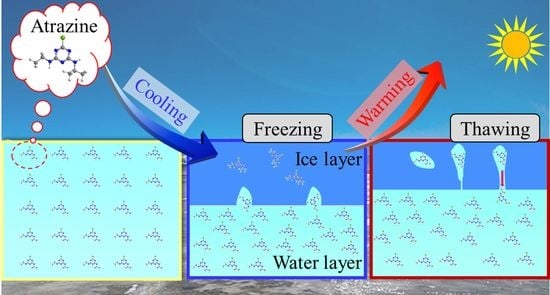
The Migration Pattern of Atrazine during the Processes of Water Freezing and Thawing
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Instruments
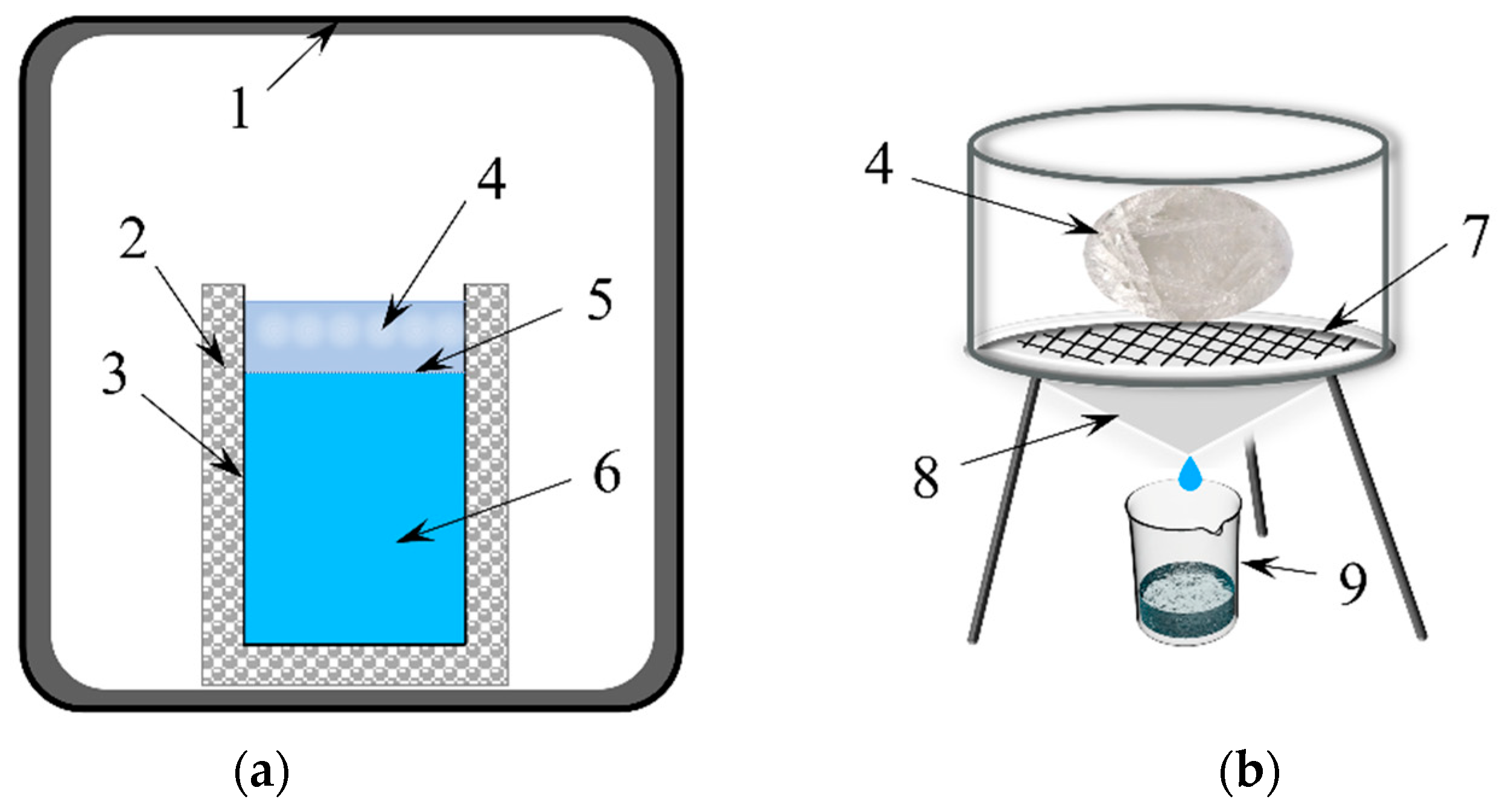
2.2. Experimental Setup
2.3. Experimental Design and Procedure
- (1)
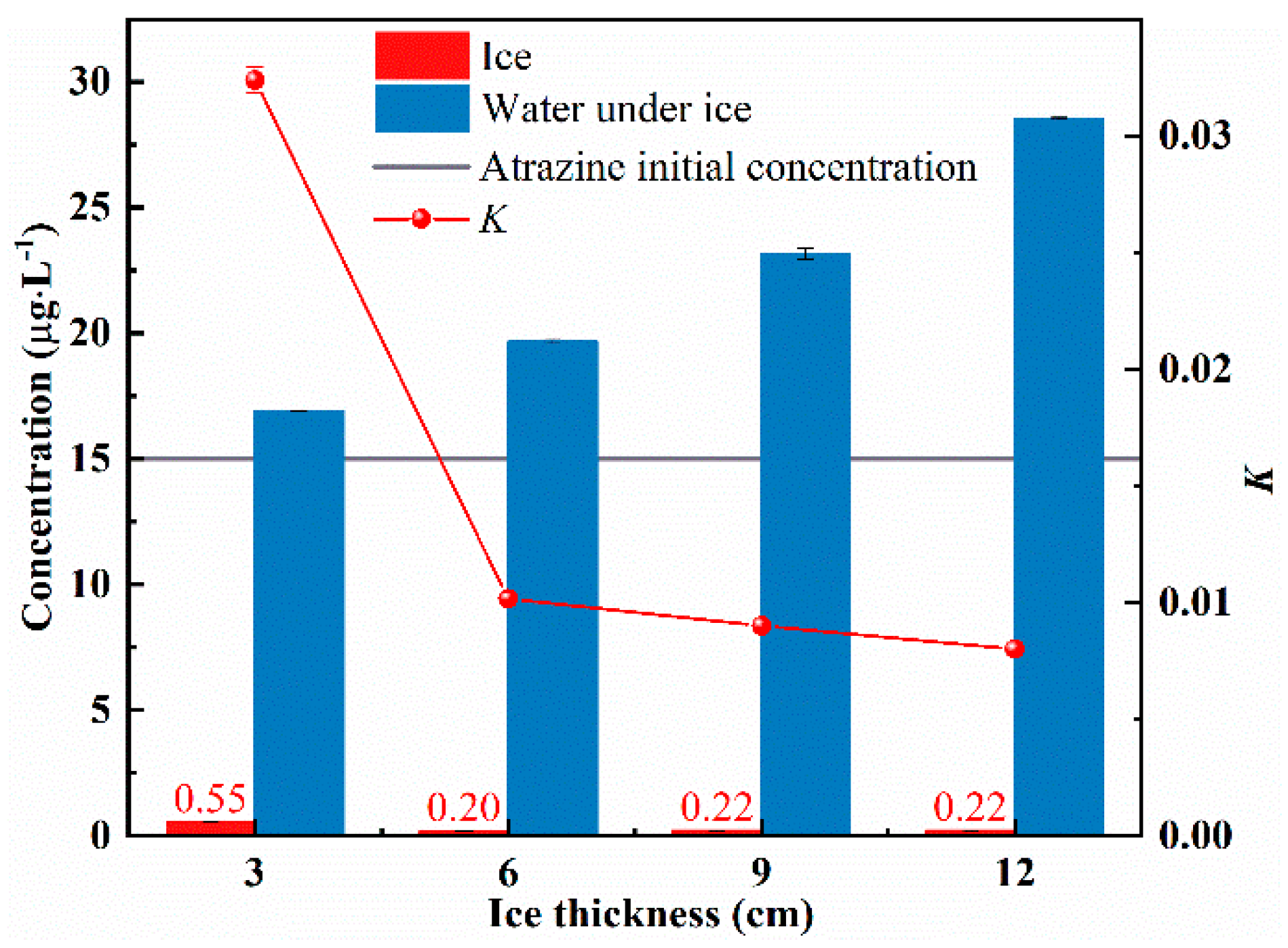
- To explore the effect of ice thickness on the migration of atrazine, four different ice thickness conditions (3 cm, 6 cm, 9 cm, and 12 cm) were set and numbered sequentially as 1–4. Four solutions of atrazine with an initial concentration of 15 μg/L were prepared. The freezing temperature was −5 °C. Freezing ended when the ice thickness reached 3 cm, 6 cm, 9 cm, and 12 cm, respectively.
- (2)
- To explore the effect of freezing temperature on the migration of atrazine, three different freezing temperature conditions (−5 °C, −10 °C, and −15 °C) were set and numbered sequentially as 5–7. Three solutions of atrazine with an initial concentration of 15 μg/L were prepared. Freezing ended when the ice thickness reached 9 cm.
