Characterization of the Toxicological Impact of Heavy Metals on Human Health in Conjunction with Modern Analytical Methods
Abstract
1. Introduction
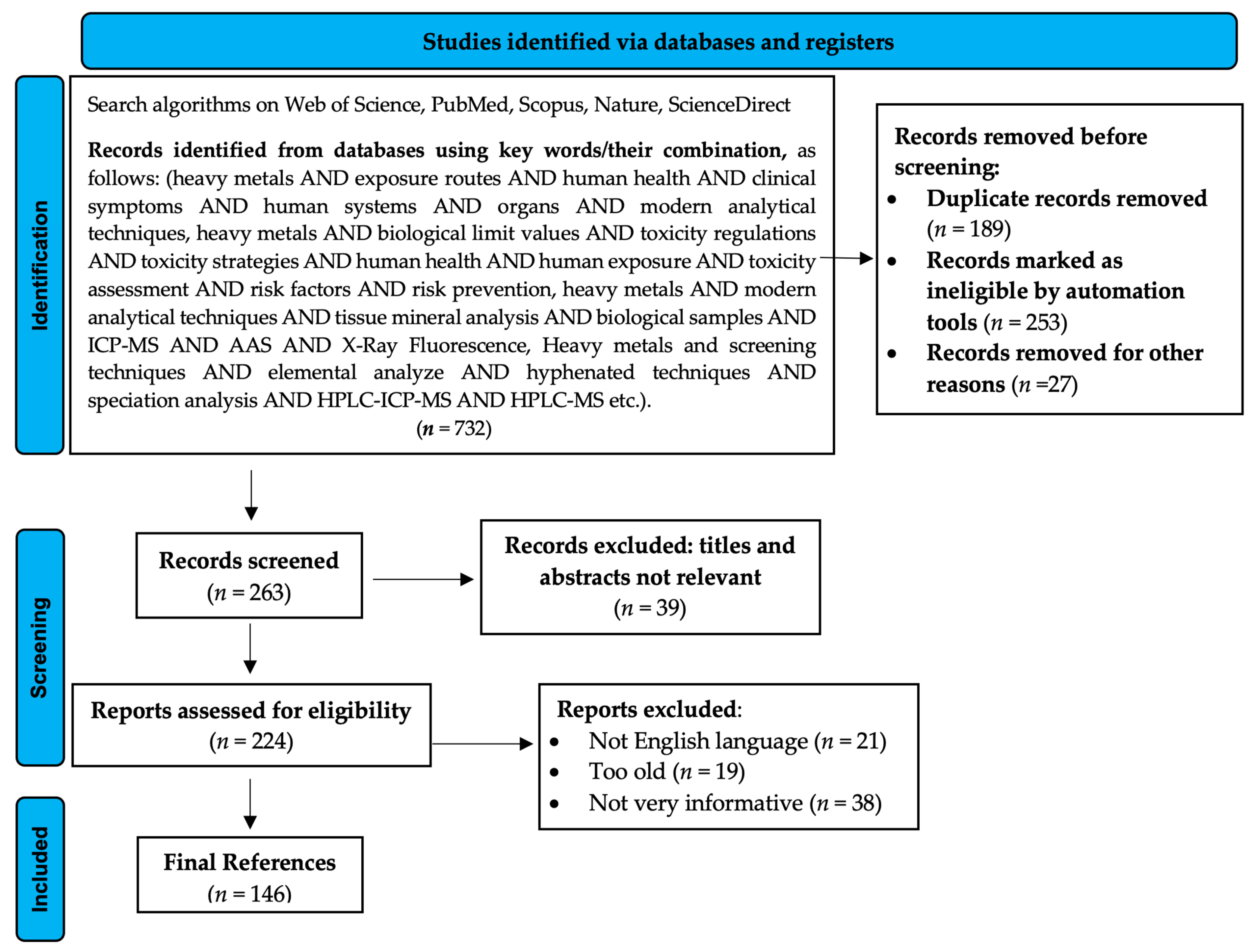
2. Criteria for Literature Selection
3. Bibliometric Analysis of the Topic in the Literature
3.1. Data Collection
3.2. Literature Results Obtained and Discussion
3.2.1. From 1975 to 2010
3.2.2. From 2011 to Present
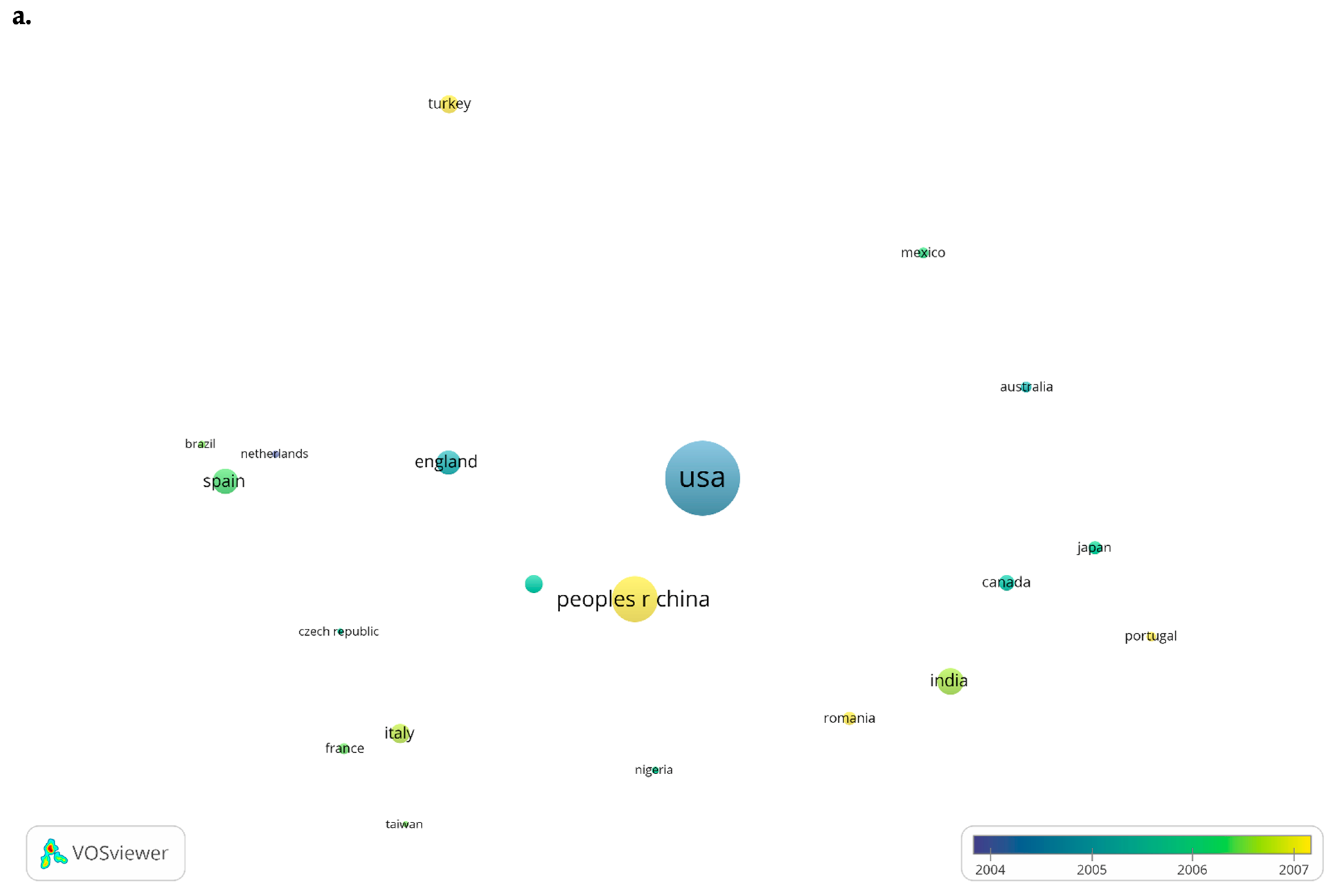
3.2.3. Leading Countries in Publishing Papers Regarding Heavy Metal Detection
- The red cluster includes Belgium, Brazil, Czech Republic, England, France, Germany, Greece, Iran, Italy, Mexico, Poland, Portugal, Romania, Russia, Spain, Sweden, Switzerland, and Turkey;
- The green cluster includes Australia, Bangladesh, Egypt, India, Japan, Malaysia, Saudi Arabia, South Korea, Taiwan, and Thailand;
- The blue cluster includes Canada, Pakistan, China, and the United States;
- The yellow cluster includes Nigeria and South Africa.
