen, T.; et al. Prenatal exposure to perfluoroalkyl substances and birth outcomes in a Spanish birth cohort. Environ. Int. 2017, 108, 278–284. [Google Scholar] [CrossRef]
- Yu, Y.; Qin, X.-D.; Bloom, M.S.; Chu, C.; Dai, X.; Li, Q.-Q.; Chen, Z.-X.; Kong, M.-L.; Xie, Y.-Q.; Meng, W.-J.; et al. Associations of prenatal exposure to perfluoroalkyl substances with preterm birth: A family-based birth cohort study. Environ. Res. 2022, 214 Pt 1, 113803. [Google Scholar] [CrossRef]
- Huo, X.N.; Zhang, L.; Huang, R.; Feng, L.P.; Wang, W.Y.; Zhang, J.; Shanghai Birth Cohort. Perfluoroalkyl substances exposure in early pregnancy and preterm birth in singleton pregnancies: A prospective cohort study. Environ. Health 2020, 19, 60. [Google Scholar] [CrossRef]
- Wu, K.; Xu, X.; Peng, L.; Liu, J.; Guo, Y.; Huo, X. Association between maternal exposure to perfluorooctanoic acid (PFOA) from electronic waste recycling and neonatal health outcomes. Environ. Int. 2012, 48, 1–8. [Google Scholar] [CrossRef]
- Fei, C.; McLaughlin, J.K.; Lipworth, L.; Olsen, J. Prenatal exposure to perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) and maternally reported developmental milestones in infancy. Environ. Health Perspect. 2008, 116, 1391–1395. [Google Scholar] [CrossRef]
- Chen, L.; Tong, C.L.; Huo, X.N.; Zhang, J.; Tian, Y.; Shanghai Birth Cohort. Prenatal exposure to perfluoroalkyl and polyfluoroalkyl substances and birth outcomes: A longitudinal cohort with repeated measurements. Chemosphere 2021, 267, 128899. [Google Scholar] [CrossRef]
- Gardener, H.; Sun, Q.; Grandjean, P. PFAS concentration during pregnancy in relation to cardiometabolic health and birth outcomes. Environ. Res. 2021, 192, 110287. [Google Scholar] [CrossRef]
- Li, M.; Zeng, X.W.; Qian, Z.M.; Vaughn, M.G.; Sauve, S.; Paul, G.; Lin, S.; Lu, L.; Hu, L.W.; Yang, B.Y.; et al. Isomers of perfluorooctanesulfonate (PFOS) in cord serum and birth outcomes in China: Guangzhou Birth Cohort Study. Environ. Int. 2017, 102, 1–8. [Google Scholar] [CrossRef]
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.-B.; Narwal, R.; Adler, A.; Vera Garcia, C.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef]
- Woudneh, M.B.; Chandramouli, B.; Hamilton, C.; Grace, R. Effect of Sample Storage on the Quantitative Determination of 29 PFAS: Observation of Analyte Interconversions during Storage. Environ. Sci. Technol. 2019, 53, 12576–12585. [Google Scholar] [CrossRef] [PubMed]