- (3)
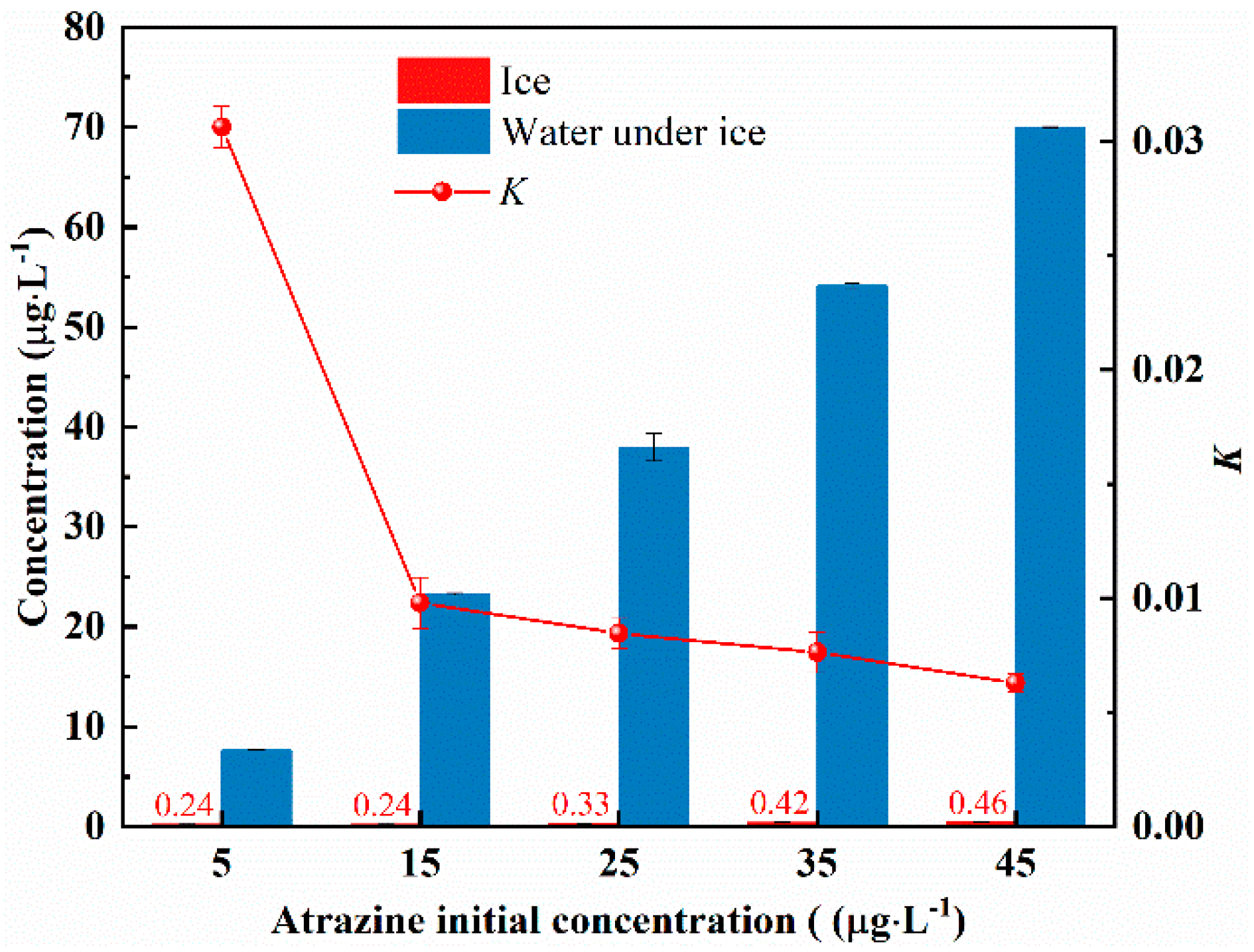
- To explore the effect of initial concentration on the migration pattern of atrazine, five different concentrations (5 μg/L, 15 μg/L, 25 μg/L, 35 μg/L, and 45 μg/L) of atrazine solutions were prepared and numbered sequentially as 8–12. The freezing temperature was −5 °C. Freezing ended when the ice thickness reached 9 cm.