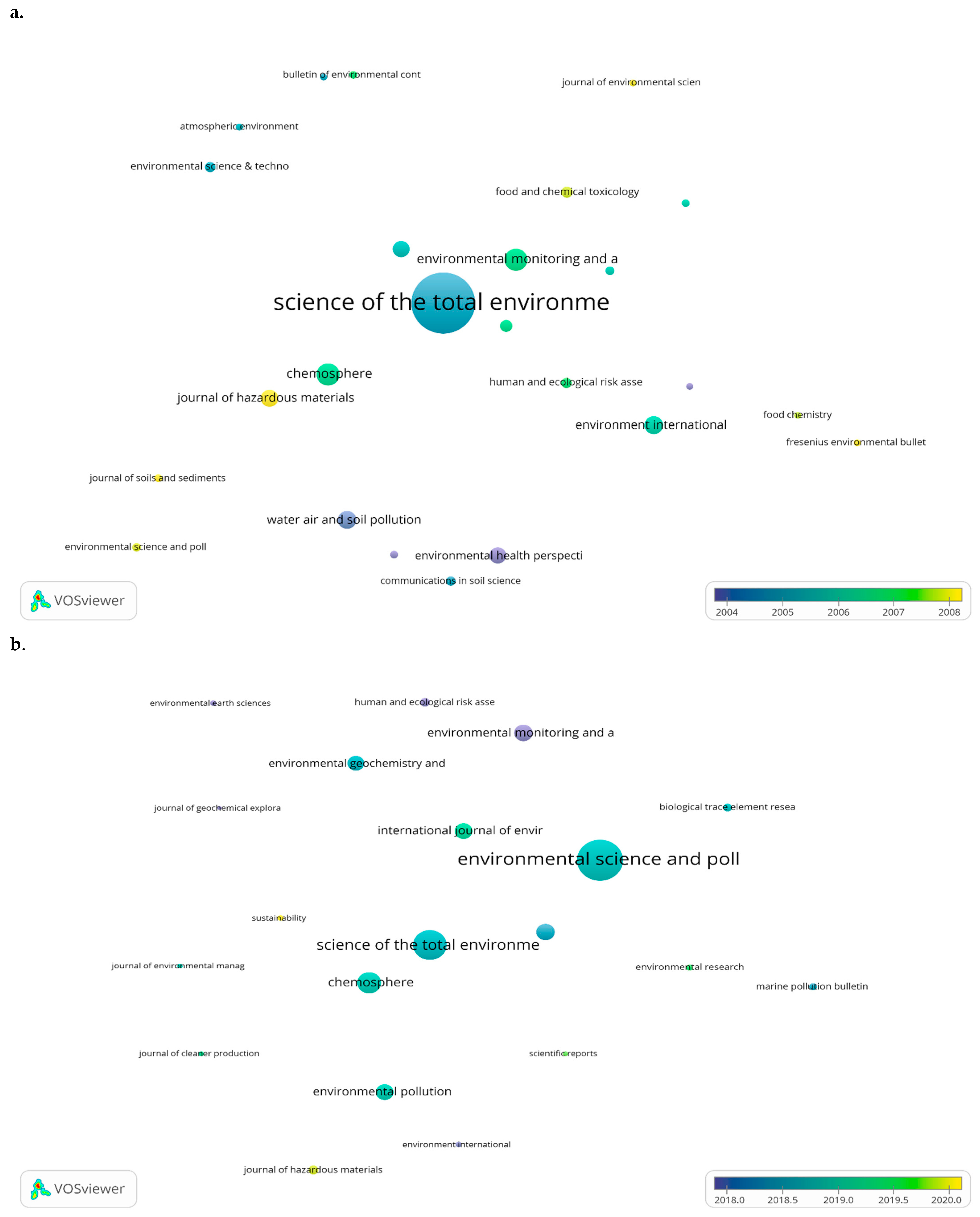
3.2.4. Most Cited Articles and Leading Journals in Publishing Papers Regarding Heavy Metal Detection
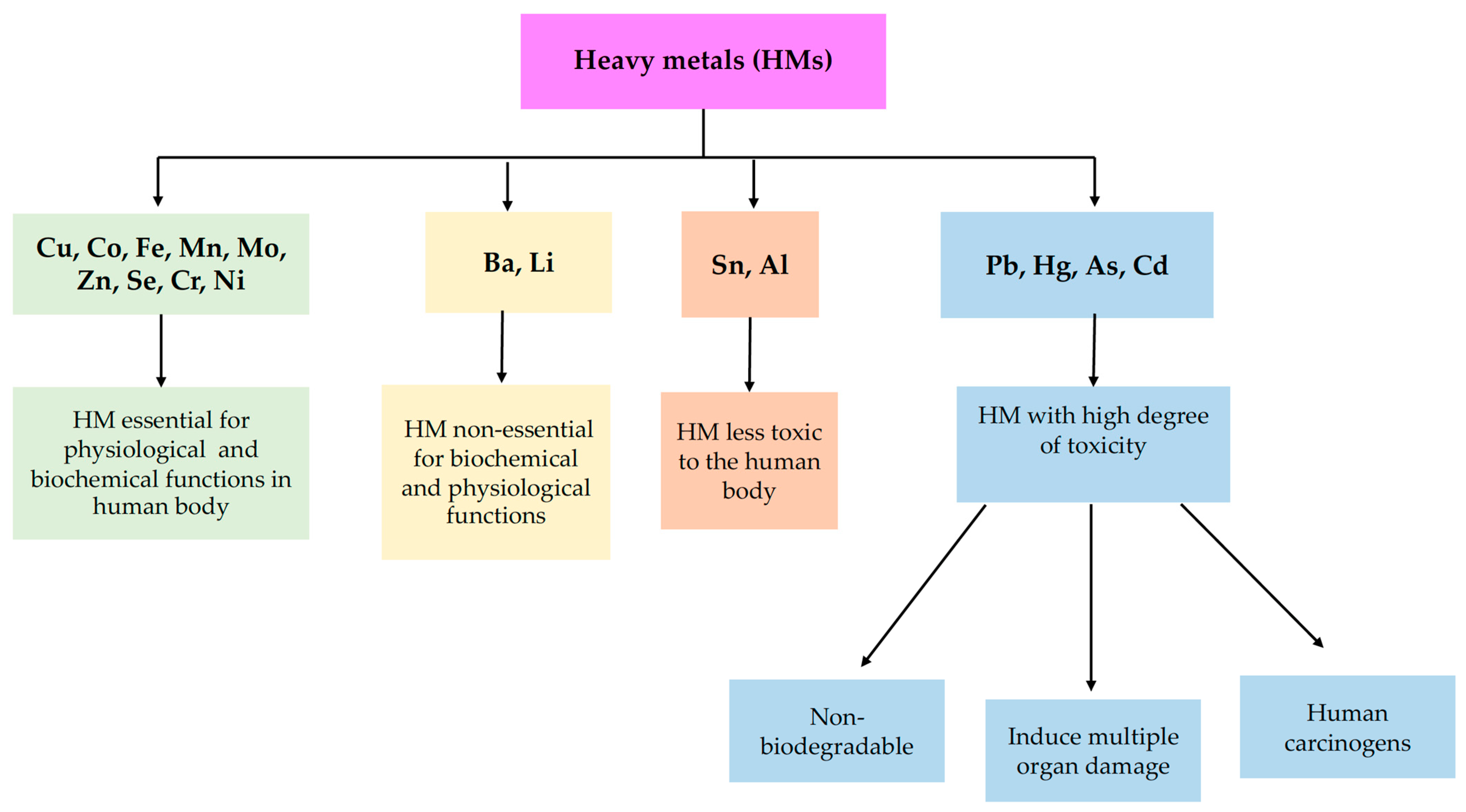
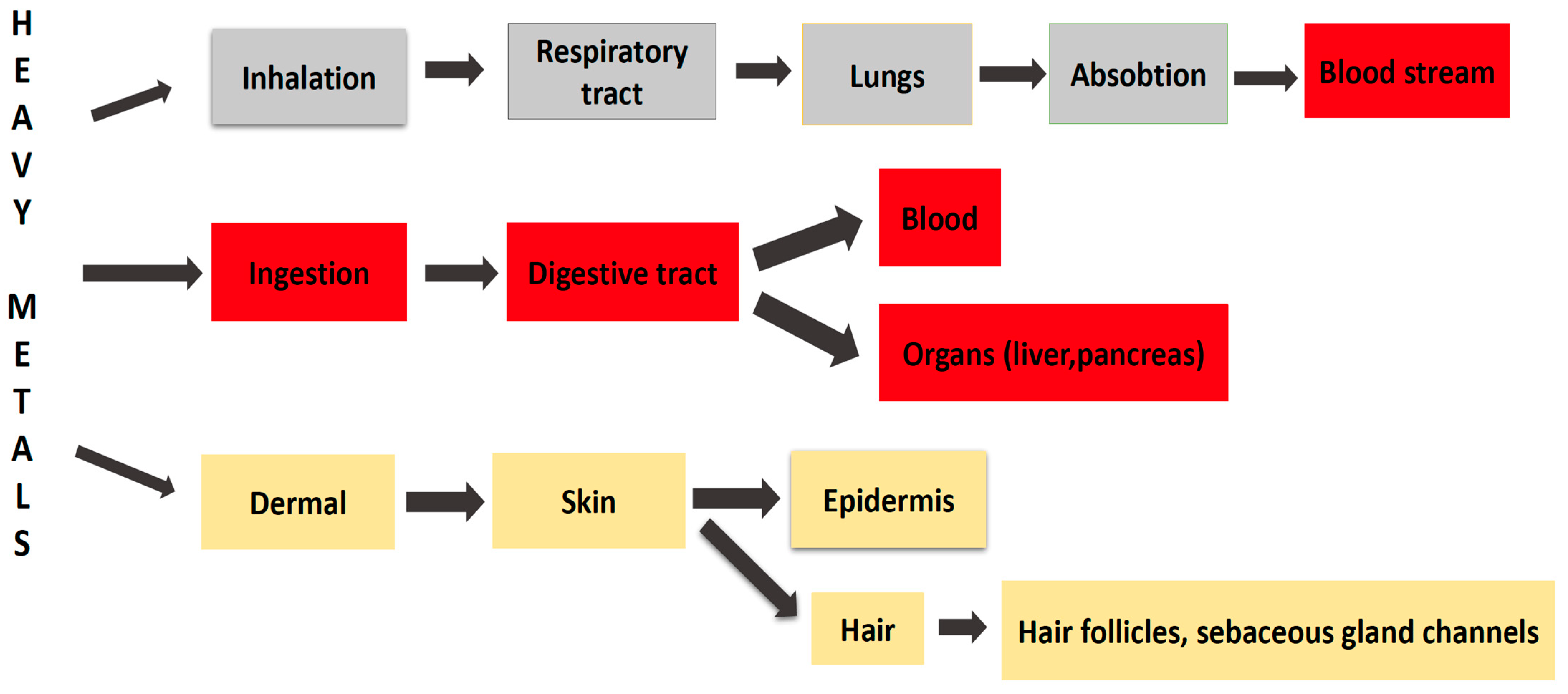
4. Exposure Routes to HMs and Toxicity Regulations
- ingestion (via food or water, reaching the bloodstream and various organs, such as the pancreas, and liver, etc., through the absorption process), as is the case for As, Pb, Hg, and Cd;
- inhalation (through the inhalation of air, vapors or aerosols, toxic metals enter the respiratory tract, reach the lungs and then the bloodstream), as is the case for Pb, Hg, and Al;
5. The Importance of Tissue Mineral Analysis
6. Human Biomarkers—An Assessing Health Status Factor
6.1. Hair, Nails, Urine, Blood, or Vitreous Humor Analysis
6.1.1. Human Hair and the Trace Elements Stored in It
6.1.2. Finger- and Toenails
6.1.3. Vitreous Humor and Epithelial Tissues (Human Eyes)
6.1.4. Blood and Urine
6.2. Different Tissues and Organs
6.2.1. Teeth and Bones
6.2.2. Tissues and Organs
7. Modern Analytical Methods of Determination
7.1. Lymphoblastic Transformation Test—HMs
7.2. X-ray Fluorescence
7.3. Atomic Absorption Spectrometry
7.4. Inductively Coupled Plasma Mass Spectrometry
7.5. Hyphenated Techniques and Speciation Analytics
8. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singhal, H.R.; Mani, N.K.; Kodgi, A.; Mehendale, N.; Sharma, S.; Garlapati, V.K. Chapter 10-Miniaturized microfluidic heuristics for the detection of polluting molecules in the environment. In Handbook on Miniaturization in Analytical Chemistry; Hussain, C.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 221–235. [Google Scholar]
- Griffin, R.M. WebMD, Heavy Metal Poisoning. Available online: https://www.webmd.com/a-to-z-guides/what-is-heavy-metal-poisoning (accessed on 17 April 2022).
