Embryonic Nicotine Exposure Disrupts Adult Social Behavior and Craniofacial Development in Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Zebrafish Husbandry
2.3. Embryo Exposure and Experimental Design
2.4. Cotinine Assay
2.5. Dual Bone and Cartilage Staining
2.6. Behavioral Assay
2.7. Data Analysis
3. Results
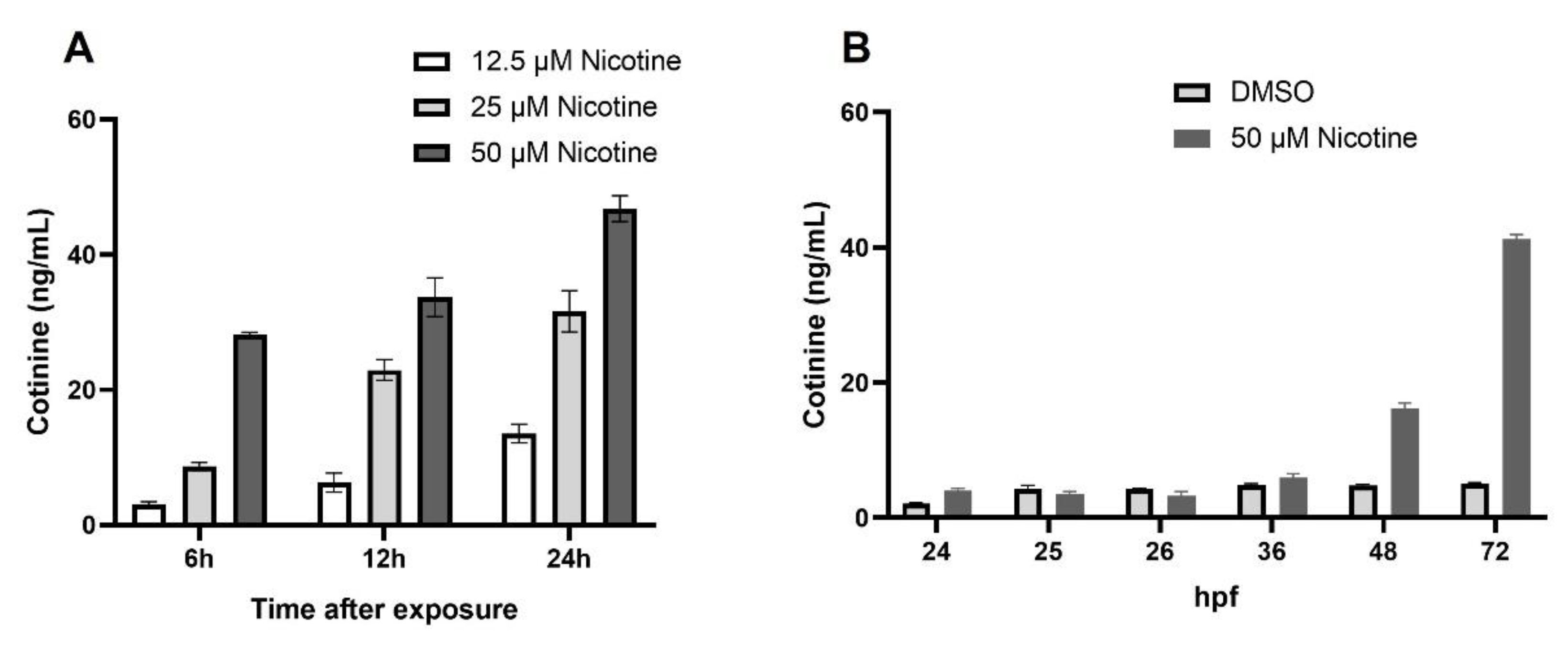
3.1. Nicotine Is Metabolized by Zebafish Embryo
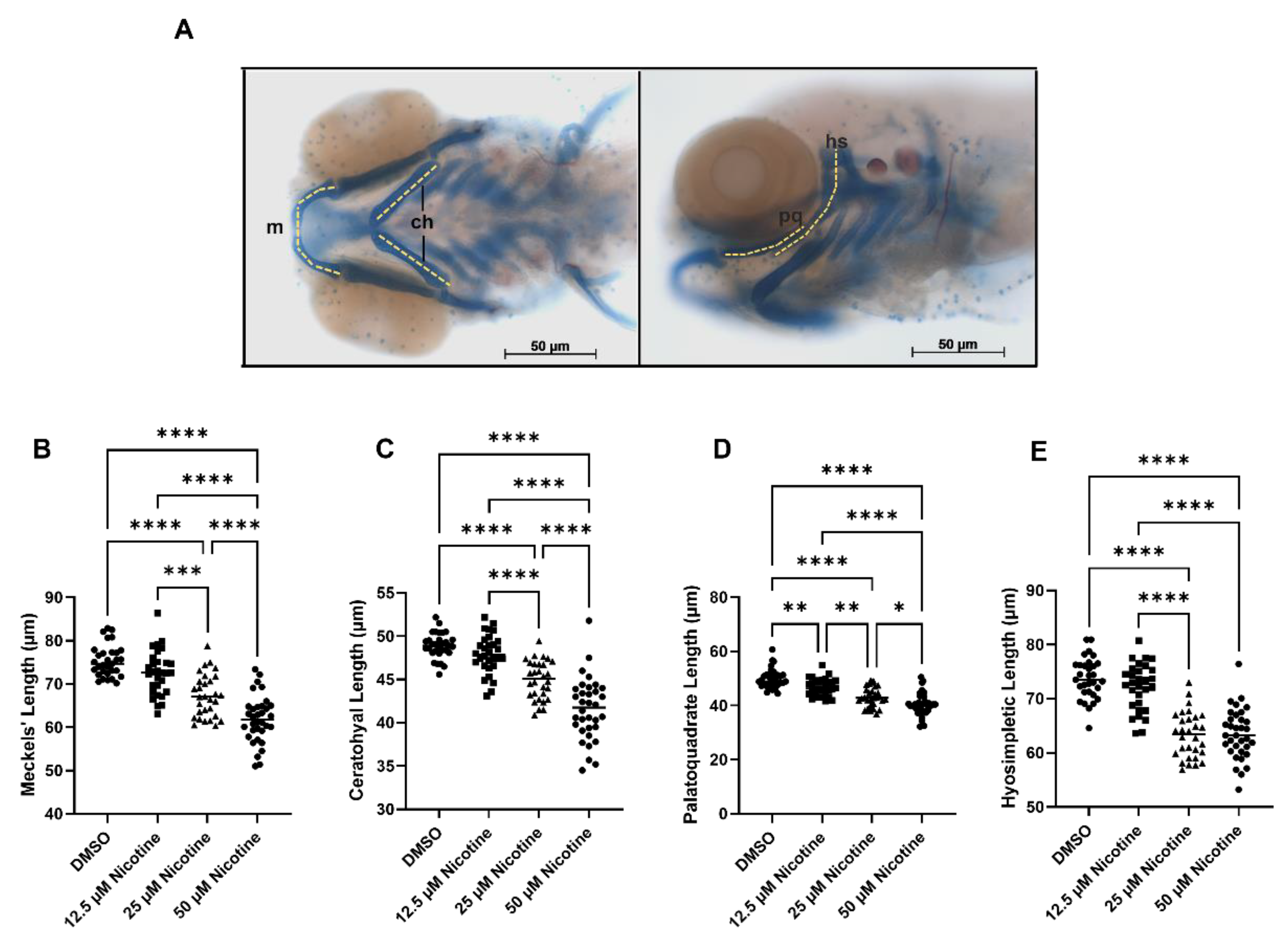