2.4. UPLC Method Parameters and Precision
2.5. Date Analysis
3. Results
3.1. Effect of Ice Thickness on the Migration of Atrazine
3.2. Effect of Freezing Temperature on the Migration of Atrazine
3.3. Effect of Initial Concentration on the Migration of Atrazine
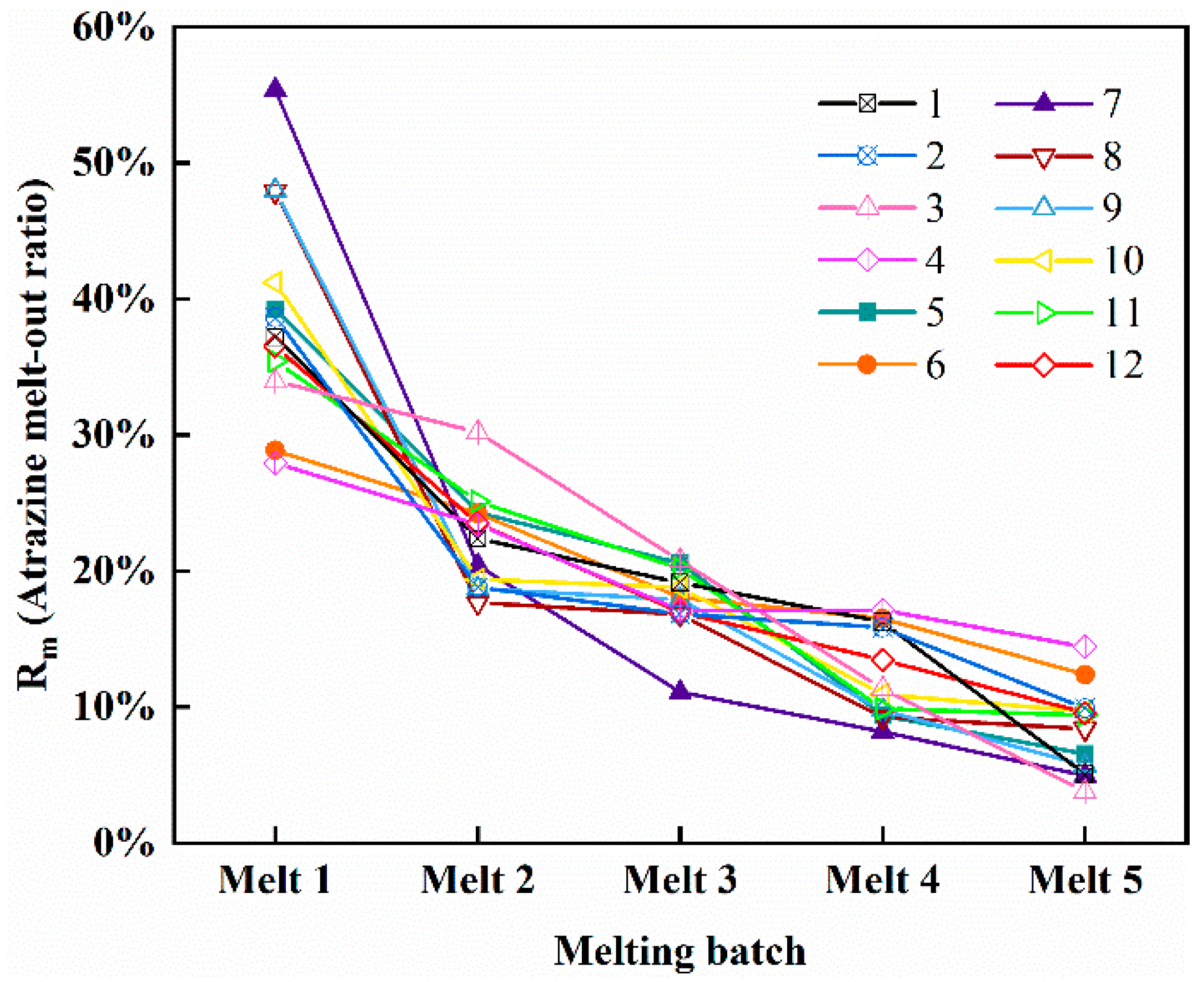
3.4. Release Pattern of Atrazine during Ice Melting Process
4. Discussion
4.1. Migration Mechanism of Atrazine between Ice and Water during Freezing
4.2. Influence of Various Factors on the Migration Capacity of Atrazine
4.3. Mechanism of Atrazine Release from Ice during Melting
5. Conclusions
- (1)
- Freezing causes redistribution of atrazine in both ice and water phases, and its concentration relationship is expressed as ice < water before freezing < water under the ice. Atrazine continuously migrated from the ice to the water under the ice during the freezing process, which reminded us that we need to pay more attention to the quality of under-ice water in the natural water body during the freezing period.
- (2)
- During the initial stages of thawing, atrazine trapped in the ice exhibits a massive release into the water. Moreover, as thawing continued, the concentration of atrazine in the meltwater became lower and lower the unmelted ice becomes purer. In areas where the water environment is prone to freezing in winter, the water environment may deteriorate instantaneously due to the concentrated release of atrazine in the early stages of ice thawing.