- Huamain, C.; Chunrong, Z.; Cong, T.; Yongguan, Z. Heavy metal pollution in soils in China: Status and countermeasures. Ambio 1999, 28, 130–134. [Google Scholar]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-C.; Priambodo, R.; Huang, R.-L.; Huang, Y.-H. The Effective Electrolytic Recovery of Dilute Copper from Industrial Wastewater. J. Waste Manag. 2013, 2013, 164780. [Google Scholar] [CrossRef]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef]
- Bolan, S.; Kunhikrishnan, A.; Seshadri, B.; Choppala, G.; Naidu, R.; Bolan, N.S.; Ok, Y.S.; Zhang, M.; Li, C.-G.; Li, F.; et al. Sources, distribution, bioavailability, toxicity, and risk assessment of heavy metal(loid)s in complementary medicines. Environ. Int. 2017, 108, 103–118. [Google Scholar] [CrossRef]
- Nabulo, G.; Black, C.; Young, S. Trace metal uptake by tropical vegetables grown on soil amended with urban sewage sludge. Environ. Pollut. 2011, 159, 368–376. [Google Scholar] [CrossRef]
- Savic Gajic, I.; Savic, I.; Gajic, D. The role and health risk of heavy metals in human organism. In Heavy Metals and Health; Castillo, L., Ed.; Nova Science Publisher: Hauppauge, NY, USA, 2016; pp. 47–89. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef]
- Ali, H.; Khan, E.; Sajad, M.A. Phytoremediation of heavy metals—Concepts and applications. Chemosphere 2013, 91, 869–881. [Google Scholar] [CrossRef]
- Salt, D.E.; Smith, R.D.; Raskin, I. PHYTOREMEDIATION. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998, 49, 643–668. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; van der Kuijp, T.J.; Yuan, Z.; Huang, L. A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment. Sci. Total Environ. 2014, 468, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Engwa, G.; Okeke, P.; Nwalo, N.; Unachukwu, M. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In Poisoning in the Modern World—New Tricks for an Old Dog; Intechopen: London, UK, 2019; pp. 1–23. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Flora, S.J. Arsenic-induced oxidative stress and its reversibility. Free Radic. Biol. Med. 2011, 51, 257–281. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.L. Trace elements and neuropsychological problems as reflected in tissue mineral analysis (TMA) patterns. J. Orthomol. Med. 1990, 5, 159–166. [Google Scholar]
- Behl, T.; Kaur, G.; Sehgal, A.; Bhardwaj, S.; Singh, S.; Buhas, C.; Judea-Pusta, C.; Uivarosan, D.; Munteanu, M.A.; Bungau, S. Multifaceted Role of Matrix Metalloproteinases in Neurodegenerative Diseases: Pathophysiological and Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 1413. [Google Scholar] [CrossRef]
- Kabir, M.T.; Uddin, M.S.; Zaman, S.; Begum, Y.; Ashraf, G.M.; Bin-Jumah, M.N.; Bungau, S.G.; Mousa, S.A.; Abdel-Daim, M.M. Molecular Mechanisms of Metal Toxicity in the Pathogenesis of Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 1–20. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicol. 2012, 5, 47. [Google Scholar] [CrossRef]
- Karagas, M.R.; Choi, A.L.; Oken, E.; Horvat, M.; Schoeny, R.; Kamai, E.; Cowell, W.; Grandjean, P.; Korrick, S. Evidence on the human health effects of low-level methylmercury exposure. Environ. Health Perspect. 2012, 120, 799–806. [Google Scholar] [CrossRef]
- Hong, Y.-S.; Kim, Y.-M.; Lee, K.-E. Methylmercury exposure and health effects. J. Prev. Med. Public Health 2012, 45, 353. [Google Scholar] [CrossRef]
- Charkiewicz, A.; Backstrand, J. Lead Toxicity and Pollution in Poland. Int. J. Environ. Res. Public Health 2020, 17, 4385. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Chen, C.; Ahn, W.-S. Chromium terephthalate metal–organic framework MIL-101: Synthesis, functionalization, and applications for adsorption and catalysis. RSC Adv. 2014, 4, 52500–52525. [Google Scholar] [CrossRef]
- Andrade, V.; Aschner, M.; Marreilha Dos Santos, A. Neurotoxicity of metal mixtures. Neurotox. Met. 2017, 18, 227–265. [Google Scholar]
- Ratnaike, R.N. Acute and chronic arsenic toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef]
- Bjørklund, G.; Oliinyk, P.; Lysiuk, R.; Rahaman, M.; Antonyak, H.; Lozynska, I.; Lenchyk, L.; Peana, M. Arsenic intoxication: General aspects and chelating agents. Arch. Toxicol. 2020, 94, 1879–1897. [Google Scholar] [CrossRef]
- Ikechukwu, U.R.; Okpashi, V.E.; Oluomachi, U.N.; Paulinus, N.C.; Obiageli, N.F.; Precious, O. Evaluation of heavy metals in selected fruits in Umuahia market, Nigeria: Associating toxicity to effect for improved metal risk assessment. J. Appl. Biol. Biotechnol. 2019, 7, 3–5. [Google Scholar]
- Syversen, T.; Kaur, P. The toxicology of mercury and its compounds. J. Trace Elem. Med. Biol. 2012, 26, 215–226. [Google Scholar] [CrossRef]
- Jiao, J.; Wang, M.; Wang, Y.; Sun, N.; Li, C. Lead exposure increases blood pressure by increasing angiotensinogen expression. J. Environ. Sci. Health A Tox. Hazard. Subst. Environ. Eng. 2016, 51, 434–439. [Google Scholar] [CrossRef]
- Gambelunghe, A.; Sallsten, G.; Borné, Y.; Forsgard, N.; Hedblad, B.; Nilsson, P.; Fagerberg, B.; Engström, G.; Barregard, L. Low-level exposure to lead, blood pressure, and hypertension in a population-based cohort. Environ. Res. 2016, 149, 157–163. [Google Scholar] [CrossRef]
- Sharma, B.; Singh, S.; Siddiqi, N. Biomedical Implications of Heavy Metals Induced Imbalances in Redox Systems. BioMed Res. Int. 2014, 2014, 640754. [Google Scholar] [CrossRef]
- Dongre, N.N.; Suryakar, A.N.; Patil, A.J.; Hundekari, I.A.; Devarnavadagi, B.B. Biochemical effects of lead exposure on battery manufacture workers with reference to blood pressure, calcium metabolism and bone mineral density. Indian J. Clin. Biochem. 2013, 28, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Kall, J.; Just, A.; Aschner, M. What is the risk? Dental amalgam, mercury exposure, and human health risks throughout the life span. In Epigenetics, the Environment, and Children’s Health Across Lifespans; Springer: Berlin/Heidelberg, Germany, 2016; pp. 159–206. [Google Scholar]
- Agency for Toxic Substances and Disease Registry, Toxicological Profile for Mercury. Available online: https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=115&tid=24 (accessed on 10 December 2021).