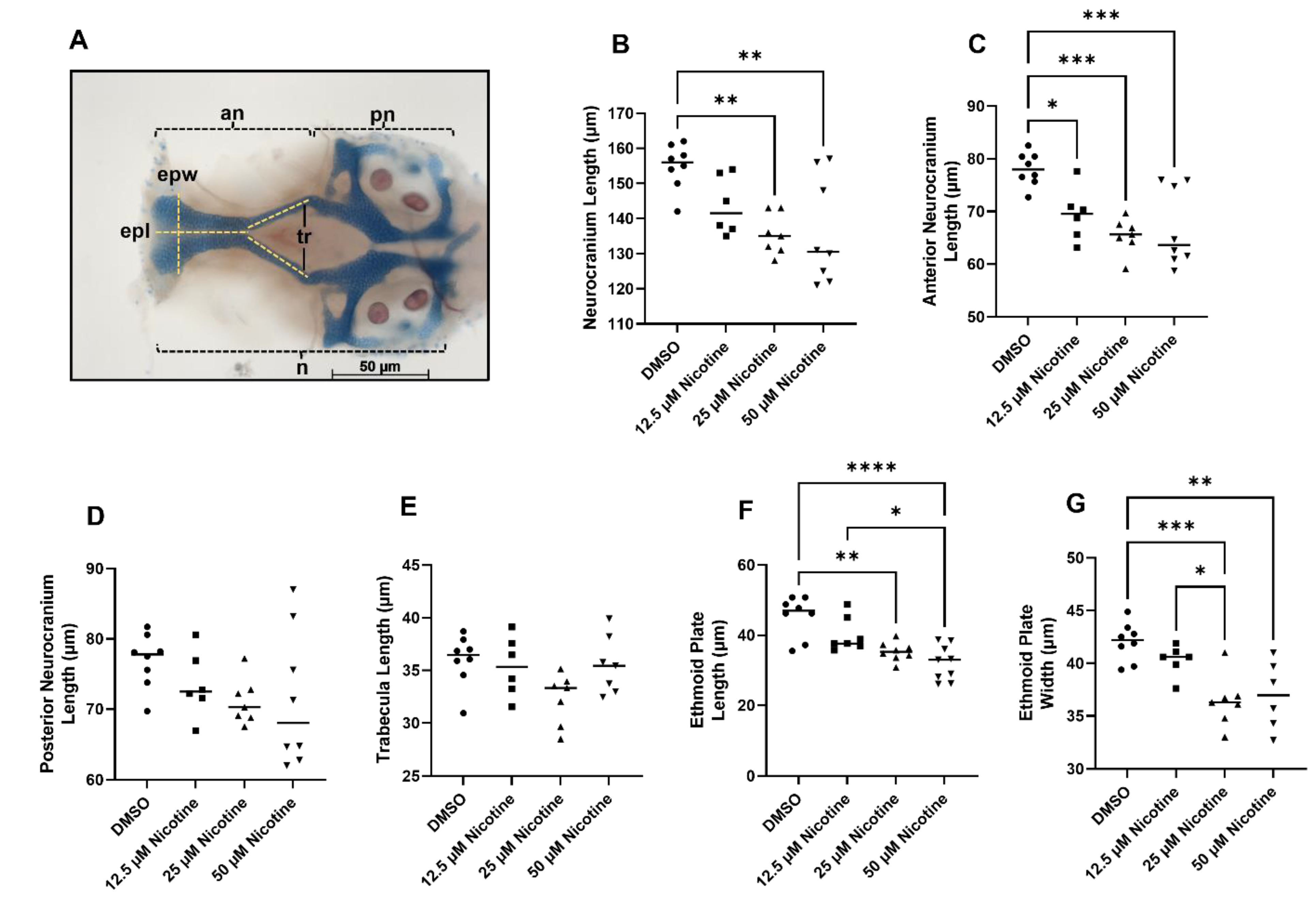
3.2. Embryonic Nicotine Exposure Disrupts Craniofacial Development
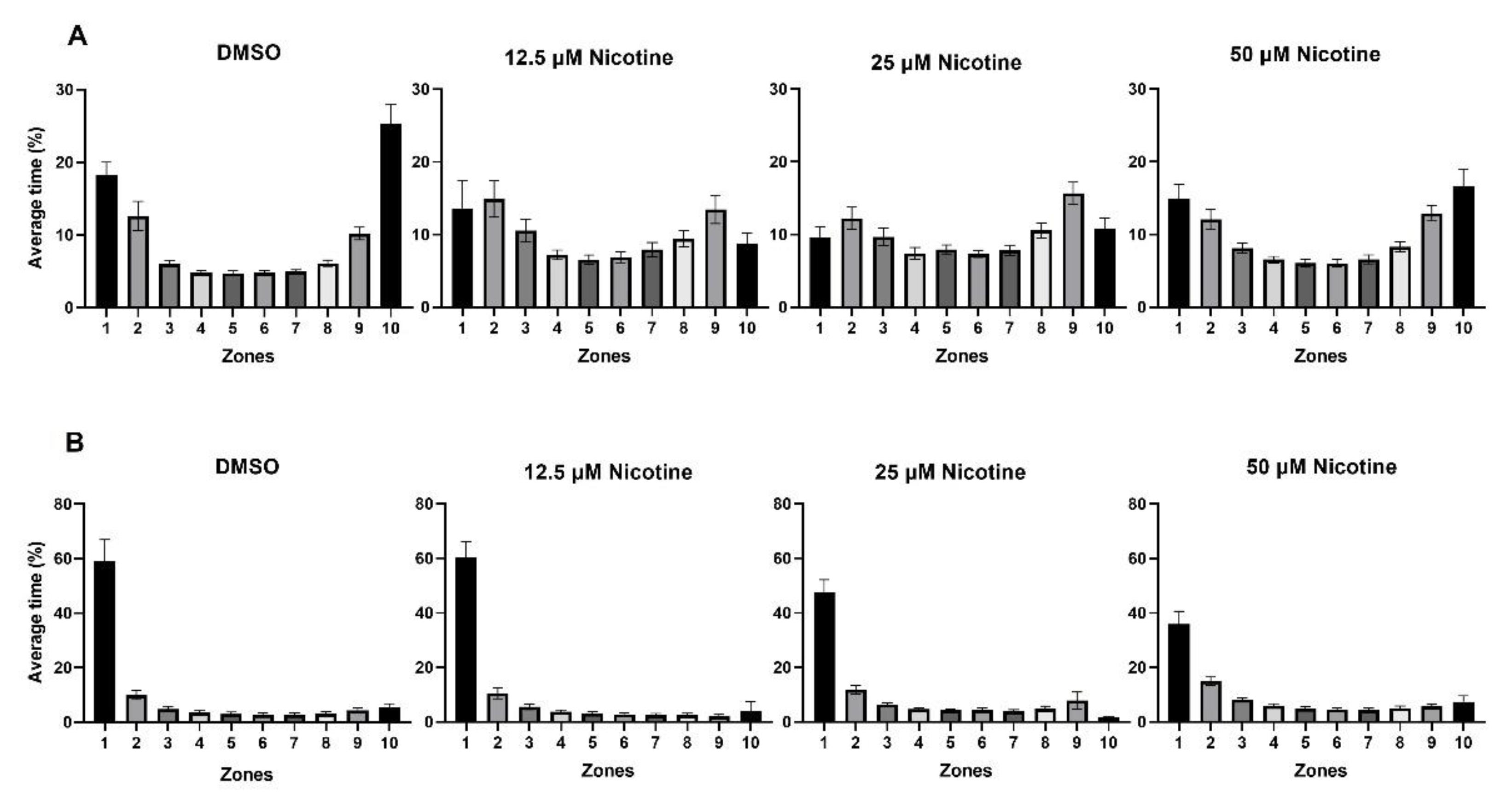
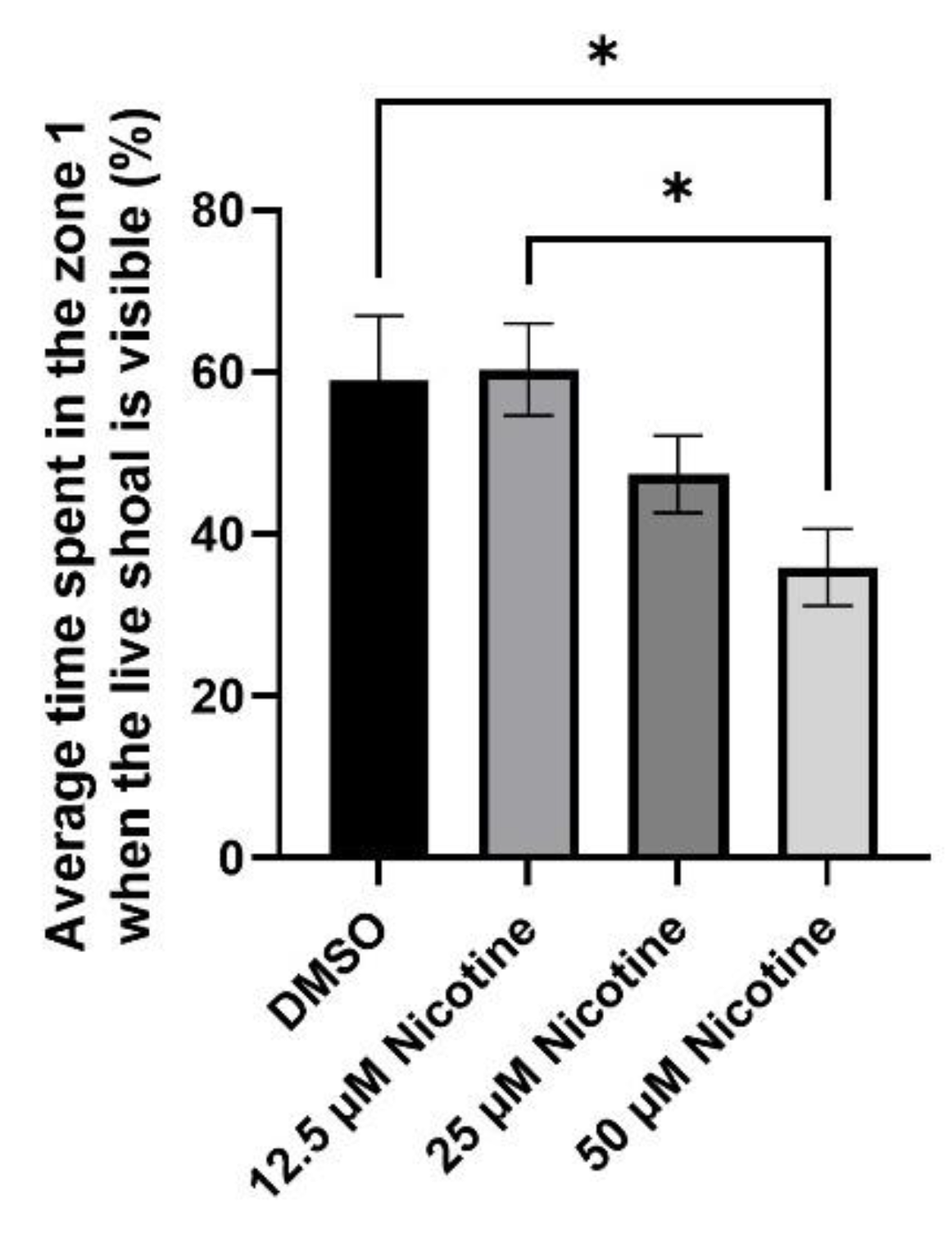
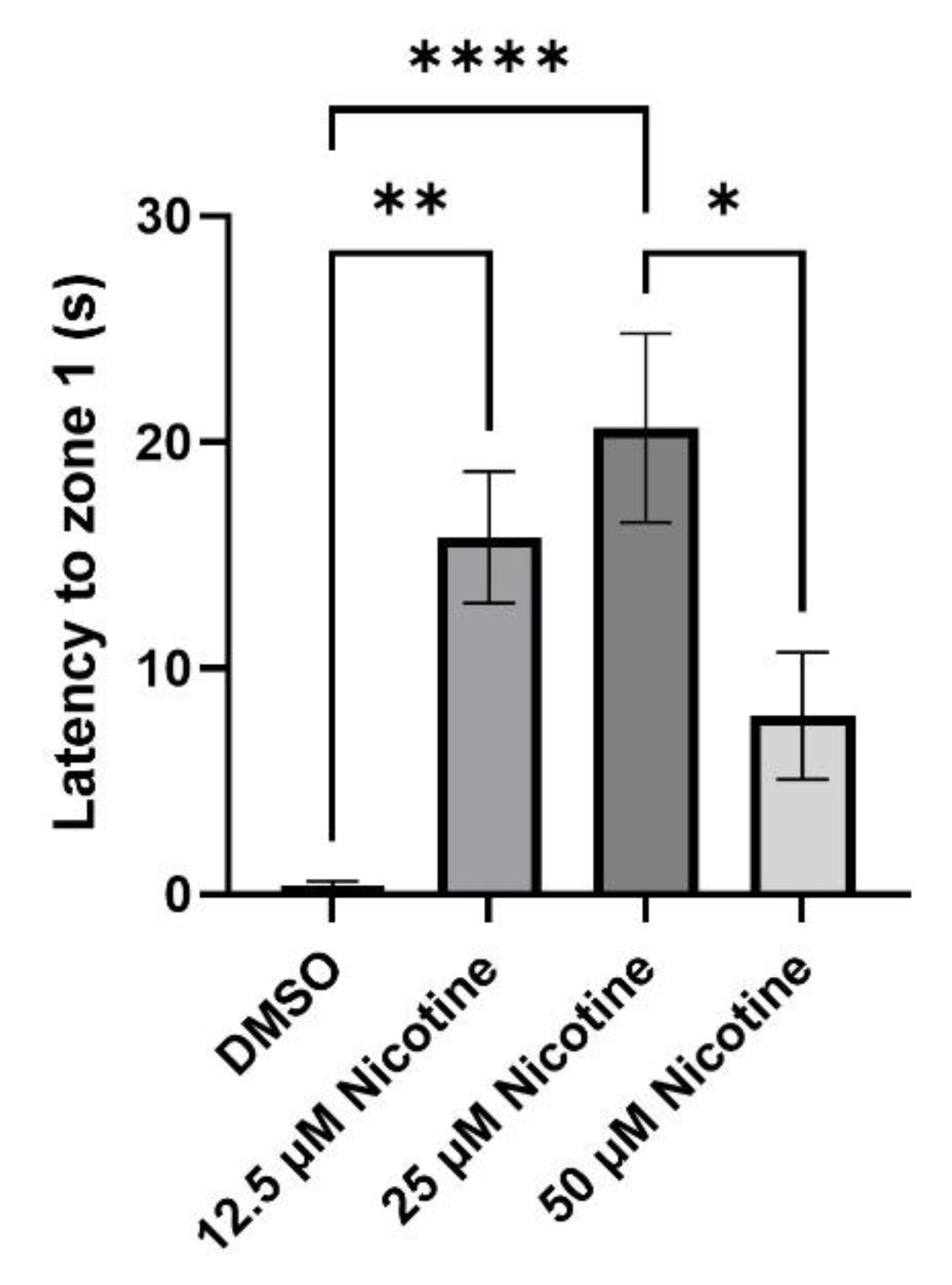