- (3)
- The K value decreased with increasing ice thickness, freezing temperature, and atrazine initial concentration. The migration ability of atrazine was positively correlated with ice thickness, freezing temperature, and atrazine initial concentration. Atrazine has an extremely low distribution coefficient (K = 0.006) when a solution with an initial concentration of 45 μg/L is frozen at −10 °C to a thickness of 9 cm. The migration ability of atrazine was strongest under this condition.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weyhenmeyer, G.A.; Livingstone, D.M.; Meili, M.; Jensen, O.; Benson, B.; Magnuson, J.J. Large geographical differences in the sensitivity of ice-covered lakes and rivers in the Northern Hemisphere to temperature changes. Glob. Chang. Biol. 2011, 17, 268–275. [Google Scholar] [CrossRef]
- Verpoorter, C.; Kutser, T.; Seekell, D.A.; Tranvik, L.J. A global inventory of lakes based on high-resolution satellite imagery. Geophys. Res. Lett. 2014, 41, 6396–6402. [Google Scholar] [CrossRef]
- Fang, X.; Stefan, H.G. Simulations of climate effects on water temperature, dissolved oxygen, and ice and snow covers in lakes of the contiguous United States under past and future climate scenarios. Limnol. Oceanogr. 2009, 54, 2359–2370. [Google Scholar] [CrossRef]
- Belzile, C.; Gibson, J.A.E.; Vincent, W.F. Colored dissolved organic matter and dissolved organic carbon exclusion from lake ice: Implications for irradiance transmission and carbon cycling. Limnol. Oceanogr. 2002, 47, 1283–1293. [Google Scholar] [CrossRef]
- Powers, S.M.; Labou, S.G.; Baulch, H.M.; Hunt, R.J.; Lottig, N.R.; Hampton, S.E.; Stanley, E.H. Ice duration drives winter nitrate accumulation in north temperate lakes. Limnol. Oceanogr. Lett. 2017, 2, 177–186. [Google Scholar] [CrossRef]
- Terry, J.A.; Sadeghian, A.; Lindenschmidt, K.E. Modelling dissolved oxygen/sediment oxygen demand under ice in a shallow eutrophic prairie reservoir. Water 2017, 9, 131. [Google Scholar] [CrossRef]
- Hampton, S.E.; Galloway, A.W.E.; Powers, S.M.; Ozersky, T.; Woo, K.H.; Batt, R.D.; Labou, S.G.; O’Reilly, C.M.; Sharma, S.; Lottig, N.R.; et al. Ecology under lake ice. Ecol. Lett. 2017, 20, 98–111. [Google Scholar] [CrossRef]
- Hampton, S.E.; Moore, M.V.; Ozersky, T.; Stanley, E.H.; Polashenski, C.M.; Galloway, A.W.E. Heating up a cold subject: Prospects for under-ice plankton research in lakes. J. Plankton Res. 2015, 37, 277–284. [Google Scholar] [CrossRef]
- Huang, W.; Han, H.; Niu, F.; Li, Z. Field observations on water temperature and stratification in a seasonally ice-covered shallow thermokarst lake. Shuikexue Jinzhan/Adv. Water Sci. 2016, 27, 280–289. [Google Scholar] [CrossRef]
- Yang, F.; Cen, R.; Feng, W.; Zhu, Q.; Leppäranta, M.; Yang, Y.; Wang, X.; Liao, H. Dynamic simulation of nutrient distribution in lakes during ice cover growth and ablation. Chemosphere 2021, 281, 130781. [Google Scholar] [CrossRef]
- Xue, S.; Chen, J.; Tie, M. Release of Dissolved Organic Matter from Melting Ice. Environ. Prog. Sustain. Energy 2016, 35, 1458–1467. [Google Scholar] [CrossRef]
- Bukin, Y.S.; Bondarenko, N.A.; Rusanov, I.I.; Pimenov, N.V.; Bukin, S.V.; Pogodaeva, T.V.; Chernitsyna, S.M.; Shubenkova, O.V.; Ivanov, V.G.; Zakharenko, A.S.; et al. Interconnection of bacterial and phytoplanktonic communities with hydrochemical parameters from ice and under-ice water in coastal zone of Lake Baikal. Sci. Rep. 2020, 10, 11087. [Google Scholar] [CrossRef] [PubMed]