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. In Molecular, Clinical and Environmental Toxicology: Volume 3: Environmental Toxicology; Luch, A., Ed.; Springer: Basel, Switzerland, 2012; pp. 133–164. [Google Scholar]
- Wong, C.; Roberts, S.M.; Saab, I.N. Review of regulatory reference values and background levels for heavy metals in the human diet. Regul. Toxicol. Pharmacol. 2022, 130, 105122. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Cao, Q.; Zheng, Y.M.; Huang, Y.Z.; Zhu, Y.G. Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut. 2008, 152, 686–692. [Google Scholar] [CrossRef]
- Naz, A.; Chowdhury, A.; Mishra, B.K.; Karthikeyan, K. Distribution of heavy metals and associated human health risk in mine, agricultural and roadside soils at the largest chromite mine of India. Environ. Geochem. Health 2018, 40, 2155–2175. [Google Scholar] [CrossRef] [PubMed]
- Kafka, Z.; Punčochárová, J.; Švadlenka, J.; Kuraš, M. Determination of acute toxicity of heavy metals. Toxicol. Environ. Chem. 1997, 63, 119–124. [Google Scholar] [CrossRef]
- Alam, M.K.; Maughan, O.E. Acute toxicity of heavy metals to common carp (Cyprinus carpio). J. Environ. Sci. Health Part A Environ. Sci. Eng. Toxicol. 1995, 30, 1807–1816. [Google Scholar] [CrossRef]
- Aldoghachi, M.; Rahman, M.; Yusoff, I.; Azirun, M. Acute toxicity and bioaccumulation of heavy metals in red tilapia fish. JAPS J. Anim. Plant Sci. 2016, 26, 507–513. [Google Scholar]
- Cai, L.-M.; Xu, Z.-C.; Qi, J.-Y.; Feng, Z.-Z.; Xiang, T.-S. Assessment of exposure to heavy metals and health risks among residents near Tonglushan mine in Hubei, China. Chemosphere 2015, 127, 127–135. [Google Scholar] [CrossRef]
- Tang, J.; Huang, Z.; Pan, X.-D. Exposure assessment of heavy metals (Cd, Hg, and Pb) by the intake of local foods from Zhejiang, China. Environ. Geochem. Health 2014, 36, 765–771. [Google Scholar] [CrossRef]
- Government Decision no. 1216 of 2006 on the Establishment of Minimum Occupational Safety and Health Requirements to Ensure the Protection of Workers against Risks Related to the Presence of Chemical Agents. Available online: https://legislatie.just.ro/Public/DetaliiDocument/75978 (accessed on 26 September 2022).
- Omrane, F.; Gargouri, I.; Khadhraoui, M.; Elleuch, B.; Zmirou-Navier, D. Risk assessment of occupational exposure to heavy metal mixtures: A study protocol. BMC Public Health 2018, 18, 314. [Google Scholar] [CrossRef]
- Schenk, L. and Johanson, G. Will worker DNELs derived under the European REACH regulation extend the landscape of occupational exposure guidance values? Arch Toxicol 2019, 93, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- European Commission; Directorate-General for Employment; Social Affairs and Inclusion. Methodology for Derivation of Occupational Exposure Limits of Chemical Agents: The General Decision-Making Framework of the Scientific Committee on Occupational Exposure Limits (SCOEL) 2017; Publications Office of the European Union: Luxembourg, 2018. [Google Scholar]
- European Agency for Safety and Health at Work. Information about the European Opinion Polls on Safety and Health at Work; 2016. Available online: https://osha.europa.eu/en (accessed on 10 September 2022).
- MAK- und BAT-Werte-Liste 2020. Available online: https://series.publisso.de/sites/default/files/documents/series/mak/lmbv/Vol2020/Iss1/Doc001/mbwl_2020_deu.pdf (accessed on 10 November 2022).
- Sakai, T. Biomarkers of lead exposure. Ind. Health 2000, 38, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Asadauskaite, R.; Naginiene, R.; Abdrachmanovas, O. δ-Aminolevulinic Acid Dehydratase in Blood as a Biomarker for Low-Level Lead Exposure. Cent. Eur. J. Public Health 2007, 15. Available online: https://hdl.handle.net/20.500.12512/83745 (accessed on 20 November 2022).
- World Health Organization. Recommended Health-Based Limits in Occupational Exposure to Heavy Metals: Report of a WHO Study Group [Meeting Held in Geneva from 5 to 11 June 1979]; World Health Organization: Geneva, Switzerland, 1980. [Google Scholar]
- Lead Toxicity Workup. Available online: https://emedicine.medscape.com/article/1174752-workup (accessed on 11 October 2022).
- Flora, S. Metals. In Biomarkers in Toxicology; Gupta, R.C., Ed.; Academic Press: San Diego, CA, USA, 2014; Volume 1, pp. 485–519. [Google Scholar]
- Daphnelab. Available online: https://www.daphnelab.com/ro/home-ro/ (accessed on 19 January 2022).
- Dubey, P.; Thakur, V.; Chattopadhyay, M. Role of minerals and trace elements in diabetes and insulin resistance. Nutrients 2020, 12, 1864. [Google Scholar] [CrossRef]
- Shahid, M.; Pourrut, B.; Dumat, C.; Nadeem, M.; Aslam, M.; Pinelli, E. Heavy-metal-induced reactive oxygen species: Phytotoxicity and physicochemical changes in plants. Rev. Environ. Contam. Toxicol. 2014, 232, 1–44. [Google Scholar]
- Soetan, K.; Olaiya, C.O.; Oyewole, O.E. The importance of mineral elements for humans, domestic animals and plants—Review. Afr. J. Food Sci. 2010, 4, 200–222. [Google Scholar]
- Ghorbani, A.; Darmani kuhi, H.; Mohit, A. A review Hair tissue analysis: An analytical method for determining essential elements, toxic elements, hormones and drug use and abuse. Int. Res. J. Appl. Basic Sci. 2013, 4, 3675–3688. [Google Scholar]
- Mohmand, J.; Eqani, S.A.M.A.S.; Fasola, M.; Alamdar, A.; Mustafa, I.; Ali, N.; Liu, L.; Peng, S.; Shen, H. Human exposure to toxic metals via contaminated dust: Bio-accumulation trends and their potential risk estimation. Chemosphere 2015, 132, 142–151. [Google Scholar] [CrossRef]
- Tamás, M.; Sharma, S.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy Metals and Metalloids As a Cause for Protein Misfolding and Aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef]
- Ab Razak, N.H.; Praveena, S.M.; Hashim, Z. Toenail as a biomarker of heavy metal exposure via drinking water: A systematic review. Rev. Environ. Health 2015, 30, 1–7. [Google Scholar] [CrossRef]
- Cabral, M.; Toure, A.; Garçon, G.; Diop, C.; Bouhsina, S.; Dewaele, D.; Cazier, F.; Courcot, D.; Tall-Dia, A.; Shirali, P. Effects of environmental cadmium and lead exposure on adults neighboring a discharge: Evidences of adverse health effects. Environ. Pollut. 2015, 206, 247–255. [Google Scholar] [CrossRef] [PubMed]
- He, K. Trace elements in nails as biomarkers in clinical research. Eur. J. Clin. Investig. 2011, 41, 98–102. [Google Scholar] [CrossRef]
- Katz, S.A. On the use of hair analysis for assessing arsenic intoxication. Int. J. Environ. Res. Public Health 2019, 16, 977. [Google Scholar] [CrossRef] [PubMed]
- Chatt, A.; Katz, S.A. Hair Analysis: Applications in the Biomedical and Environmental Sciences; VCH: Weinheim, Germany, 1989. [Google Scholar]
- Quig, D. Trace element analysis in hair: Factors determining accuracy, precision, and reliability. Altern. Med. Rev. 2001, 6, 472–481. [Google Scholar]
- Park, S.B.; Choi, S.W.; Nam, A.Y. Hair tissue mineral analysis and metabolic syndrome. Biol. Trace Elem. Res. 2009, 130, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Yoshida, K.; Segawa, M.; Tokuda, R.; Tsutsui, T.; Yasuda, Y.; Magara, S. Metallomics study using hair mineral analysis and multiple logistic regression analysis: Relationship between cancer and minerals. Environ. Health Prev. Med. 2009, 14, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Kintz, P.; Ginet, M.; Marques, N.; Cirimele, V. Arsenic speciation of two specimens of Napoleon’s hair. Forensic Sci. Int. 2007, 170, 204–206. [Google Scholar] [CrossRef]