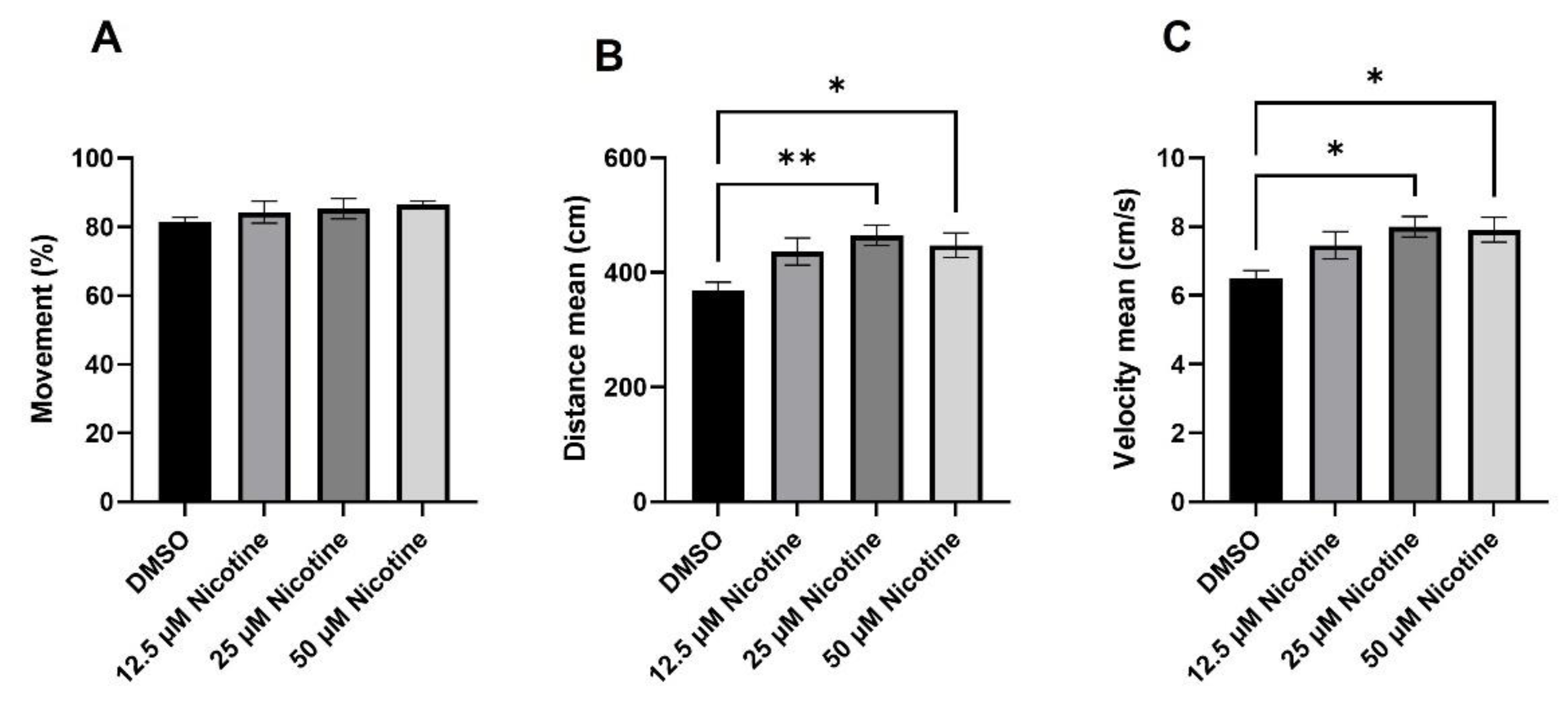
3.3. Social Behavior Is Affected by Embryonic Nicotine Exposure
4. Discussion
4.1. Nicotine Metabolism in Zebrafish
4.2. Nicotine and Craniofacial Defects
4.3. Embryonic Nicotine Exposure and Adult Zebrafish Behavior
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tobacco. Available online: https://www.who.int/news-room/fact-sheets/detail/tobacco (accessed on 27 August 2022).