- Poothong, S.; Thomsen, C.; Padilla-Sanchez, J.A.; Papadopoulou, E.; Haug, L.S. Distribution of Novel and Well-Known Poly- and Perfluoroalkyl Substances (PFASs) in Human Serum, Plasma, and Whole Blood. Environ. Sci. Technol. 2017, 51, 13388–13396. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, Q.; Shen, L.; Zhao, S.; Pang, W.; Zhang, J. Perfluoroalkyl and polyfluoroalkyl substances in cord blood of newborns in Shanghai, China: Implications for risk assessment. Environ. Int. 2016, 97, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.M.; Goin, D.E.; Cushing, L.; DeMicco, E.; Park, J.S.; Wang, Y.Z.; Smith, S.; Padula, A.M.; Woodruff, T.J.; Morello-Frosch, R. Mixture effects of prenatal exposure to per- and polyfluoroalkyl substances and polybrominated diphenyl ethers on maternal and newborn telomere length. Environ. Health 2021, 20, 76. [Google Scholar] [CrossRef]
- Luo, F.; Chen, Q.; Yu, G.; Huo, X.; Wang, H.; Nian, M.; Tian, Y.; Xu, J.; Zhang, J.; Zhang, J.; et al. Exposure to perfluoroalkyl substances and neurodevelopment in 2-year-old children: A prospective cohort study. Environ. Int. 2022, 166, 107384. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.M.; Enright, E.A.; Padula, A.M.; Aung, M.; Geiger, S.D.; Cushing, L.; Trowbridge, J.; Keil, A.P.; Baek, H.G.; Smith, S.; et al. Prenatal PFAS and psychosocial stress exposures in relation to fetal growth in two pregnancy cohorts: Applying environmental mixture methods to chemical and non-chemical stressors. Environ. Int. 2022, 163, 107238. [Google Scholar] [CrossRef]
- Cao, T.; Qu, A.; Li, Z.; Wang, W.; Liu, R.; Wang, X.; Nie, Y.; Sun, S.; Zhang, X.; Liu, X. The relationship between maternal perfluoroalkylated substances exposure and low birth weight of offspring: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2021, 28, 67053–67065. [Google Scholar] [CrossRef]
- Liao, Q.; Tang, P.; Song, Y.; Liu, B.; Huang, H.; Liang, J.; Lin, M.; Shao, Y.; Liu, S.; Pan, D.; et al. Association of single and multiple prefluoroalkyl substances exposure with preterm birth: Results from a Chinese birth cohort study. Chemosphere 2022, 307, 135741. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Wang, Z.; Zhang, L.; Qi, X.; Zhang, Y.; Chang, X.; Wu, C.; Zhou, Z. Umbilical cord serum perfluoroalkyl substance mixtures in relation to thyroid function of newborns: Findings from Sheyang Mini Birth Cohort Study. Chemosphere 2021, 273, 129664. [Google Scholar] [CrossRef]
- Birukov, A.; Andersen, L.B.; Andersen, M.S.; Nielsen, J.H.; Nielsen, F.; Kyhl, H.B.; Jorgensen, J.S.; Grandjean, P.; Dechend, R.; Jensen, T.K. Exposure to perfluoroalkyl substances and blood pressure in pregnancy among 1436 women from the Odense Child Cohort. Environ. Int. 2021, 151, 106442. [Google Scholar] [CrossRef]