- Morton, J.; Carolan, V.A.; Gardiner, P.H. Removal of exogenously bound elements from human hair by various washing procedures and determination by inductively coupled plasma mass spectrometry. Anal. Chim. Acta 2002, 455, 23–34. [Google Scholar] [CrossRef]
- Chojnacka, K.; Górecka, H.; Chojnacki, A.; Górecki, H. Inter-element interactions in human hair. Environ. Toxicol. Pharmacol. 2005, 20, 368–374. [Google Scholar] [CrossRef]
- Kaur, N. Photochemical mediated reactions in five-membered O-heterocycles synthesis. Synth. Commun. 2018, 48, 2119–2149. [Google Scholar] [CrossRef]
- Rashed, M.N.; Hossam, F. Heavy Metals in Fingernails and Scalp Hair of Children, Adults and Workers from Environmentally Exposed Areas at Aswan, Egypt. Environ. Bioindic. 2007, 2, 131–145. [Google Scholar] [CrossRef]
- Erie, J.C.; Butz, J.A.; Good, J.A.; Erie, E.A.; Burritt, M.F.; Cameron, J.D. Heavy Metal Concentrations in Human Eyes. Am. J. Ophthalmol. 2005, 139, 888–893. [Google Scholar] [CrossRef] [PubMed]
- Tamahkar, E.; Denizli, A. Metal ion coordination interactions for biomolecule recognition: A review. Hittite J. Sci. Eng. 2014, 1, 21–26. [Google Scholar] [CrossRef]
- Eichenbaum, J.W.; Zheng, W. Distribution of lead and transthyretin in human eyes. J. Toxicol. Clin. Toxicol. 2000, 38, 377–381. [Google Scholar] [CrossRef]
- Alomary, A.; Al-Momani, I.; Massadeh, A. Lead and cadmium in human teeth from Jordan by atomic absorption spectrometry: Some factors influencing their concentrations. Sci. Total Environ. 2006, 369, 69–75. [Google Scholar] [CrossRef]
- Bryła, E.; Dobrzyński, M.; Konkol, D.; Kuropka, P.; Styczyńska, M.; Korczyński, M. Toxic Metals Content in Impacted Third Molars and Adjacent Bone Tissue in Different Groups of Patients. Materials 2021, 14, 793. [Google Scholar] [CrossRef]
- Sharma, S.; Kaur, G.; Kaur, C. Trend in the analysis of heavy metals in human teeth dentine: A review. J. Indo-Pac. Acad. Forensic Odontol. 2020, 9, 93. [Google Scholar]
- Asaduzzaman, K.; Khandaker, M.U.; Baharudin, N.A.B.; Amin, Y.B.M.; Farook, M.S.; Bradley, D.; Mahmoud, O. Heavy metals in human teeth dentine: A bio-indicator of metals exposure and environmental pollution. Chemosphere 2017, 176, 221–230. [Google Scholar] [CrossRef]
- Chang, L.; Shen, S.; Zhang, Z.; Song, X.; Zhang, P. Study on the relationship between age and the concentrations of heavy metal elements in human bone. Ann. Transl. Med. 2018, 6, 320. [Google Scholar] [CrossRef]
- Jan, A.T.; Azam, M.; Siddiqui, K.; Ali, A.; Choi, I.; Haq, Q.M. Heavy metals and human health: Mechanistic insight into toxicity and counter defense system of antioxidants. Int. J. Mol. Sci. 2015, 16, 29592–29630. [Google Scholar] [CrossRef]
- Klotz, K.; Göen, T. Human biomonitoring of lead exposure. Met. Ions Life Sci. 2017, 17, 99–121. [Google Scholar]
- Mikulewicz, M.; Chojnacka, K.; Wołowiec, P. Release of metal ions from fixed orthodontic appliance: An in vitro study in continuous flow system. Angle Orthod. 2014, 84, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Waseem, A.; Arshad, J. A review of Human Biomonitoring studies of trace elements in Pakistan. Chemosphere 2016, 163, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, M.; Soares, M.C.F.; Baisch, P.R.M.; Baisch, A.L.M.; da Silva Júnior, F.M.R. Biomonitoring of trace elements in urine samples of children from a coal-mining region. Chemosphere 2018, 197, 622–626. [Google Scholar] [CrossRef]
- Park, S.J.; Kwon, K.-E.; Choi, N.-K.; Park, K.; Woo, S.J. Prevalence and Incidence of Exudative Age-Related Macular Degeneration in South Korea: A Nationwide Population-Based Study. Ophthalmology 2015, 122, 2063–2070. [Google Scholar] [CrossRef]
- Schmeling, M.; Davidson, J.; Eng, P.; Stubbs, J.; Jurewicz, A.; Burnett, D. Grazing Incidence X-ray Fluorescence Measurements of Genesis Sample 30580 for Determination of Manganese and Nickel Fluences. In Proceedings of the Lunar and Planetary Science Conference, Woodlands, Texas, 16–20 March 2015; p. 2238. [Google Scholar]
- Kempson, I.M.; Lombi, E. Hair analysis as a biomonitor for toxicology, disease and health status. Chem. Soc. Rev. 2011, 40, 3915–3940. [Google Scholar] [CrossRef]
- Grassin-Delyle, S.; Martin, M.; Hamzaoui, O.; Lamy, E.; Jayle, C.; Sage, E.; Etting, I.; Devillier, P.; Alvarez, J.-C. A high-resolution ICP-MS method for the determination of 38 inorganic elements in human whole blood, urine, hair and tissues after microwave digestion. Talanta 2019, 199, 228–237. [Google Scholar] [CrossRef]
- Kaur, I.; Jakhar, D. Intramatricial platelet-rich plasma therapy: A novel treatment modality in refractory nail disorders. Dermatol. Ther. 2019, 32, e12831. [Google Scholar] [CrossRef]
- Ab Razak, N.H.; Praveena, S.M.; Aris, A.Z.; Hashim, Z. Drinking water studies: A review on heavy metal, application of biomarker and health risk assessment (a special focus in Malaysia). J. Epidemiol. Glob. Health 2015, 5, 297–310. [Google Scholar] [CrossRef]
- Malara, P.; Fischer, A.; Malara, B. Selected toxic and essential heavy metals in impacted teeth and the surrounding mandibular bones of people exposed to heavy metals in the environment. J. Occup. Med. Toxicol. 2016, 11, 56. [Google Scholar] [CrossRef]
- Tvinnereim, H.M.; Eide, R.; Riise, T. Heavy metals in human primary teeth: Some factors influencing the metal concentrations. Sci. Total Environ. 2000, 255, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, J.L.; Hall, A.J. Mercury in cetaceans: Exposure, bioaccumulation and toxicity. Sci. Total Environ. 2019, 694, 133683. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.; Costa, F.; Pereira, M. Heavy Metals and Human Health. In Environmental Health—Emerging Issues and Practice; Oosthuizen, J., Ed.; IntechOpen: London, UK, 2012; pp. 227–246. [Google Scholar]
- Davidson, C.M. Methods for the Determination of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils: Trace Metals and Metalloids in Soils and Their Bioavailability; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 97–140. [Google Scholar]
- Batista, B.L.; Rodrigues, J.L.; de Oliveira Souza, V.C.; Barbosa, F. A fast ultrasound-assisted extraction procedure for trace elements determination in hair samples by ICP-MS for forensic analysis. Forensic Sci. Int. 2009, 192, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Bolann, B.J.; Rahil-Khazen, R.; Henriksen, H.; Isrenn, R.; Ulvik, R.J. Evaluation of methods for trace-element determination with emphasis on their usability in the clinical routine laboratory. Scand. J. Clin. Lab. Investig. 2007, 67, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Schalock, P.C.; Crawford, G.; Nedorost, S.; Scheinman, P.L.; Atwater, A.R.; Mowad, C.; Brod, B.; Ehrlich, A.; Watsky, K.L.; Sasseville, D.; et al. Patch Testing for Evaluation of Hypersensitivity to Implanted Metal Devices: A Perspective From the American Contact Dermatitis Society. Dermatitis 2016, 27, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Granchi, D.; Cenni, E.; Giunti, A.; Baldini, N. Metal hypersensitivity testing in patients undergoing joint replacement: A systematic review. J. Bone Jt. Surg. Br. 2012, 94, 1126–1134. [Google Scholar] [CrossRef]