- Hughes, J.R.; Keely, J.; Naud, S. Shape of the Relapse Curve and Long-Term Abstinence among Untreated Smokers. Addiction 2004, 99, 29–38. [Google Scholar] [CrossRef]
- Stephens, S.H.; Hartz, S.M.; Hoft, N.R.; Saccone, N.L.; Corley, R.C.; Hewitt, J.K.; Hopfer, C.J.; Breslau, N.; Coon, H.; Chen, X.; et al. Distinct Loci in the CHRNA5/CHRNA3/CHRNB4 Gene Cluster Are Associated with Onset of Regular Smoking. Genet. Epidemiol. 2013, 37, 846–859. [Google Scholar] [CrossRef] [Green Version]
- Dani, J.A. Neuronal Nicotinic Acetylcholine Receptor Structure and Function and Response to Nicotine. Int. Rev. Neurobiol. 2015, 124, 3–19. [Google Scholar] [CrossRef] [Green Version]
- Xavier, J.; Singh, S.; Kumari, P.; Ravichandiran, V. Neurological Repercussions of Neonatal Nicotine Exposure: A Review. Int. J. Dev. Neurosci. 2022, 82, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Burg, M.L.; Chai, Y.; Yao, C.A.; Magee, W.; Figueiredo, J.C. Epidemiology, Etiology, and Treatment of Isolated Cleft Palate. Front. Physiol. 2016, 7, 67. [Google Scholar] [CrossRef] [Green Version]
- Sabbagh, H.J.; Hassan, M.H.A.; Innes, N.P.T.; Elkodary, H.M.; Little, J.; Mossey, P.A. Passive Smoking in the Etiology of Non-Syndromic Orofacial Clefts: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0116963. [Google Scholar] [CrossRef] [Green Version]
- Dolan, C.V.; Geels, L.; Vink, J.M.; van Beijsterveldt, C.E.M.; Neale, M.C.; Bartels, M.; Boomsma, D.I. Testing Causal Effects of Maternal Smoking During Pregnancy on Offspring’s Externalizing and Internalizing Behavior. Behav. Genet. 2016, 46, 378–388. [Google Scholar] [CrossRef] [Green Version]
- Park, A.; O’Malley, S.S.; King, S.L.; Picciotto, M.R. Mediating Role of Stress Reactivity in the Effects of Prenatal Tobacco Exposure on Childhood Mental Health Outcomes. Nicotine Tob. Res. 2014, 16, 174–185. [Google Scholar] [CrossRef] [Green Version]
- Lotfipour, S.; Ferguson, E.; Leonard, G.; Miettunen, J.; Perron, M.; Pike, G.B.; Richer, L.; Séguin, J.R.; Veillette, S.; Jarvelin, M.R.; et al. Maternal Cigarette Smoking during Pregnancy Predicts Drug Use via Externalizing Behavior in Two Community-Based Samples of Adolescents. Addiction 2014, 109, 1718–1729. [Google Scholar] [CrossRef] [Green Version]
- Behnke, M.; Smith, V.C. Prenatal Substance Abuse: Short- and Long-Term Effects on the Exposed Fetus. Pediatrics 2013, 131, e1009–e1024. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.E.; Ben Lovely, C.; Eberhart, J.K. Variation in Phenotypes from a Bmp-Gata3 Genetic Pathway Is Modulated by Shh Signaling. PLoS Genet. 2021, 17, e1009579. [Google Scholar] [CrossRef] [PubMed]
- Mccarthy, N.; Liu, J.S.; Richarte, A.M.; Eskiocak, B.; Lovely, C.B.; Tallquist, M.D.; Eberhart, J.K. Pdgfra and Pdgfrb Genetically Interact during Craniofacial Development. Dev. Dyn. 2016, 245, 641–652. [Google Scholar] [CrossRef] [Green Version]
- Fish, E.W.; Tucker, S.K.; Peterson, R.L.; Eberhart, J.K.; Parnell, S.E. Loss of Tumor Protein 53 Protects against Alcohol-Induced Facial Malformations in Mice and Zebrafish. Alcohol. Clin. Exp. Res. 2021, 45, 1965–1979. [Google Scholar] [CrossRef]
- Everson, J.L.; Batchu, R.; Eberhart, J.K. Multifactorial Genetic and Environmental Hedgehog Pathway Disruption Sensitizes Embryos to Alcohol-Induced Craniofacial Defects. Alcohol. Clin. Exp. Res. 2020, 44, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Y.; Rampersad, M.; Jones, E.M.; Eberhart, J.K. Social Deficits Following Embryonic Ethanol Exposure Arise in Post-Larval Zebrafish. Addict. Biol. 2018, 24, 898–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, Y.; Rampersad, M.; Gerlai, R. Impairment of Social Behaviour Persists Two Years after Embryonic Alcohol Exposure in Zebrafish: A Model of Fetal Alcohol Spectrum Disorders. Behav. Brain Res. 2015, 292, 102–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandes, Y.; Rampersad, M.; Eberhart, J.K. Social Behavioral Phenotyping of the zebrafish Casper Mutant Following Embryonic Alcohol Exposure. Behav. Brain Res. 2019, 356, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish; University of Oregon Press: Corvallis, OR, USA, 1993. [Google Scholar]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of Embryonic Development of the Zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Walker, M.B.; Kimmel, C.B. A Two-Color Acid-Free Cartilage and Bone Stain for Zebrafish Larvae. Biotech. Histochem. 2007, 82, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Hukkanen, J.; Iii, P.J.; Benowitz, N.L. Metabolism and Disposition Kinetics of Nicotine. Pharmacol. Rev. 2005, 57, 79–115. [Google Scholar] [CrossRef]
- Benjamin, J. Wilkins and Michael Pack Zebrafish Models of Human Liver Development and Disease. Physiol. Behav. 2016, 176, 139–148. [Google Scholar] [CrossRef]
- McCarthy, N.; Sidik, A.; Bertrand, J.Y.; Eberhart, J.K. An Fgf-Shh Signaling Hierarchy Regulates Early Specification of the Zebrafish Skull. Dev. Biol. 2016, 415, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Mayor, R. Mechanisms of Neural Crest Migration. Annu. Rev. Genet. 2018, 52, 43–63. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2015; Volume 26. [Google Scholar]
- Hossain, M.M.; Khan, N.; Sultana, A.; Ma, P.; McKyer, E.L.J.; Ahmed, H.U.; Purohit, N. Prevalence of Comorbid Psychiatric Disorders among People with Autism Spectrum Disorder: An Umbrella Review of Systematic Reviews and Meta-Analyses. Psychiatry Res. 2020, 287, 112922. [Google Scholar] [CrossRef]
- Gordon-Lipkin, E.; Marvin, A.R.; Law, J.K.; Lipkin, P.H. Anxiety and Mood Disorder in Children with Autism Spectrum Disorder and ADHD. Pediatrics 2018, 141, e20171377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robbins, L.S.; Blanchard, C.T.; Sinkey, R.G.; Harris, S.L.; Tita, A.T.; Harper, L.M. Prenatal Tobacco Exposure and Childhood Neurodevelopment among Infants Born Prematurely. Am. J. Perinatol. 2021, 38, 218–223. [Google Scholar] [CrossRef]
- Haustein, K.O. Cigarette Smoking, Nicotine and Pregnancy. Int. J. Clin. Pharm. 1999, 37, 417–427. [Google Scholar]
- Vaglenova, J.; Parameshwaran, K.; Suppiramaniam, V.; Breese, C.R.; Pandiella, N.; Birru, S. Long-Lasting Teratogenic Effects of Nicotine on Cognition: Gender Specificity and Role of AMPA Receptor Function. Neurobiol. Learn. Mem. 2008, 90, 527–536. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services; Public Health Service; Office of the Surgeon General; Rockville, M.D. The Health Consequences of Smoking: 50 Years of Progress. A Report of the Surgeon General; U.S. Department of Health and Human Services: Washington, DC, USA, 2014.