- Kashino, I.; Sasaki, S.; Okada, E.; Matsuura, H.; Goudarzi, H.; Miyashita, C.; Okada, E.; Ito, Y.M.; Araki, A.; Kishi, R. Prenatal exposure to 11 perfluoroalkyl substances and fetal growth: A large-scale, prospective birth cohort study. Environ. Int. 2020, 136, 105355. [Google Scholar] [CrossRef]
- Wang, H.; Yang, J.; Du, H.; Xu, L.; Liu, S.; Yi, J.; Qian, X.; Chen, Y.; Jiang, Q.; He, G. Perfluoroalkyl substances, glucose homeostasis, and gestational diabetes mellitus in Chinese pregnant women: A repeat measurement-based prospective study. Environ. Int. 2018, 114, 12–20. [Google Scholar] [CrossRef]
- Marks, K.J.; Cutler, A.J.; Jeddy, Z.; Northstone, K.; Kato, K.; Hartman, T.J. Maternal serum concentrations of perfluoroalkyl substances and birth size in British boys. Int. J. Hyg. Environ. Health 2019, 222, 889–895. [Google Scholar] [CrossRef]
- Gao, K.; Zhuang, T.; Liu, X.; Fu, J.; Zhang, J.; Fu, J.; Wang, L.; Zhang, A.; Liang, Y.; Song, M.; et al. Prenatal Exposure to Per- and Polyfluoroalkyl Substances (PFASs) and Association between the Placental Transfer Efficiencies and Dissociation Constant of Serum Proteins-PFAS Complexes. Environ. Sci. Technol. 2019, 53, 6529–6538. [Google Scholar] [CrossRef]
- Tatum-Gibbs, K.; Wambaugh, J.F.; Das, K.P.; Zehr, R.D.; Strynar, M.J.; Lindstrom, A.B.; Delinsky, A.; Lau, C. Comparative pharmacokinetics of perfluorononanoic acid in rat and mouse. Toxicology 2011, 281, 48–55. [Google Scholar] [CrossRef]
- Du, X.; Alipanahrostami, M.; Wang, W.; Tong, T. Long-Chain PFASs-Free Omniphobic Membranes for Sustained Membrane Distillation. ACS Appl. Mater. Interfaces 2022, 14, 23808–23816. [Google Scholar] [CrossRef]
- Bjorke-Monsen, A.L.; Varsi, K.; Averina, M.; Brox, J.; Huber, S. Perfluoroalkyl substances (PFASs) and mercury in never-pregnant women of fertile age: Association with fish consumption and unfavorable lipid profile. BMJ Nutr. Prev. Health 2020, 3, 277–284. [Google Scholar] [CrossRef]
- Manzano-Salgado, C.B.; Casas, M.; Lopez-Espinosa, M.J.; Ballester, F.; Martinez, D.; Ibarluzea, J.; Santa-Marina, L.; Schettgen, T.; Vioque, J.; Sunyer, J.; et al. Variability of perfluoroalkyl substance concentrations in pregnant women by socio-demographic and dietary factors in a Spanish birth cohort. Environ. Int. 2016, 92–93, 357–365. [Google Scholar] [CrossRef]
- Brantsaeter, A.L.; Whitworth, K.W.; Ydersbond, T.A.; Haug, L.S.; Haugen, M.; Knutsen, H.K.; Thomsen, C.; Meltzer, H.M.; Becher, G.; Sabaredzovic, A.; et al. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environ. Int. 2013, 54, 74–84. [Google Scholar] [CrossRef]