- Valentine-Thon, E.; Schiwara, H.-W. Validity of MELISA for metal sensitivity testing. Neuro Endocrinol. Lett. 2003, 24, 57–64. [Google Scholar] [PubMed]
- Cerghizan, D.; Iulia, N.A.; Maris, M.; Laura, B.D.; Condurache, G.; Gabriela, T.; Matei, M. Interrelation between Prosthetic and Orthodontic Treatment. Rom. J. Oral Rehabil. 2020, 12, 145–156. [Google Scholar]
- Valentine-Thon, E.; Müller, K.; Guzzi, G.; Kreisel, S.; Ohnsorge, P.; Sandkamp, M. LTT-MELISA is clinically relevant for detecting and monitoring metal sensitivity. Neuro Endocrinol. Lett. 2006, 27 (Suppl. S1), 17–24. [Google Scholar]
- Jones, S.L.; Campbell, B.; Hart, T. Laboratory tests commonly used in complementary and alternative medicine: A review of the evidence. Ann. Clin. Biochem. 2019, 56, 310–325. [Google Scholar] [CrossRef] [PubMed]
- Vrbova, R.; Podzimek, S.; Himmlova, L.; Roubickova, A.; Janovska, M.; Janatova, T.; Bartos, M.; Vinsu, A. Titanium and Other Metal Hypersensitivity Diagnosed by MELISA® Test: Follow-Up Study. BioMed Res. Int. 2021, 2021, 5512091. [Google Scholar] [CrossRef] [PubMed]
- Koene, R. The’memory lymphocyte immunostimulation assay’(MELISA) is useless for the detection of metal allergy. Ned. Tijdschr. Voor Geneeskd. 2005, 149, 2090–2092. [Google Scholar]
- Vadalà, M.; Laurino, C.; Palmieri, B. The memory lymphocyte immunostimulation assay in immune system disorders: Is useful or useless? J. Lab. Physicians 2017, 9, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Pushie, M.J.; Pickering, I.J.; Korbas, M.; Hackett, M.J.; George, G.N. Elemental and Chemically Specific X-ray Fluorescence Imaging of Biological Systems. Chem. Rev. 2014, 114, 8499–8541. [Google Scholar] [CrossRef]
- Mikušová, P.P.V.; Oľga, S.; Sýkorová, M.; Havránek, E. Determination of heavy metals in samples of different states by radionuclide x-ray fluorescence. Acta Fac. Pharm. Univ. Comen. 2006, 53, 268–274. [Google Scholar]
- Roux, C.; Lennard, C. X-ray Fluorescence in Forensic Science. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Brettell, T.C., Eds.; John Wiley & Sons: Oxford, UK, 2006; pp. 1–9. [Google Scholar]
- Ene, A.; Bosneaga Sion, A.; Georgescu, L. Determination of heavy metals in soils using XRF technique. Rom. J. Phys. 2010, 55, 815–820. [Google Scholar]
- Zhang, R.; Li, L.; Sultanbawa, Y.; Xu, Z.P. X-ray fluorescence imaging of metals and metalloids in biological systems. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 169–188. [Google Scholar]
- Taylor, A.; Day, M.; Hill, S.; Marshall, J.; Patriarca, M.; White, M. Atomic spectrometry update: Review of advances in the analysis of clinical and biological materials, foods and beverages. J. Anal. At. Spectrom. 2014, 29, 386. [Google Scholar] [CrossRef]
- Tiwari, M.K.; Singh, A.K.; Sawhney, K.J.S. Sample Preparation for Evaluation of Detection Limits in X-ray Fluorescence Spectrometry. Anal. Sci. 2005, 21, 143–147. [Google Scholar] [CrossRef][Green Version]
- Goullé, J.P.; Mahieu, L.; Castermant, J.; Neveu, N.; Bonneau, L.; Lainé, G.; Bouige, D.; Lacroix, C. Metal and metalloid multi-elementary ICP-MS validation in whole blood, plasma, urine and hair. Reference values. Forensic Sci. Int. 2005, 153, 39–44. [Google Scholar] [CrossRef]
- Parsons, P.J.; Barbosa, F. Atomic spectrometry and trends in clinical laboratory medicine. Spectrochim. Acta 2007, 62, 992–1003. [Google Scholar] [CrossRef]
- Freedman, J.C. Biophysical Chemistry of Physiological Solutions. In Cell Physiology Source Book, 4th ed.; Chapter 1; Sperelakis, N., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 3–17. [Google Scholar]
- Jin, M.; Yuan, H.; Liu, B.; Peng, J.; Xu, L.; Yang, D. Review of the distribution and detection methods of heavy metals in the environment. Anal. Methods 2020, 12, 5747–5766. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Kumar, K.; Anghore, D.; Rawal, K.R. ICP-MS: Analytical Method for Identification and Detection of Elemental Impurities. Curr. Drug Discov. Technol. 2017, 14, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Jakubowski, N. The synergy of elemental and biomolecular mass spectrometry: New analytical strategies in life sciences. Chem. Soc. Rev. 2009, 38, 1969–1983. [Google Scholar] [CrossRef] [PubMed]
- Cavey, T.; Ropert, M.; Loréal, O.; Bendavid, C.; Peoc’h, K. Métaux: Applications cliniques courantes en spectrométrie de masse à plasma à couplage inductif. In Proceedings of the Annales de Biologie Clinique, Paris, France, 1 October 2019; pp. 495–504. [Google Scholar]
- Goullé, J.-P.; Saussereau, E.; Mahieu, L.; Guerbet, M. Current role of ICP–MS in clinical toxicology and forensic toxicology: A metallic profile. Bioanalysis 2014, 6, 2245–2259. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; LaFrance, D.R.; Hao, Y. Elemental Testing Using Inductively Coupled Plasma Mass Spectrometry in Clinical Laboratories: An ACLPS Critical Review. Am. J. Clin. Pathol. 2021, 156, 167–175. [Google Scholar] [CrossRef]
- Laur, N.; Kinscherf, R.; Pomytkin, K.; Kaiser, L.; Knes, O.; Deigner, H.-P. ICP-MS trace element analysis in serum and whole blood. PLoS ONE 2020, 15, e0233357. [Google Scholar] [CrossRef]
- Krachler, M.; Irgolic, K. The potential of inductively coupled plasma mass spectrometry (ICP-MS) for the simultaneous determination of trace elements in whole blood, plasma and serum. J. Trace Elem. Med. Biol. 1999, 13, 157–169. [Google Scholar] [CrossRef]
- Sigel, A.; Sigel, H.; Sigel, R. Cadmium: From Toxicity to Essentiality; Springer: New York, NY, USA; Dordrecht, The Netherlands, 2013; Volume 1. [Google Scholar]
- Suh, J.H.; Lee, Y.Y.; Lee, H.J.; Kang, M.; Hur, Y.; Lee, S.N.; Yang, D.-H.; Han, S.B. Dispersive liquid–liquid microextraction based on solidification of floating organic droplets followed by high performance liquid chromatography for the determination of duloxetine in human plasma. J. Pharm. Biomed. Anal. 2013, 75, 214–219. [Google Scholar] [CrossRef]
- Abduljabbar, T.N.; Sharp, B.L.; Reid, H.J.; Barzegar-Befroeid, N.; Peto, T.; Lengyel, I. Determination of Zn, Cu and Fe in human patients’ serum using micro-sampling ICP-MS and sample dilution. Talanta 2019, 204, 663–669. [Google Scholar] [CrossRef]
- González-Domínguez, R.; García-Barrera, T.; Gómez-Ariza, J.L. Characterization of metal profiles in serum during the progression of Alzheimer’s disease. Metallomics 2014, 6, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.N.; Patel, J.K.; Patel, M.P.; Rajput, G.C.; Patel, H.A. Introduction to hyphenated techniques and their applications in pharmacy. Pharm Methods 2010, 1, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Sarker, S.D.; Nahar, L. Hyphenated Techniques. In Natural Products Isolation; Sarker, S.D., Latif, Z., Gray, A.I., Eds.; Humana Press: Totowa, NJ, USA, 2005; pp. 233–267. [Google Scholar]
- Meermann, B.; Sperling, M. Hyphenated techniques as tools for speciation analysis of metal-based pharmaceuticals: Developments and applications. Anal. Bioanal. Chem. 2012, 403, 1501–1522. [Google Scholar] [CrossRef]