- Polli, F.S.; Kohlmeier, K.A. Prenatal Nicotine Exposure in Rodents: Why Are There so Many Variations in Behavioral Outcomes? Nicotine Tob. Res. 2021, 22, 1694–1710. [Google Scholar] [CrossRef]
- Zhang, C.; Fan, S.I.J.; Sun, A.N.B.; Liu, Z.Z.; Liu, L. Prenatal Nicotine Exposure Induces Depression—like Behavior in Adolescent Female Rats via Modulating Neurosteroid in the Hippocampus. Mol. Med. Rep. 2019, 19, 4185–4194. [Google Scholar] [CrossRef] [Green Version]
- Polli, F.S.; Scharff, M.B.; Ipsen, T.H.; Aznar, S.; Kohlmeier, K.A.; Andreasen, J.T. Prenatal Nicotine Exposure in Mice Induces Sex-Dependent Anxiety-like Behavior, Cognitive Deficits, Hyperactivity, and Changes in the Expression of Glutamate Receptor Associated-Genes in the Prefrontal Cortex. Pharmacol. Biochem. Behav. 2020, 195, 172951. [Google Scholar] [CrossRef]
- Chirico, D.; Romano, E.; Famele, M.; Draisci, R.; Mancinelli, R.; Pascucci, T.; Adriani, W. Forced but Not Free-Choice Nicotine during Lactation Alters Maternal Behavior and Noradrenergic System of Pups: Impact on Social Behavior of Adolescent Isolated Male Rats. Neuroscience 2017, 361, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Vrana, K.; Ding, Z.-M. Cotinine: Pharmacologically Active Metabolite of Nicotine and Neural Mechanisms for Its Actions. Front. Behav. Neurosci. 2021, 15, 758252. [Google Scholar] [CrossRef]
- Alzualde, A.; Jaka, O.; Latino, D.A.R.S.; Alijevic, O.; Iturria, I.; de Mendoza, J.H.; Pospisil, P.; Frentzel, S.; Peitsch, M.C.; Hoeng, J.; et al. Effects of Nicotinic Acetylcholine Receptor-Activating Alkaloids on Anxiety-like Behavior in Zebrafish. J. Nat. Med. 2021, 75, 926–941. [Google Scholar] [CrossRef]
- Baldwin, K.V.; Racowsky, C. Nicotine and Cotinine Effects on Development of Two-Cell Mouse Embryos in Vitro. Reprod. Toxicol. 1987, 1, 173–178. [Google Scholar] [CrossRef]
- Dawson, D.A.; Fort, D.J.; Smith, G.J.; Newell, D.L.; Bantle, J.A. Evaluation of the Developmental Toxicity of Nicotine and Cotinine with Frog Embryo Teratogenesis Assay: Xenopus. Teratog. Carcinog. Mutagen. 1988, 8, 329–338. [Google Scholar] [CrossRef]
- Bastianini, S.; Martire, V.L.; Alvente, S.; Berteotti, C.; Matteoli, G.; Rullo, L.; Stamatakos, S.; Silvani, A.; Candeletti, S.; Romualdi, P.; et al. Early-Life Nicotine or Cotinine Exposure Produces Long-Lasting Sleep Alterations and Downregulation of Hippocampal Corticosteroid Receptors in Adult Mice. Sci. Rep. 2021, 11, 23897. [Google Scholar] [CrossRef]
- Vyhlidal, C.A.; Riffel, A.K.; Haley, K.J.; Sharma, S.; Dai, H.; Tantisira, K.G.; Weiss, S.T.; Leeder, J.S. Cotinine in Human Placenta Predicts Induction of Gene Expression in Fetal Tissues. Drug Metab. Dispos. 2013, 41, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Rahimabad, P.K.; Anthony, T.M.; Jones, A.D.; Eslamimehr, S.; Mukherjee, N.; Ewart, S.; Holloway, J.W.; Arshad, H.; Commodore, S.; Karmaus, W. Nicotine and Its Downstream Metabolites in Maternal and Cord Sera: Biomarkers of Prenatal Smoking Exposure Associated with Offspring DNA Methylation. Int. J. Environ. Res. Public Health 2020, 17, 9552. [Google Scholar] [CrossRef]
- Li, Z.; Liu, J.; Ye, R.; Zhang, L.; Zheng, X.; Ren, A. Maternal Passive Smoking and Risk of Cleft Lip with or without Cleft Palate. Epidemiology 2010, 21, 240–242. [Google Scholar] [CrossRef]
- Honein, M.A.; Rasmussen, S.A.; Reefhuis, J.; Romitti, P.A.; Lammer, E.J.; Sun, L.; Correa, A. Maternal Smoking and Environmental Tobacco Smoke Exposure and the Risk of Orofacial Clefts. Epidemiology 2007, 18, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Eshete, M.; Butali, A.; Abate, F.; Hailu, T.; Hailu, A.; Degu, S.; Demissie, Y.; Gravem, P.E.; Derbew, M.; Mossey, P.; et al. The Role of Environmental Factors in the Etiology of Nonsyndromic Orofacial Clefts. J. Craniofacial Surg. 2020, 31, 113–116. [Google Scholar] [CrossRef] [PubMed]
- Durham, E.L.; Balog, C.; Howie, R.N.; Boyce, M.A.; Arand, J.R.; Warren, G.; Larue, A.C.; Cray, J.J. Effects of Nicotine Exposure on Murine Mandibular Development. PLoS ONE 2019, 14, e0218376. [Google Scholar] [CrossRef] [Green Version]