- Gomez-Canela, C.; Fernandez-Sanjuan, M.; Farres, M.; Lacorte, S. Factors affecting the accumulation of perfluoroalkyl substances in human blood. Environ. Sci. Pollut. Res. 2015, 22, 1480–1486. [Google Scholar] [CrossRef]
- Callan, A.C.; Rotander, A.; Thompson, K.; Heyworth, J.; Mueller, J.F.; Odland, J.O.; Hinwood, A.L. Maternal exposure to perfluoroalkyl acids measured in whole blood and birth outcomes in offspring. Sci. Total Environ. 2016, 569–570, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yin, S.; Zhang, J.; Guo, F.; Aamir, M.; Liu, S.; Liu, K.; Liu, W. Exposure patterns, chemical structural signatures, and health risks of pesticides in breast milk: A multicenter study in China. Sci. Total Environ. 2022, 830, 154617. [Google Scholar] [CrossRef] [PubMed]
- Salihovic, S.; Karrman, A.; Lind, L.; Lind, P.M.; Lindstrom, G.; van Bavel, B. Perfluoroalkyl substances (PFAS) including structural PFOS isomers in plasma from elderly men and women from Sweden: Results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). Environ. Int. 2015, 82, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Luebker, D.J.; York, R.G.; Hansen, K.J.; Moore, J.A.; Butenhoff, J.L. Neonatal mortality from in utero exposure to perfluorooctanesulfonate (PFOS) in Sprague-Dawley rats: Dose-response, and biochemical and pharamacokinetic parameters. Toxicology 2005, 215, 149–169. [Google Scholar] [CrossRef] [PubMed]
- Marks, K.J.; Howards, P.P.; Smarr, M.M.; Flanders, W.D.; Northstone, K.; Daniel, J.H.; Sjodin, A.; Calafat, A.M.; Hartman, T.J. Prenatal Exposure to Mixtures of Persistent Endocrine-disrupting Chemicals and Birth Size in a Population-based Cohort of British Girls. Epidemiology 2021, 32, 573–582. [Google Scholar] [CrossRef]
- Itoh, S.; Araki, A.; Mitsui, T.; Miyashita, C.; Goudarzi, H.; Sasaki, S.; Cho, K.; Nakazawa, H.; Iwasaki, Y.; Shinohara, N.; et al. Association of perfluoroalkyl substances exposure in utero with reproductive hormone levels in cord blood in the Hokkaido Study on Environment and Children’s Health. Environ. Int. 2016, 94, 51–59. [Google Scholar] [CrossRef]
- Marinello, W.P.; Mohseni, Z.S.; Cunningham, S.J.; Crute, C.; Huang, R.; Zhang, J.J.; Feng, L. Perfluorobutane sulfonate exposure disrupted human placental cytotrophoblast cell proliferation and invasion involving in dysregulating preeclampsia related genes. FASEB J. 2020, 34, 14182–14199. [Google Scholar] [CrossRef]