- Das, A.K.; de la Guardia, M.; Cervera, M.L. Literature survey of on-line elemental speciation in aqueous solutions. Talanta 2001, 55, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Michalski, R.; Szopa, S.; Jabłońska, M.; Łyko, A. Application of hyphenated techniques in speciation analysis of arsenic, antimony, and thallium. Sci. World J. 2012, 2012, 902464. [Google Scholar] [CrossRef]
- Alonso, E.; Santos, A.; Callejón, M.; Jiménez, J.C. Speciation as a screening tool for the determination of heavy metal surface water pollution in the Guadiamar river basin. Chemosphere 2004, 56, 561–570. [Google Scholar] [CrossRef]
- Van Landeghem, G.F.; De Broe, M.E.; D’Haese, P.C. Al and Si: Their speciation, distribution, and toxicity. Clin. Biochem. 1998, 31, 385–397. [Google Scholar] [CrossRef]
- Morton, J.; Leese, E. Arsenic speciation in clinical samples: Urine analysis using fast micro-liquid chromatography ICP-MS. Anal. Bioanal. Chem. 2011, 399, 1781–1788. [Google Scholar] [CrossRef]
- Jitaru, P.; Goenaga-Infante, H.; Vaslin-Reimann, S.; Fisicaro, P. A systematic approach to the accurate quantification of selenium in serum selenoalbumin by HPLC–ICP-MS. Anal. Chim. Acta 2010, 657, 100–107. [Google Scholar] [CrossRef]
- Dunemann, L.; Hajimiragha, H.; Begerow, J. Simultaneous determination of Hg(II) and alkylated Hg, Pb, and Sn species in human body fluids using SPME-GC/MS-MS. Fresenius J. Anal. Chem. 1999, 363, 466–468. [Google Scholar] [CrossRef]
- Seger, C.; Sturm, S. NMR-Based Chromatography Readouts: Indispensable Tools to “Translate” Analytical Features into Molecular Structures. Cells 2022, 11, 3526. [Google Scholar] [CrossRef] [PubMed]
- Barrett, S. Commercial hair analysis. Science or scam? JAMA 1985, 254, 1041–1045. [Google Scholar] [CrossRef] [PubMed]


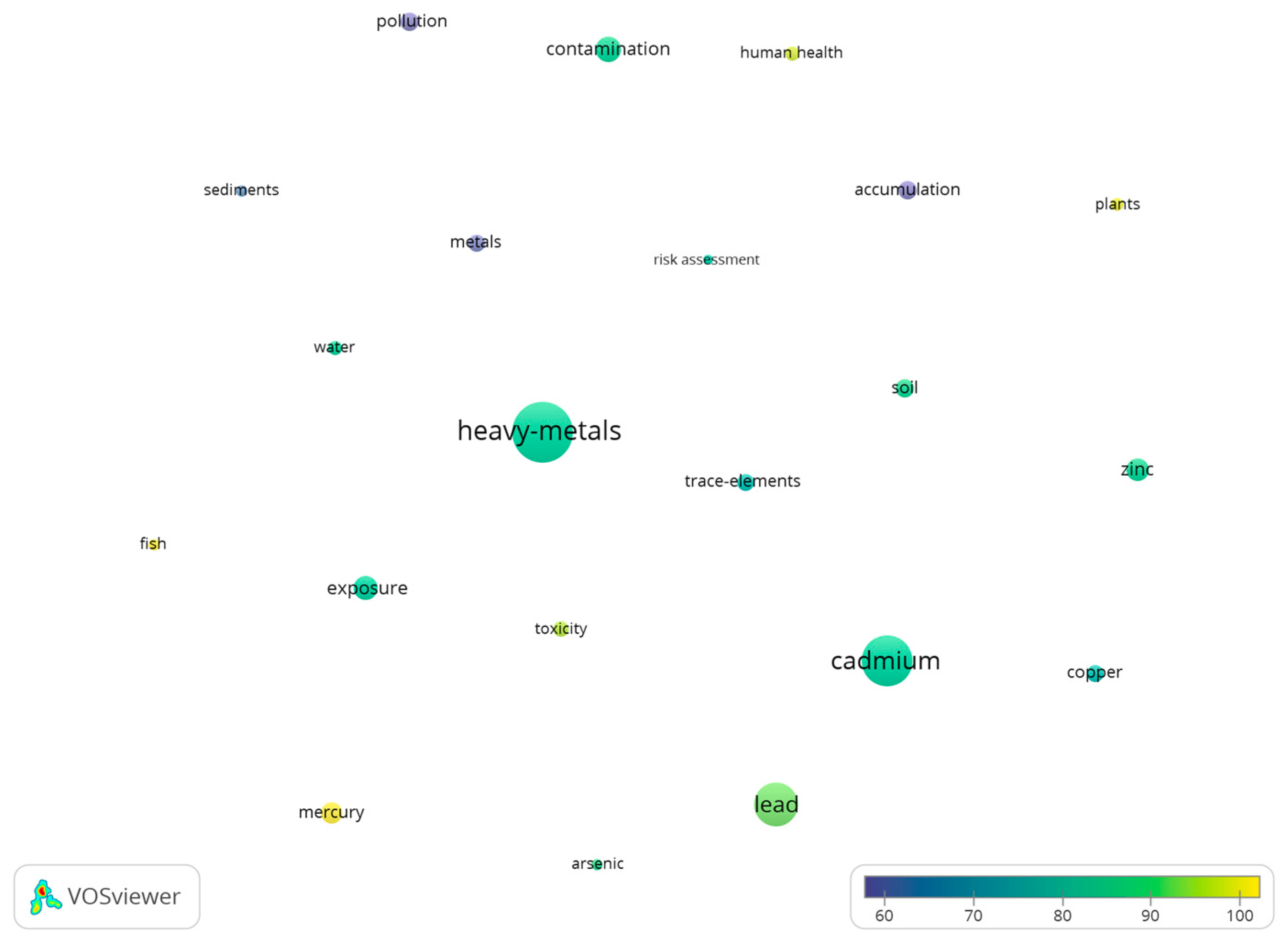
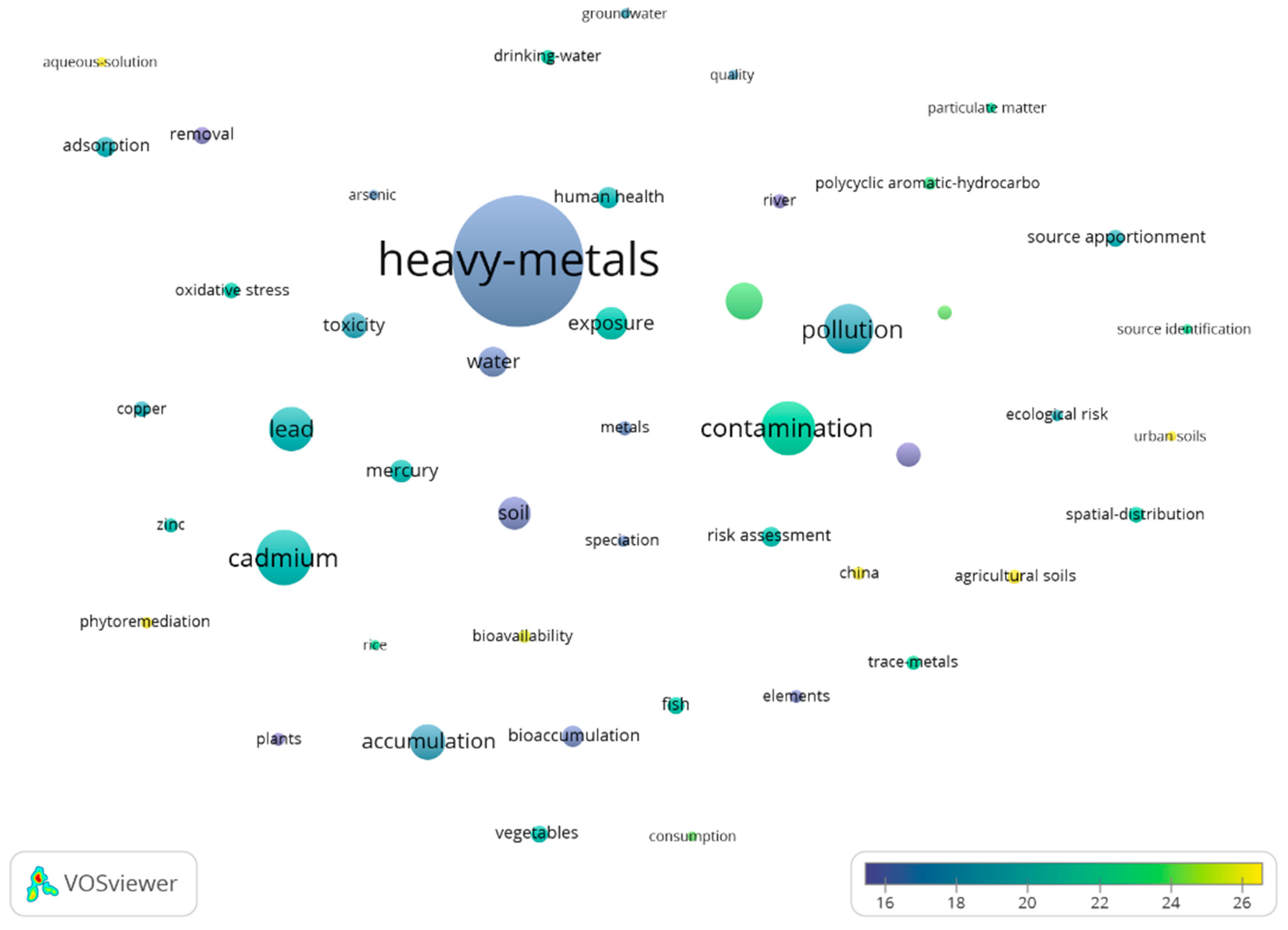



| Word/Term | Average Citation/ Article | Occurrence | Word/Term | Average Citation/ Article | Occurrence |
|---|---|---|---|---|---|
| 1975–2010 | 2011-to Present | ||||
| Heavy metals | 84.15 | 361 | Heavy metals | 17.07 | 3920 |
| Cadmium | 83.94 | 302 | Cadmium | 20.05 | 1693 |
| Lead | 90.56 | 252 | Contamination | 21.77 | 1628 |
| Heavy metal contamination | 84.84 | 138 | Pollution | 18.78 | 1514 |
| Exposure | 83.29 | 128 | Lead | 19.68 | 1345 |
| Zinc | 85.05 | 127 | Trace-elements | 23.02 | 1127 |
| Mercury | 101.54 | 112 | Accumulation | 18.39 | 1074 |
| Accumulation | 53.28 | 102 | Exposure | 20.78 | 997 |
| Pollution | 54.79 | 101 | Soil | 16.41 | 994 |
| Soil | 85.51 | 95 | Water | 16.73 | 915 |
| Country | Articles No. | Citations No. | Average Citation/Document | Total Link Strength |
|---|---|---|---|---|
| China | 3944 | 106,114 | 26.91 | 1400 |
| United States | 1346 | 52,942 | 39.33 | 971 |
| India | 1157 | 29,987 | 25.92 | 513 |
| Pakistan | 647 | 20,070 | 31.02 | 629 |
| Iran | 617 | 11,328 | 18.36 | 286 |
| Italy | 596 | 17,895 | 30.03 | 278 |
| Spain | 477 | 14,915 | 31.27 | 256 |
| Turkey | 447 | 7222 | 16.16 | 141 |
| Australia | 424 | 14,624 | 34.49 | 475 |
| Brazil | 377 | 5006 | 13.28 | 162 |
| Author (Year) | Title of the Article | Journal | IF | Citations | Ref. |
|---|---|---|---|---|---|
| Jarup (2003) | Hazards of heavy metal contamination | British Medical Bulletin | 4.291 | 3507 | [4] |
| Kampa (2008) | Human health effects of air pollution | Environmental Pollution | 8.071 | 2188 | [11] |
| Ali (2013) | Phytoremediation of heavy metals–Concepts and applications | Chemosphere | 7.086 | 2118 | [12] |
| Salt (1998) | Phytoremediation | Annual Review of Plant Biology | 22.192 | 1663 | [13] |
| Li (2014) | A review of soil heavy metal pollution from mines in China: Pollution and health risk assessment | Science of the Total Environment | 7.963 | 1523 | [14] |
| Source | Documents No. | Total Citations | Average Citation/Article | Impact Factor | Publisher |
|---|---|---|---|---|---|
| Environmental Science and Pollution Research | 750 | 10,916 | 14.55 | 4.223 | Springer |
| Science of the Total Environment | 630 | 33,435 | 53.07 | 7.963 | Elsevier |
| Chemosphere | 409 | 16,734 | 40.91 | 7.086 | Elsevier |
| Environmental Monitoring and Assessment | 333 | 6538 | 19.63 | 2.513 | Springer |
| Ecotoxicology and Environmental Safety | 309 | 10,759 | 34.82 | 6.291 | Elsevier |
| Environmental Pollution | 305 | 15,755 | 51.66 | 8.071 | Elsevier |
| International Journal of Environmental Research and Public Health | 291 | 4481 | 15.40 | 3.39 | MDPI |
| Environmental Geochemistry and Health | 290 | 4829 | 16.65 | 4.609 | Springer |
| Journal of Hazardous Materials | 188 | 7919 | 42.12 | 10.588 | Elsevier |
| Human and Ecological Risk Assessment | 177 | 2541 | 14.36 | 5.19 | Taylor and Francis Ltd. |
| Toxicity Effects Summary | Heavy Metals | Refs. |
|---|---|---|
| Central nervous system (CNS) | ||
Brain injuries:
Sensory disturbances, loss of peripheral vision, gait disturbance, incoordination, hearing, and speech impairment; Trembling, sleeplessness, tugging, weakness, headaches, and muscular atrophy; Mercury poisoning: “Hatter’s Shakes” Syndrome; Neurodegenerative diseases: Parkinson’s, Alzheimer’s, Lou Gehrig‘s, dialysis dementia and neurotoxicity; nervousness, somnolence, memory loss, and intellectual disability; Cyanosis, toxically polyneuropathy, and paresis. | Pb, MeHg, Hg, Al, As | [18,19,22,23,24,25,26,27,28,29] |
| Respiratory system | ||
| Pulmonary fibrosis, interstitial and granulomatous pneumonia, asthma, pulmonary edema; Pulmonary edema, asthma, and tuberculosis; Burning pain in the chest, cough, dyspnea, fibrosis, pneumonia, and pulmonary edema; Obstructive pulmonary disease, and asthma. | Al, As, Hg, Pb | [28,30,31] |
| Cardiovascular system | ||
| High blood pressure, oxidative stress, fatigue, and high risk of cardiovascular accident; Cardiovascular lesion, toxic myocarditis, dysfunction and inflammation of the myocardium, and congenital heart defects; Myocardial injuries: cardiomyopathy, low blood pressure, cardiac arrhythmia, and heart failure. | Pb, Al, As | [29,30,32,33,34] |
| Skeletal system | ||
Most lead is stored in the bones:
| Pb, Al | [18,25,30,35,36] |
| Gastrointestinal system | ||
| Abdominal and gastric dysfunction: loss appetite, abdominal pain, weakness, vomiting, diarrhea, constipation, and metallic taste in the mouth; Excessive intestinal inflammation, and damage to the intestinal microbiota; Metallic taste in mouth, garlic-smelling breath, gastric ulcers, heartburns, nausea, vomiting, abdominal pain, and bloody rice water diarrhea. | Pb, Al, As | [18,25,29,30,34] |
| Hepatorenal system | ||
| Liver effects, disturbance of normal kidney function (proteinuria); Hepatic lesions, oxidative injuries → necrosis, tissue degeneration, and biochemical derangement; Metallic taste in the mouth. | Hg, Al, As | [29,30,37] |
| Reproductive system | ||
| In women: high risk of miscarriage, low birth weight, stillbirth, and children—developmental problems; In men: impotency, sterility, reduction in sperm count and motility; Spontaneous abortion, birth defects, miscarriage, and low birth weight. | Pb, As | [25,29] |
| Hematopoietic system | ||
| Adversely effects the metabolism of blood cell and blood, heme synthesis is disturbed; Side effects of red blood cell metabolism, anemia, and leukopenia; Modifies blood-biochemical parameters, anemia, “metal fume fever” syndrome → fever, chills, fatigue, and elevated leukocyte count. | Pb, As, Al, Hg | [18,22,25,30] |