- Pieters, R.; Lawrence, G.A. Effect of salt exclusion from lake ice on seasonal circulation. Limnol. Oceanogr. 2009, 54, 401–412. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, T.; Tang, Y.; Zhao, W.; Ren, F.; Zhao, T.; Liu, Y. The migration law of magnesium ions during freezing and melting processes. Environ. Sci. Pollut. Res. 2022, 29, 26675–26687. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, Y.; Tang, Y.; Wang, X.; Zhao, C.; Wang, N.; Liu, Y. Application of progressive freeze concentration in the removal of Ca2+ from wastewater. J. Water Process Eng. 2022, 46, 102619. [Google Scholar] [CrossRef]
- Lazarcik, J.; Dibb, J.E.; Adolph, A.C.; Amante, J.M.; Wake, C.P.; Scheuer, E.; Mineau, M.M.; Albert, M.R. Major fraction of black carbon is flushed from the melting New Hampshire snowpack nearly as quickly as soluble impurities. J. Geophys. Res. 2017, 122, 537–553. [Google Scholar] [CrossRef]
- Szpaczyñski, J.A.; White, J.A.; Côté, C.L. Separation of contaminants in the freeze/thaw process. Chem. Process Eng.-Inz. Chem. Proces. 2017, 38, 249–264. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Du, S.; Zhang, X.; Lyu, G.; Dong, D.; Hua, X.; Zhang, W.; Guo, Z. Occurrence, distribution, and ecological risk of pharmaceuticals in a seasonally ice-sealed river: From ice formation to melting. J. Hazard. Mater. 2020, 389, 122083. [Google Scholar] [CrossRef]
- Li, Z.J.; Wang, X.; Li, Q.S.; Xu, S.G.; Xu, X.Z.; Bai, Y. Study on nitrobenzene ratio in water-ice system under different conditions. Sci. China, Ser. E Technol. Sci. 2008, 51, 2013–2020. [Google Scholar] [CrossRef]
- Moreira, A.J.; Pinheiro, B.S.; Araújo, A.F.; Freschi, G.P.G. Evaluation of atrazine degradation applied to different energy systems. Environ. Sci. Pollut. Res. 2016, 23, 18502–18511. [Google Scholar] [CrossRef]
- Ouyang, W.; Zhang, Y.; Lin, C.; Wang, A.; Tysklind, M.; Wang, B. Metabolic process and spatial partition dynamics of Atrazine in an estuary-to-bay system, Jiaozhou bay. J. Hazard. Mater. 2021, 414, 125530. [Google Scholar] [CrossRef] [PubMed]
- Perkins, D.B.; Stone, Z.; Jacobson, A.; Chen, W.; Szarka, A.Z.; White, M.; Christensen, B.; Ghebremichael, L.; Brain, R.A. Development of a US national-scale, mixed-source, pesticide, rural well database for use in drinking water risk assessment: An atrazine case study. Environ. Monit. Assess. 2022, 194, 578. [Google Scholar] [CrossRef]
- Rippy, M.A.; Deletic, A.; Black, J.; Aryal, R.; Lampard, J.L.; Tang, J.Y.M.; McCarthy, D.; Kolotelo, P.; Sidhu, J.; Gernjak, W. Pesticide occurrence and spatio-temporal variability in urban run-off across Australia. Water Res. 2017, 115, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Peng, Y.; Muir, D.; Lin, J.; Zhang, X. A critical review of synthetic chemicals in surface waters of the US, the EU and China. Environ. Int. 2019, 131, 104994. [Google Scholar] [CrossRef] [PubMed]
- Akpinar, I.; Drout, R.J.; Islamoglu, T.; Kato, S.; Lyu, J.; Farha, O.K. Exploiting π-π Interactions to Design an Efficient Sorbent for Atrazine Removal from Water. ACS Appl. Mater. Interfaces 2019, 11, 6097–6103. [Google Scholar] [CrossRef]
- Jablonowski, N.D.; Schäffer, A.; Burauel, P. Still present after all these years: Persistence plus potential toxicity raise questions about the use of atrazine. Environ. Sci. Pollut. Res. 2011, 18, 328–331. [Google Scholar] [CrossRef]
- Xie, D.; Chen, C.; Li, C.; Wang, Q. Influence of Cd on atrazine degradation and the formation of three primary metabolites in water under the combined pollution. Environ. Sci. Pollut. Res. 2021, 28, 16081–16091. [Google Scholar] [CrossRef]
- Johnson, J.A.; Ihunwo, A.; Chimuka, L.; Mbajiorgu, E.F. Cardiotoxicity in African clawed frog (Xenopus laevis) sub-chronically exposed to environmentally relevant atrazine concentrations: Implications for species survival. Aquat. Toxicol. 2019, 213, 105218. [Google Scholar] [CrossRef]