- Kvalem, H.E.; Nygaard, U.C.; Lodrup Carlsen, K.C.; Carlsen, K.H.; Haug, L.S.; Granum, B. Perfluoroalkyl substances, airways infections, allergy and asthma related health outcomes—Implications of gender, exposure period and study design. Environ. Int. 2020, 134, 105259. [Google Scholar] [CrossRef]
- Nian, M.; Luo, K.; Luo, F.; Aimuzi, R.; Huo, X.N.; Chen, Q.; Tian, Y.; Zhang, J. Association between Prenatal Exposure to PFAS and Fetal Sex Hormones: Are the Short-Chain PFAS Safer? Environ. Sci. Technol. 2020, 54, 8291–8299. [Google Scholar] [CrossRef]
- Meng, Q.; Inoue, K.; Ritz, B.; Olsen, J.; Liew, Z. Prenatal Exposure to Perfluoroalkyl Substances and Birth Outcomes; An Updated Analysis from the Danish National Birth Cohort. Int. J. Environ. Res. Public Health 2018, 15, 1729. [Google Scholar] [CrossRef]
- Fei, C.; McLaughlin, J.K.; Tarone, R.E.; Olsen, J. Perfluorinated chemicals and fetal growth: A study within the Danish National Birth Cohort. Environ. Health Perspect. 2007, 115, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, D.; Wang, B.; Xu, F.; Pang, Y.; Zhang, L.; Zhang, Y.; Jin, L.; Li, Z.; Ren, A. Does Low Maternal Exposure to Per- and Polyfluoroalkyl Substances Elevate the Risk of Spontaneous Preterm Birth? A Nested Case-Control Study in China. Environ. Sci. Technol. 2020, 54, 8259–8268. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.J.; Xu, C.Y.; Zhou, Q.; Shen, C.S.; Ma, C.Y.; Liu, S.R.; Yin, S.S.; Li, F. Congener- and isomer-specific Perfluorinated compounds in textile wastewater from Southeast China. J. Clean. Prod. 2021, 320, 128897. [Google Scholar] [CrossRef]
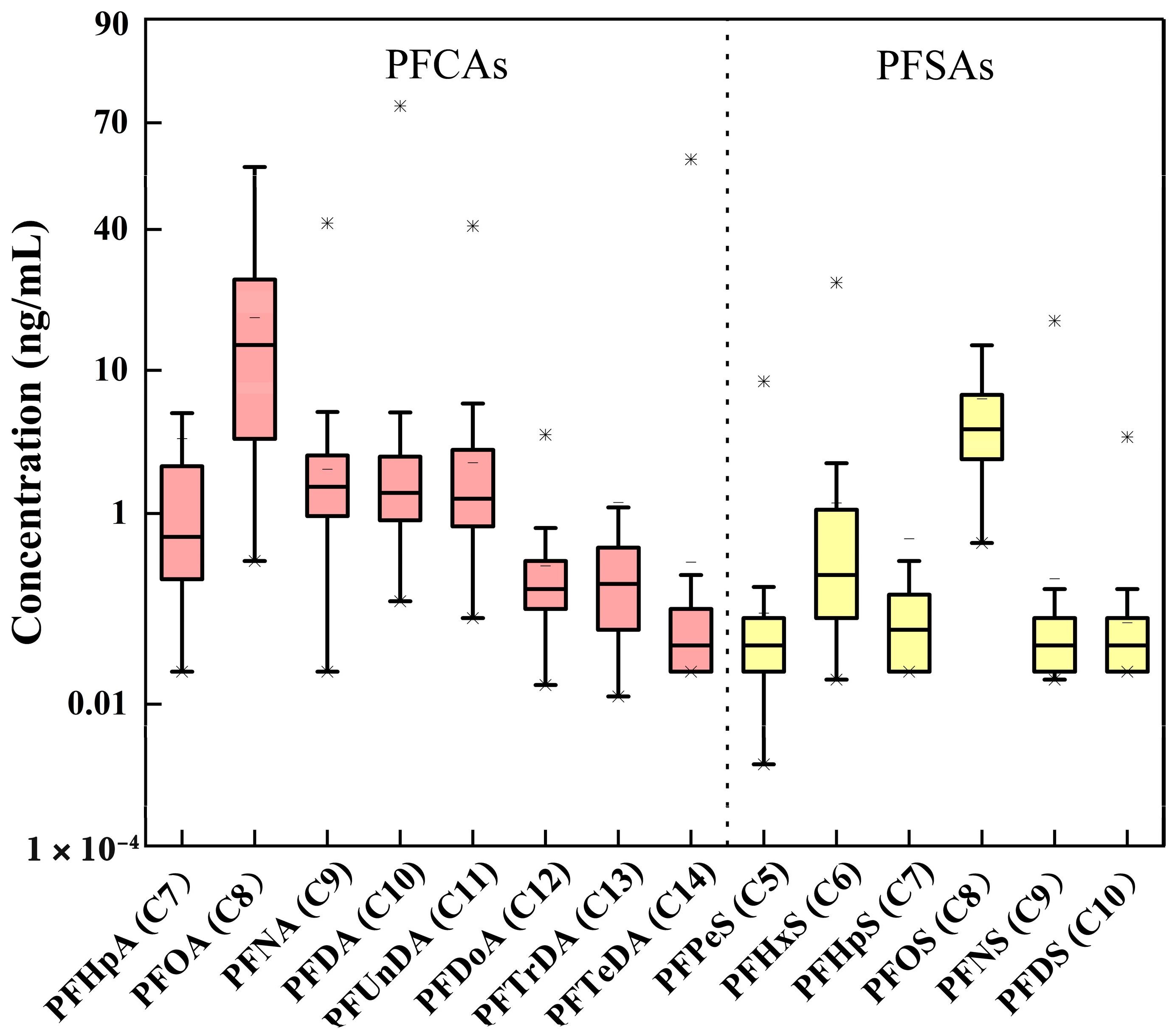
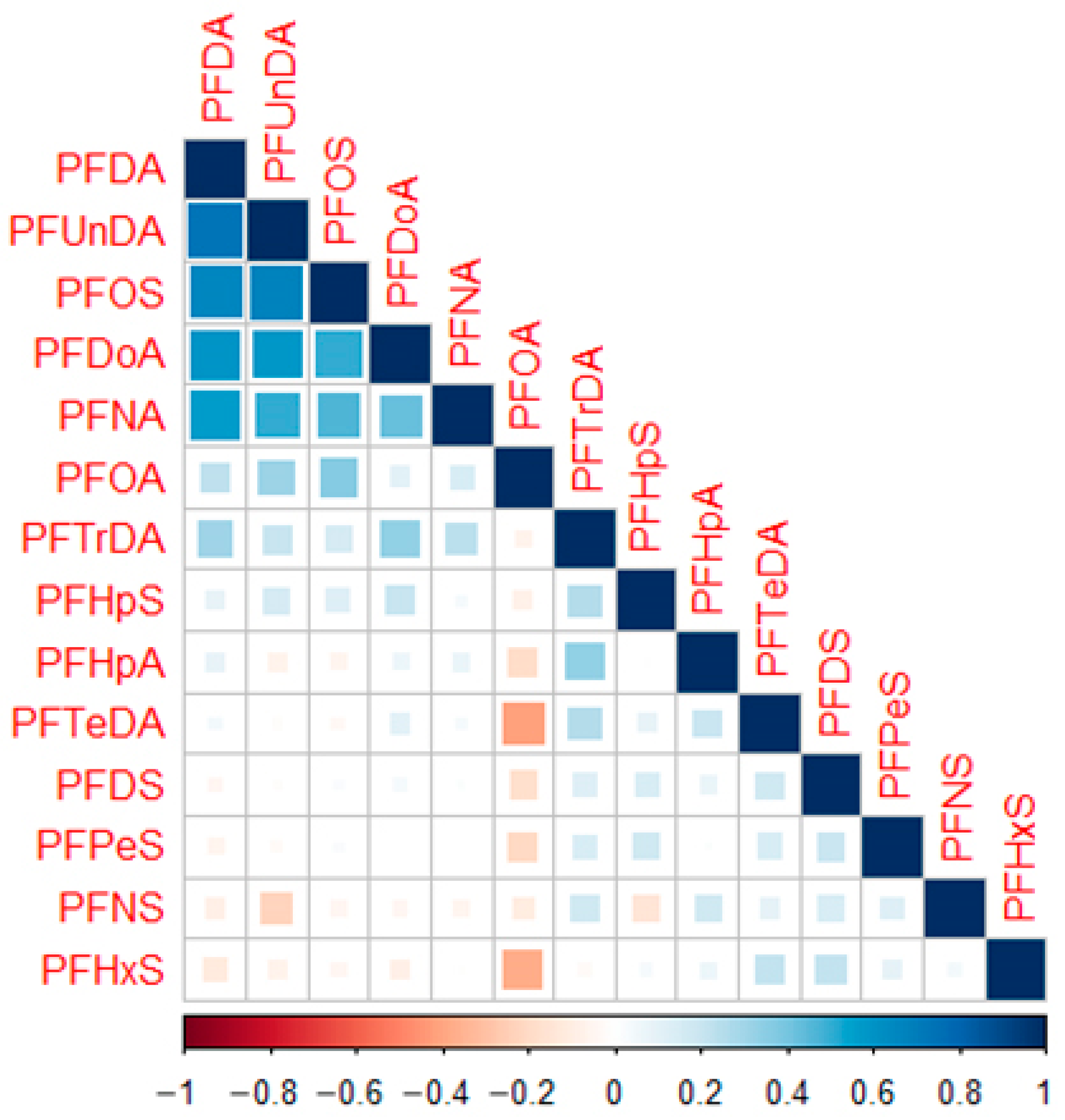


| PFHpA | PFOA | PFNA | PFDA | PFUnDA | PFDoA | PFTrDA | |
|---|---|---|---|---|---|---|---|
| Maternal age | |||||||
| <30 | 0.045 ± 0.756 | 0.957 ± 0.526 | 0.140 ± 0.374 | 0.163 ± 0.399 | 0.091 ± 0.419 | 0.711 ± 0.410 | 0.648 ± 0.578 |
| 30–35 | 0.142 ± 0.763 | 0.963 ± 0.561 | 0.212 ± 0.366 | 0.192 ± 0.374 | 0.174 ± 0.446 | 0.675 ± 0.411 | 0.644 ± 0.642 |
| >35 | 0.259 ± 0.757 | 1.22 ± 0.415 | 0.299 ± 0.364 | 0.340 ± 0.455 | 0.368 ± 0.420 | 0.578 ± 0.372 | 0.634 ± 0.514 |
| p | 0.123 | 0.000 ** | 0.003 * | 0.003 * | 0.000 ** | 0.043 * | 0.986 |
| Prenatal BMI | |||||||
| <25 | 0.068 ± 0.726 | 0.938 ± 0.569 | 0.217 ± 0.335 | 0.237 ± 0.354 | 0.196 ± 0.414 | 0.650 ± 0.393 | 0.607 ± 0.555 |
| 25–30 | 0.125 ± 0.773 | 1.023 ± 0.510 | 0.190 ± 0.393 | 0.179 ± 0.406 | 0.171 ± 0.455 | 0.687 ± 0.405 | 0.667 ± 0.629 |
| >30 | 0.279 ± 0.765 | 1.098 ± 0.508 | 0.191 ± 0.388 | 0.220 ± 0.482 | 0.136 ± 0.452 | 0.678 ± 0.446 | 0.655 ± 0.580 |