| Dermal | ||
| Skin lesions: skin depigmentation/nuances, “rain drops on a dusty road” Syndrome (keratosis), alopecia, over-exfoliation of the skin on the extremities, and dermatitis. | As | [18,29] |
| Substances | Biological Indicator | Biological Matrix | Sample Collection | BBLV |
|---|---|---|---|---|
| Aluminium | Aluminium | Urine | At the end of the shift | 200 μg/L |
| Arsenic and AsH3 | Arsenic | Urine | At the end of the week | 50 μg/gC |
| Hair | At the end of the week | 0,5 mg/100 g | ||
| Mercury and its compounds | Mercury | Blood | At the end of the shift | 10 μg/L |
| Mercury | Urine | At the beginning of the next shift | 30 μg/gC | |
| Lead | Lead | Blood | At the end of the shift | 70 μg/100 mL |
| Lead | Urine | At the end of the shift | 150μg/L | |
| Lead | Hair | At the end of the shift | 3 mg/cm | |
| δ-aminolevulinic acid | Urine | At the end of the shift | 10 mg/L | |
| Coproporphyrins | Urine | At the end of the shift | 300 μg/L | |
| Free Erythrocyte protoporphyrin | Blood | At the end of the shift | 100 μg/100 mL erythocytes | |
| Lead tetraethyl | Diethyl Lead | Urine | At the end of the shift | 25 μg/L |
| Total Lead | Urine | At the end of the shift | 50 μg/L |
| Advantages | Disadvantages | Refs. |
|---|---|---|
| Blood | ||
| The most-used biological matrix; Releases information about HM at the present moment; Preferable to identify Pb and Hg salts; Short residence times 2–3 h or 3–4 days. | Invasive sampling method; Does not show the cumulative degree of exposure; Risk of contamination during collecting and storage; Requires cold storage; Blood levels are transient, independent of those from the tissues; The anticoagulant may interfere with the method of determination; Very low concentrations cannot be detectable by common analytical methods. | [65,87,88,89] |
| Human urine | ||
| Shows how the HM is excreted at the time of collecting; Simple/easy method of collecting; Preferable for the determination of certain HMs: inorganic forms of Hg and As; Accessible and available in large Volumes. | Provides information only on relatively recent exposure; Risk of contamination during collecting and storage; If the sample is not processed immediately, it must be frozen. | [87,89,90] |
| Vitreous humor/Retinal tissues | ||
| High affinity for HM; Shows the cumulative degree of exposure. | Difficult to collect (preferably from dead bodies); High risk of contamination during collecting and storage; Available in small quantities (2–4 mL). | [78,89,91,92] |
| Hair | ||
| The most important biomarker for health assessment; Provides information on long-term exposure time (from few weeks to years); Simple, frank, and non-invasive method of collecting; Shows the direct correlation between hair analysis and various diseases. | Some elements may be deposited by different cosmetic procedures (dyeing, blenching, permanent waving, and smoke); Seasonal variations; The lack of reference ranges for the interpretation of results. | [65,68,93,94] |
| Nails | ||
| Shows long-term exposure and intake of HM; Easy to collect, transport, store and prepare for analysis; It does not require a large sample; Toenails have been used as a biomarker in forensic, clinical, and environmental Studies. | Can be easily contaminated with some medications or nail polish; Small nail amount → low detection limit; Differences in the analysis of the toenails and fingernails; Use of nails as a biomarker for some elements is not well characterized. | [95,96] |
| Bones | ||
| Bioindicator of a long-term exposure; Accumulates HMs with a high affinity; In particular, femurs and tibiae can fix HM very well; Chemically stable; Ideal calcified tissues for long-term Exposure. | Not very accessible for sampling and measurement; The mineral phase is subject to turnover; Release and fluctuation of different minerals from the bones to blood; Significant differences in HM concentrations between different bones. | [25,87,97] |
| Human teeth | ||
| Provides a cumulative and permanent record of recent/past exposure to HMs; Dentine and enamel also suitable as bioindicators of exposure; Offer several advantages over other bioindicators of exposure (liver, and kidney); Readily accessible materials; Low-rate elimination of HM. | Primary teeth may be used as a bioindicator of long-term exposure, but they have a short lifespan; Use the non-carious teeth toreduce the confounding factors; The chemical composition of the teeth may change after the influence of some factors (drugs, food, and medicines); The extraction of permanent teeth is a problem. | [82,83,84,97,98] |
| Human organs/tissues | ||
| Kidney, brain, lung, liver, heart, and muscles are targeted tissues for HM deposit. | Difficult sampling. | [25,92,99] |
| Analytical Methods | Biological Matrix | Limit of Detection (LOD) | Applications |
|---|---|---|---|
| LTT MELISA HMs | Blood | SI < 2, NEGATIVE, there is no type IV, sensitization for tested metals; SI = 2–3, HIGHLY suggestive result for a POSITIVE reaction SI > 3, POSITIVE | Metal allergies |
| X-ray Fluorescence | Blood, soft tissues, bones, organs, and hair | 0.1–1 mg/g | Clinical, forensic toxicology Occupational exposure |
| AAS | Blood, urine, and vitreous humor; Soft tissues; Hair, bones, nails, organs (liver, lung, kidney, brain, and skin). | 0.001–0.5 μg/L | Clinical and forensic toxicology Occupational exposure Acute/chronic intoxications |
| ICP-MS | Blood, urine, and serum; Vitreous humour, soft tissues, hair, nails, and bones; Organs (liver, lung, kidney, brain, and skin). | 0.001–0.1 μg/L | Clinical and forensic toxicology Occupational exposure Acute or chronic intoxications |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filipoiu, D.C.; Bungau, S.G.; Endres, L.; Negru, P.A.; Bungau, A.F.; Pasca, B.; Radu, A.-F.; Tarce, A.G.; Bogdan, M.A.; Behl, T.; et al. Characterization of the Toxicological Impact of Heavy Metals on Human Health in Conjunction with Modern Analytical Methods. Toxics 2022, 10, 716. https://doi.org/10.3390/toxics10120716
Filipoiu DC, Bungau SG, Endres L, Negru PA, Bungau AF, Pasca B, Radu A-F, Tarce AG, Bogdan MA, Behl T, et al. Characterization of the Toxicological Impact of Heavy Metals on Human Health in Conjunction with Modern Analytical Methods. Toxics. 2022; 10(12):716. https://doi.org/10.3390/toxics10120716
Chicago/Turabian StyleFilipoiu, Dana Claudia, Simona Gabriela Bungau, Laura Endres, Paul Andrei Negru, Alexa Florina Bungau, Bianca Pasca, Andrei-Flavius Radu, Alexandra Georgiana Tarce, Mihaela Alexandra Bogdan, Tapan Behl, and et al. 2022. "Characterization of the Toxicological Impact of Heavy Metals on Human Health in Conjunction with Modern Analytical Methods" Toxics 10, no. 12: 716. https://doi.org/10.3390/toxics10120716
APA StyleFilipoiu, D. C., Bungau, S. G., Endres, L., Negru, P. A., Bungau, A. F., Pasca, B., Radu, A.-F., Tarce, A. G., Bogdan, M. A., Behl, T., Nechifor, A. C., Hassan, S. S. u., & Tit, D. M. (2022). Characterization of the Toxicological Impact of Heavy Metals on Human Health in Conjunction with Modern Analytical Methods. Toxics, 10(12), 716. https://doi.org/10.3390/toxics10120716