- Kang, P.; Svoboda, K.K.H. Nicotine inhibits palatal fusion and modulates nicotinic receptors and the PI-3 kinase pathway in medial edge epithelia. Orthod. Craniofacial Res. 2003, 6, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, F.; Sheldon, E.; Sharma, J.; Canturk, K.M.; Otu, H.H.; Nawshad, A. Nicotine Exposure during Pregnancy Results in Persistent Midline Epithelial Seam with Improper Palatal Fusion. Nicotine Tob. Res. 2016, 18, 604–612. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Cao, H.; Cu, F.; Xu, D.; Lei, Y.; Tan, Y.; Magdalou, J.; Wang, H.; Chen, L. Nicotine-Induced Retardation of Chondrogenesis through down-Regulation of IGF-1 Signaling Pathway to Inhibit Matrix Synthesis of Growth Plate Chondrocytes in Fetal Rats. Toxicol. Appl. Pharmacol. 2013, 269, 25–33. [Google Scholar] [CrossRef]
- Le Douarin, N.M.; Brito, J.M.; Creuzet, S. Role of the Neural Crest in Face and Brain Development. Brain Res. Rev. 2007, 55, 237–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swartz, M.E.; Sheehan-Rooney, K.; Dixon, M.J.; Eberhart, J.K. Examination of a Palatogenic Gene Program in Zebrafish. Dev. Dyn. 2011, 240, 2204–2220. [Google Scholar] [CrossRef] [Green Version]
- Parker, B.; Connaughton, V.P. Effects of Nicotine on Growth and Development in Larval Zebrafish. Zebrafish 2007, 4, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.T.; Welsh, L.; Galvez, F.; Svoboda, K.R. Acute Nicotine Exposure and Modulation of a Spinal Motor Circuit in Embryonic Zebrafish. Toxicol. Appl. Pharmacol. 2009, 239, 129–142. [Google Scholar] [CrossRef] [PubMed]
- Svoboda, K.R.; Vijayaraghavan, S.; Tanguay, R.L. Nicotinic Receptors Mediate Changes in Spinal Motoneuron Development and Axonal Pathfinding in Embryonic Zebrafish Exposed to Nicotine. J. Neurosci. 2005, 10731–10741, Erratum in J. Neurosci. 2007, 27, 3356. [Google Scholar] [CrossRef] [Green Version]
- Mora-Zamorano, F.X.; Svoboda, K.R.; Carvan, M.J. The Nicotine-Evoked Locomotor Response: A Behavioral Paradigm for Toxicity Screening in Zebrafish (Danio Rerio) Embryos and Eleutheroembryos Exposed to Methylmercury. PLoS ONE 2016, 11, e0154570. [Google Scholar] [CrossRef] [Green Version]
- Victoria, S.; Hein, M.; Harrahy, E.; King-Heiden, T.C. Potency Matters: Impacts of Embryonic Exposure to NAChR Agonists Thiamethoxam and Nicotine on Hatching Success, Growth, and Neurobehavior in Larval Zebrafish. J. Toxicol. Environ. Health Part A 2022, 85, 767–782. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.M.; Grossman, L.; Collier, A.D.; Echevarria, D.J.; Kalueff, A.V. Anxiogenic-like Effects of Chronic Nicotine Exposure in Zebrafish. Pharm. Biochem. Behav. 2015, 139 Pt B, 112–120. [Google Scholar] [CrossRef]
- Hawkey, A.B.; Hoeng, J.; Peitsch, M.C.; Levin, E.D.; Koshibu, K. Subchronic Effects of Plant Alkaloids on Anxiety-like Behavior in Zebrafish. Pharmacol. Biochem. Behav. 2021, 207, 173223. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, Y.; Rampersad, M.; Gerlai, R. Embryonic Alcohol Exposure Impairs the Dopaminergic System and Social Behavioral Responses in Adult Zebrafish. Int. J. Neuropsychopharmacol. 2015, 18, pyu089. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borrego-Soto, G.; Eberhart, J.K. Embryonic Nicotine Exposure Disrupts Adult Social Behavior and Craniofacial Development in Zebrafish. Toxics 2022, 10, 612. https://doi.org/10.3390/toxics10100612
Borrego-Soto G, Eberhart JK. Embryonic Nicotine Exposure Disrupts Adult Social Behavior and Craniofacial Development in Zebrafish. Toxics. 2022; 10(10):612. https://doi.org/10.3390/toxics10100612
Chicago/Turabian StyleBorrego-Soto, Gissela, and Johann K. Eberhart. 2022. "Embryonic Nicotine Exposure Disrupts Adult Social Behavior and Craniofacial Development in Zebrafish" Toxics 10, no. 10: 612. https://doi.org/10.3390/toxics10100612
APA StyleBorrego-Soto, G., & Eberhart, J. K. (2022). Embryonic Nicotine Exposure Disrupts Adult Social Behavior and Craniofacial Development in Zebrafish. Toxics, 10(10), 612. https://doi.org/10.3390/toxics10100612