| p | 0.181 | 0.071 | 0.758 | 0.335 | 0.614 | 0.663 | 0.760 |
| Mode of delivery | |||||||
| Spontaneous labor | 0.057 ± 0.748 | 0.850 ± 0.543 | 0.171 ± 0.368 | 0.136 ± 0.360 | 0.109 ± 0.415 | 0.712 ± 0.412 | 0.656 ± 0.594 |
| cesarean birth | 0.176 ± 0.769 | 1.14 ± 0.487 | 0.221 ± 0.375 | 0.264 ± 0.428 | 0.229 ± 0.453 | 0.640 ± 0.399 | 0.633 ± 0.599 |
| p | 0.110 | 0.000 ** | 0.136 | 0.000 ** | 0.002 * | 0.050 * | 0.679 |
| Parity | |||||||
| 1 | 0.101 ± 0.762 | 1.01 ± 0.505 | 0.121 ± 0.368 | 0.134 ± 0.393 | 0.088 ± 0.432 | 0.726 ± 0.396 | 0.719 ± 0.619 |
| 2 | 0.167 ± 0.769 | 0.953 ± 0.584 | 0.211 ± 0.401 | 0.197 ± 0.390 | 0.176 ± 0.450 | 0.658 ± 0.382 | 0.626 ± 0.600 |
| ≥3 | 0.124 ± 0.758 | 1.04 ± 0.517 | 0.295 ± 0.325 | 0.316 ± 0.406 | 0.296 ± 0.415 | 0.610 ± 0.437 | 0.550 ± 0.544 |
| p | 0.772 | 0.002 * | 0.000 ** | 0.000 ** | 0.000 ** | 0.028 * | 0.037 * |
| PFTeDA | PFOS | PFNS | PFHxS | PFPeS | PFHpS | PFDS | |
| Maternal age | |||||||
| <30 | 1.21 ± 0.510 | 0.563 ± 0.336 | 1.18 ± 0.569 | 0.485 ± 0.722 | 1.23 ± 0.358 | 1.19 ± 0.478 | 1.28 ± 0.348 |
| 30–35 | 1.13 ± 0.527 | 0.671 ± 0.387 | 1.19 ± 0.542 | 0.503 ± 0.749 | 1.19 ± 0.453 | 1.08 ± 0.568 | 1.25 ± 0.369 |
| >35 | 1.07 ± 0.537 | 0.803 ± 0.366 | 1.19 ± 0.416 | 0.696 ± 0.736 | 1.32 ± 0.325 | 0.95 ± 0.453 | 1.34 ± 0.321 |
| p | 0.215 | 0.000 ** | 0.972 | 0.129 | 0.049 * | 0.006 ** | 0.201 |
| Prenatal BMI | |||||||
| <25 | 1.14 ± 0.530 | 0.660 ± 0.372 | 1.18 ± 0.530 | 0.411 ± 0.728 | 1.24 ± 0.351 | 1.19 ± 0.519 | 1.28 ± 0.320 |
| 25–30 | 1.15 ± 0.499 | 0.643 ± 0.377 | 1.19 ± 0.543 | 0.581 ± 0.730 | 1.19 ± 0.437 | 1.02 ± 0.528 | 1.26 ± 0.392 |
| >30 | 1.14 ± 0.596 | 0.656 ± 0.370 | 1.19 ± 0.521 | 0.579 ± 0.745 | 1.29 ± 0.353 | 1.15 ± 0.468 | 1.32 ± 0.288 |
| p | 0.221 | 0.767 | 0.985 | 0.546 | 0.067 | 0.133 | 0.501 |
| Mode of delivery | |||||||
| Spontaneous labor | 1.22 ± 0.487 | 0.586 ± 0.360 | 1.23 ± 0.508 | 0.365 ± 0.725 | 1.17 ± 0.413 | 1.16 ± 0.479 | 1.25 ± 0.336 |
| cesarean birth | 1.10 ± 0.541 | 0.705 ± 0.374 | 1.15 ± 0.550 | 0.675 ± 0.721 | 1.27 ± 0.380 | 1.05 ± 0.548 | 1.30 ± 0.368 |
| p | 0.044 * | 0.000 ** | 0.099 | 0.000 ** | 0.004 * | 0.057 | 0.101 |
| Parity | |||||||
| 1 | 1.19 ± 0.525 | 0.612 ± 0.365 | 1.12 ± 0.580 | 0.547 ± 0.737 | 1.21 ± 0.379 | 1.13 ± 0.522 | 1.27 ± 0.346 |
| 2 | 1.14 ± 0.520 | 0.644 ± 0.376 | 1.19 ± 0.563 | 0.347 ± 0.714 | 1.20 ± 0.393 | 1.18 ± 0.450 | 1.31 ± 0.340 |
| ≥3 | 1.10 ± 0.525 | 0.713 ± 0.375 | 1.27 ± 0.407 | 0.678 ± 0.733 | 1.26 ± 0.434 | 0.968 ± 0.562 | 1.25 ± 0.377 |