- Ge, L.; Chen, J.; Wei, X.; Zhang, S.; Qiao, X.; Cai, X.; Xie, Q. Aquatic photochemistry of fluoroquinolone antibiotics: Kinetics, pathways, and multivariate effects of main water constituents. Environ. Sci. Technol. 2010, 44, 2400–2405. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, P.; Halsall, C.; Li, Y.; Chen, C.E.; Li, J.; Sun, H.; Yao, Z. The importance of reactive oxygen species on the aqueous phototransformation of sulfonamide antibiotics: Kinetics, pathways, and comparisons with direct photolysis. Water Res. 2019, 149, 243–250. [Google Scholar] [CrossRef]
- Ge, L.; Li, J.; Na, G.; Chen, C.E.; Huo, C.; Zhang, P.; Yao, Z. Photochemical degradation of hydroxy PAHs in ice: Implications for the polar areas. Chemosphere 2016, 155, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Yoon, H.-I.; Lee, C.; Vetráková, L.; Heger, D.; Kim, K.; Kim, J. Activation of Periodate by Freezing for the Degradation of Aqueous Organic Pollutants. Environ. Sci. Technol. 2018, 52, 5378–5385. [Google Scholar] [CrossRef]
- Pei, G.X.; Han, Y.H.; Liu, G.Y.; Zhang, Q.; Cao, W.J.; Guo, Q.; Han, Z.; Wei, L.; Li, H.Q. Distribution and release of 2,4,5-trichlorobiphenyl in ice. Water Sci. Eng. 2015, 8, 132–138. [Google Scholar] [CrossRef][Green Version]
- Li, Y.; Li, M.; Dang, C.; Liu, X. Effects of dissolved gas on the nucleation and growth of ice crystals in freezing droplets. Int. J. Heat Mass Transf. 2022, 184, 122334. [Google Scholar] [CrossRef]
- Koop, T. Homogeneous Ice Nucleation in Water and Aqueous Solutions. Zeitschrift für Phys. Chemie 2004, 218, 1231–1258. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, C.; Rong, H.; Wei, C.; Gou, S. Experimental study on seawater desalination through supercooled water dynamic ice making. Desalination 2020, 476, 114233. [Google Scholar] [CrossRef]
- Tsironi, I.; Schlesinger, D.; Späh, A.; Eriksson, L.; Segad, M.; Perakis, F. Brine rejection and hydrate formation upon freezing of NaCl aqueous solutions. Phys. Chem. Chem. Phys. 2020, 22, 7625–7632. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cao, C.; Fei, X.; Zhao, H.; Jin, L. Solute selectivity, separation mechanism and application performance of freezing wastewater treatment: Focus on air cooling and direct contact cooling. J. Water Process Eng. 2021, 44, 102445. [Google Scholar] [CrossRef]
- Kim, K.; Chung, H.Y.; Kim, B.; Wong, G.; Nguyen, A.Q.K.; Kim, S.; Kim, J. Freezing-Induced Simultaneous Reduction of Chromate and Production of Molecular Iodine: Mechanism, Kinetics, and Practical Implications. Environ. Sci. Technol. 2020, 54, 16204–16211. [Google Scholar] [CrossRef]
- El Kadi, K.; Adeyemi, I.; Janajreh, I. Application of directional freezing for seawater desalination: Parametric analysis using experimental and computational methods. Desalination 2021, 520, 115339. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, Y.; Yu, A.; Zhao, W.; Liu, Y. Research on the migration of the total manganese during the process of water icing. Water 2019, 11, 1626. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Zhao, W.; Liu, T.; Liu, Y. The migration law of iron during the process of water icing. Water 2020, 12, 441. [Google Scholar] [CrossRef]
- Luo, S.; Jin, Y.; Tao, R.; Li, H.; Li, C.; Wang, J.; Li, Z. Molecular understanding of ion rejection in the freezing of aqueous solutions. Phys. Chem. Chem. Phys. 2021, 23, 13292–13299. [Google Scholar] [CrossRef] [PubMed]
- Weeks, W.F.; Ackley, S.F. The Growth, Structure, and Properties of Sea Ice BT-The Geophysics of Sea Ice. In The Geophysics of Sea Ice; Springer: Boston, MA, USA, 1986; pp. 9–164. [Google Scholar]
- Yuan, H.; Sun, K.; Wang, K.; Zhang, J.; Zhang, Z.; Zhang, L.; Li, S.; Li, Y. Ice crystal growth in the freezing desalination process of binary water-NaCl system. Desalination 2020, 496, 114737. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, F.; Tang, Y.; Zhao, W.; Zhao, T.; Liu, T.; Liu, Y.; Shen, H.T. Migration of iron and manganese ions during ice melting. China Environ. Sci. 2021, 41, 2391–2398. [Google Scholar] [CrossRef]