| p | 0.447 | 0.041 * | 0.046 * | 0.003 ** | 0.408 | 0.008 ** | 0.371 |
| Birth Weight | Apgar-1 | Apgar-5 | ||||
|---|---|---|---|---|---|---|
| Compounds | β (95% CI) | p | β (95% CI) | p | β (95% CI) | p |
| PFHpA | ||||||
| Univariate a | 0.072 (−0.025, 0.169) | 0.145 | 0.079 (0.015, 0.142) | 0.016 * | 0.004 (−0.013, 0.022) | 0.629 |
| Fully adjusted b | 0.091 (−0.002, 0.183) | 0.056 | 0.065 (0.002, 0.129) | 0.044 * | 0.002 (−0.016, 0.020) | 0.841 |
| PFOA | ||||||
| Univariate a | −0.153 (−0.274, −0.031) | 0.014 * | 0.049 (−0.128, 0.029) | 0.215 | −0.001 (−0021, 0.020) | 0.960 |
| Fully adjusted b | −0.110 (−0.232, 0.012) | 0.077 | −0.006 (−0.086, 0.075) | 0.887 | 0.001 (−0.021, 0.024) | 0.902 |
| PFNA | ||||||
| Univariate a | −0.076 (−0.252, 0.100) | 0.395 | −0.007 (−0.119, 0.105) | 0.907 | −0.007 (−0.037, 0.023) | 0.653 |
| Fully adjusted b | −0.021 (−0.191, 0.149) | 0.807 | −0.032 (−0.144, 0.081) | 0.582 | −0.012 (−0.043, 0.019) | 0.463 |
| PFDA | ||||||
| Univariate a | −0.051 (−0.214, 0.113) | 0.544 | 0.005 (−0.099, 0.110) | 0.918 | 0.007 (−0.021, 0.035) | 0.631 |
| Fully adjusted b | 0.034 (−0.125, 0.194) | 0.674 | 0.010 (−0.096, 0.116) | 0.855 | 0.004 (−0.025, 0.033) | 0.772 |
| PFUnDA | ||||||
| Univariate a | −0.084 (−0.232, 0.064) | 0.266 | 0.034 (−0.061, 0.128) | 0.483 | 0.010 (−0.015, 0.035) | 0.425 |
| Fully adjusted b | −0.017 (−0.163, 0.128) | 0.814 | 0.028 (−0.068, 0.124) | 0.571 | 0.008 (−0.018, 0.035) | 0.535 |
| PFDoA | ||||||
| Univariate a | 0.004 (−0.161, 0.168) | 0.964 | 0.039 (−0.063, 0.141) | 0.455 | 0.028 (0.000, 0.056) | 0.054 |
| Fully adjusted b | 0.068 (−0.089, 0.225) | 0.396 | 0.027 (−0.074, 0.129) | 0.597 | 0.027 (−0.002, 0.055) | 0.068 |
| PFTrDA | ||||||
| Univariate a | 0.009 (−0.108, 0.125) | 0.885 | 0.001 (−0.074, 0.076) | 0.976 | −0.021 (−0.032, 0.008) | 0.249 |
| Fully adjusted b | 0.023 (−0.088, 0.134) | 0.684 | −0.017 (−0.091, 0.057) | 0.654 | −0.014 (−0.034, 0.007) | 0.182 |
| PFTeDA | ||||||
| Univariate a | −0.088 (−0.260, 0.084) | 0.314 | −0.054 (−0.167, 0.059) | 0.347 | −0.004 (−0.026, 0.017) | 0.677 |
| Fully adjusted b | −0.069 (−0.240, 0.101) | 0.425 | −0.049 (−0.160, 0.063) | 0.389 | −0.004 (−0.026, 0.017) | 0.702 |
| PFHxS | ||||||
| Univariate a | 0.123 (0.024, 0.223) | 0.015 * | 0.057 (−0.008, 0.121) | 0.087 | 0.008 (−0.006, 0.022) | 0.258 |
| Fully adjusted b | 0.108 (0.012, 0.204) | 0.028 * | 0.040 (−0.024, 0.105) | 0.221 | −0.006 (−0.008, 0.021) | 0.378 |
| PFPeS | ||||||
| Univariate a | 0.226 (0.053, 0.398) | 0.010 * | 0.139 (0.027, 0.251) | 0.015 * | 0.024 (−0.008, 0.056) | 0.137 |
| Fully adjusted b | 0.171 (0.008, 0.333) | 0.039 * | 0.117 (0.006, 0.228) | 0.039 * | 0.023 (−0.009, 0.056) | 0.153 |
| PFHpS | ||||||
| Univariate a | −0.018 (0.174, 0.138) | 0.824 | 0.069 (−0.036, 0.174) | 0.194 | 0.009 (−0.020, 0.039) | 0.528 |
| Fully adjusted b | −0.014 (−0.162, 0.133) | 0.849 | 0.080 (−0.023, 0.184) | 0.129 | 0.011 (−0.019, 0.041) | 0.460 |
| PFOS | ||||||
| Univariate a | −0.217 (−0.385, −0.049) | 0.012 * | 0.008 (−0.104, 0.120) | 0.883 | 0.006 (−0.024, 0.036) | 0.678 |
| Fully adjusted b | 0.037 (−0.071, 0.145) | 0.498 | 0.073 (−0.043, 0.189) | 0.217 | 0.018 (−0.014, 0.050) | 0.272 |
| PFNS | ||||||
| Univariate a | −0.137 (−0.270, −0.003) | 0.044 * | −0.024 (−0.107, 0.059) | 0.569 | −0.011 (−0.028, 0.006) | 0.192 |
| Fully adjusted b | 0.045 (−0.037, 0.126) | 0.284 | 0.018 (−0.066, 0.102) | 0.673 | −0.005 (−0.023, 0.012) | 0.549 |
| PFDS | ||||||
| Univariate a | −0.104 (−0.234, 0.026) | 0.117 | −0.007 (−0.089, 0.075) | 0.863 | −0.008 (−0.025, 0.008) | 0.326 |
| Fully adjusted b | 0.171 (−0.018, 0.361) | 0.076 | 0.014 (−0.121, 0.149) | 0.836 | −0.001 (−0.040, 0.037) | 0.951 |
| PFAS Mixture | Estimates | 95% CI | p Value |
|---|---|---|---|
| birth weight a | 0.096 | −0.170, 0.363 | 0.479 |
| Apgar-1 a | 0.324 | 0.068, 0.579 | 0.013 * |
| Apgar-5 a | 0.128 | −0.083, 0.399 | 0.234 |
| preterm birth b | 0.356 | 0.149, 0.845 | 0.019* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.; Ding, J.; Xu, C.; Zhang, L.; Liu, S.; Tian, Y. Perfluoroalkyl Mixture Exposure in Relation to Fetal Growth: Potential Roles of Maternal Characteristics and Associations with Birth Outcomes. Toxics 2022, 10, 650. https://doi.org/10.3390/toxics10110650
Shen C, Ding J, Xu C, Zhang L, Liu S, Tian Y. Perfluoroalkyl Mixture Exposure in Relation to Fetal Growth: Potential Roles of Maternal Characteristics and Associations with Birth Outcomes. Toxics. 2022; 10(11):650. https://doi.org/10.3390/toxics10110650
Chicago/Turabian StyleShen, Chensi, Jiaxin Ding, Chenye Xu, Long Zhang, Shuren Liu, and Yonghong Tian. 2022. "Perfluoroalkyl Mixture Exposure in Relation to Fetal Growth: Potential Roles of Maternal Characteristics and Associations with Birth Outcomes" Toxics 10, no. 11: 650. https://doi.org/10.3390/toxics10110650
APA StyleShen, C., Ding, J., Xu, C., Zhang, L., Liu, S., & Tian, Y. (2022). Perfluoroalkyl Mixture Exposure in Relation to Fetal Growth: Potential Roles of Maternal Characteristics and Associations with Birth Outcomes. Toxics, 10(11), 650. https://doi.org/10.3390/toxics10110650