- Moharramzadeh, S.; Ong, S.K.; Alleman, J.; Cetin, K.S. Parametric study of the progressive freeze concentration for desalination. Desalination 2021, 510, 115077. [Google Scholar] [CrossRef]
- Terwilliger, J.P.; Dizon, S.F. Salt rejection phenomena in the freezing of saline solutions. Chem. Eng. Sci. 1970, 25, 1331–1349. [Google Scholar] [CrossRef]
- Wang, P.; Chung, T.S. A conceptual demonstration of freeze desalination-membrane distillation (FD-MD) hybrid desalination process utilizing liquefied natural gas (LNG) cold energy. Water Res. 2012, 46, 4037–4052. [Google Scholar] [CrossRef]
- Yu, T.; Ma, J.; Zhang, L.Q. Factors affecting ice crystal purity during freeze concentration process for urine treatment. J. Harbin Inst. Technol. 2007, 14, 593–597. [Google Scholar]
- Luo, C.S.; Chen, W.W.; Han, W.F. Experimental study on factors affecting the quality of ice crystal during the freezing concentration for the brackish water. Desalination 2010, 260, 231–238. [Google Scholar] [CrossRef]
- Nakagawa, K.; Maebashi, S.; Maeda, K. Freeze-thawing as a path to concentrate aqueous solution. Sep. Purif. Technol. 2010, 73, 403–408. [Google Scholar] [CrossRef]
- Shafique, U.; Anwar, J.; uz-Zaman, W.; Rehman, R.; Salman, M.; Dar, A.; Jamil, N. Forced migration of soluble and suspended materials by freezing front in aqueous systems. J. Hydro-Environ. Res. 2012, 6, 221–226. [Google Scholar] [CrossRef]
- Shum, E.; Papangelakis, V. Water recovery from inorganic solutions via natural freezing and melting. J. Water Process. Eng. 2019, 31, 100787. [Google Scholar] [CrossRef]
- Htira, T.; Cogné, C.; Gagnière, E.; Mangin, D. Experimental study of industrial wastewater treatment by freezing. J. Water Process. Eng. 2018, 23, 292–298. [Google Scholar] [CrossRef]

| Compound | Mass to Charge Ratio m/z | Collision Energy | |
|---|---|---|---|
| Parent Ion | Daughter Ion | /eV | |
| 95.8 | 25 | ||
| Atrazine | 215 | 103.73 | 28 |
| 173.9 | 17 | ||
| Number | Average Concentration (μg/L) | Melt 1 (μg/L) | Melt 2 (μg/L) | Melt 3 (μg/L) | Melt 4 (μg/L) | Melt 5 (μg/L) |
|---|---|---|---|---|---|---|
| 1 | 0.55 | 1.03 | 0.62 | 0.53 | 0.45 | 0.14 |
| 2 | 0.20 | 0.39 | 0.19 | 0.17 | 0.16 | 0.10 |
| 3 | 0.21 | 0.36 | 0.32 | 0.22 | 0.12 | 0.04 |
| 4 | 0.22 | 0.31 | 0.26 | 0.19 | 0.19 | 0.16 |
| 5 | 0.21 | 0.42 | 0.26 | 0.22 | 0.10 | 0.07 |
| 6 | 0.39 | 0.56 | 0.47 | 0.35 | 0.32 | 0.24 |
| 7 | 0.69 | 1.90 | 0.70 | 0.38 | 0.28 | 0.17 |
| 8 | 0.24 | 0.57 | 0.21 | 0.20 | 0.11 | 0.10 |
| 9 | 0.25 | 0.59 | 0.23 | 0.22 | 0.12 | 0.07 |
| 10 | 0.33 | 0.68 | 0.32 | 0.31 | 0.18 | 0.16 |
| 11 | 0.45 | 0.79 | 0.56 | 0.45 | 0.22 | 0.21 |
| 12 | 0.46 | 0.84 | 0.54 | 0.39 | 0.31 | 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhao, C.; Yu, A.; Zhao, W.; Ren, F.; Liu, Y. The Migration Pattern of Atrazine during the Processes of Water Freezing and Thawing. Toxics 2022, 10, 603. https://doi.org/10.3390/toxics10100603
Zhang Y, Zhao C, Yu A, Zhao W, Ren F, Liu Y. The Migration Pattern of Atrazine during the Processes of Water Freezing and Thawing. Toxics. 2022; 10(10):603. https://doi.org/10.3390/toxics10100603
Chicago/Turabian StyleZhang, Yan, Chen Zhao, Aixin Yu, Wanli Zhao, Fangyun Ren, and Yucan Liu. 2022. "The Migration Pattern of Atrazine during the Processes of Water Freezing and Thawing" Toxics 10, no. 10: 603. https://doi.org/10.3390/toxics10100603
APA StyleZhang, Y., Zhao, C., Yu, A., Zhao, W., Ren, F., & Liu, Y. (2022). The Migration Pattern of Atrazine during the Processes of Water Freezing and Thawing. Toxics, 10(10), 603. https://doi.org/10.3390/toxics10100603