Occurrence, Distribution, and Risk Assessment of Organophosphorus Pesticides in the Aquatic Environment of the Sele River Estuary, Southern Italy
Abstract
:1. Introduction
2. Materials and Methods
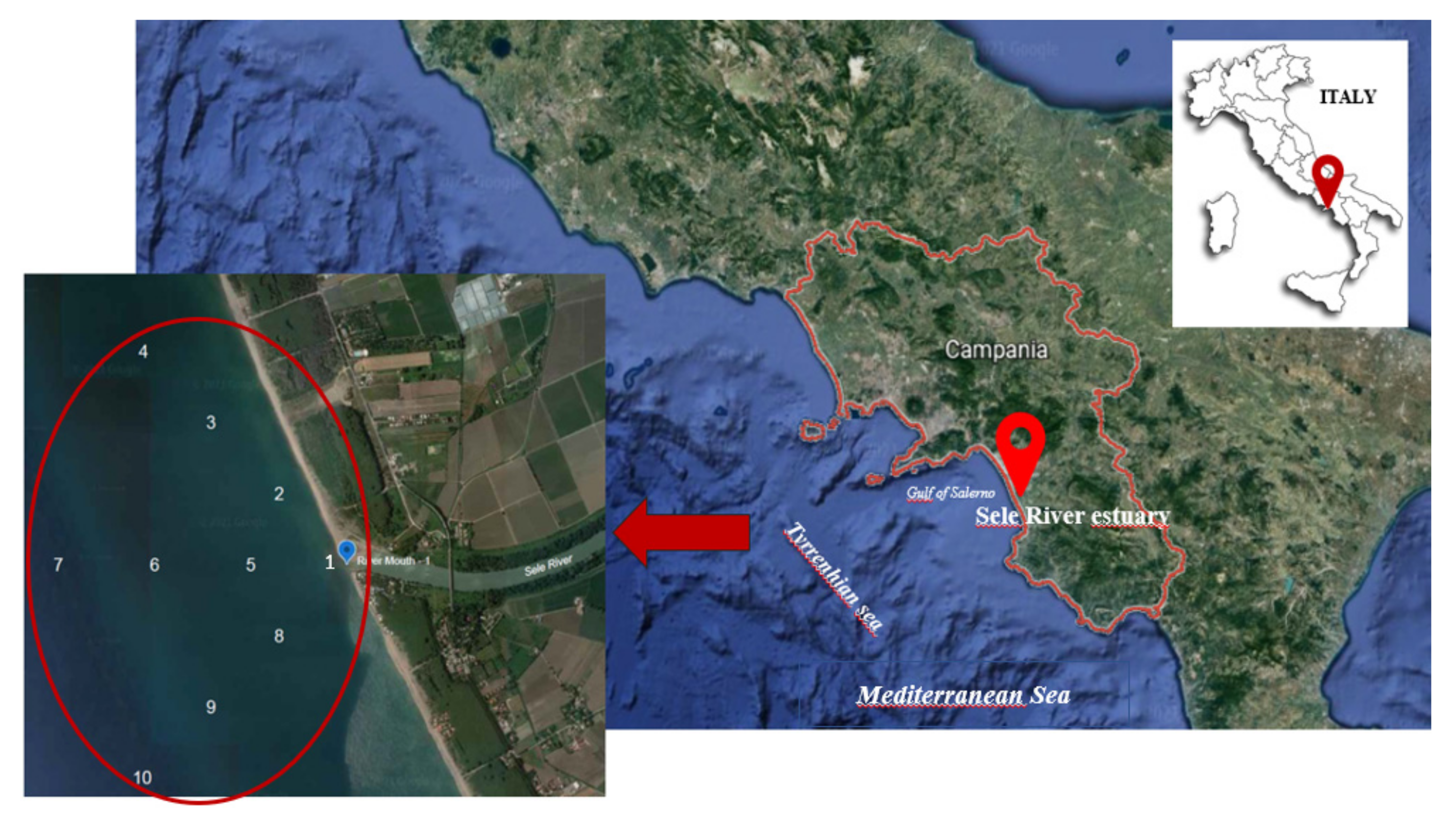
2.1. Study Area
2.2. Sampling
2.3. Extraction Procedure and Clean-Up
2.4. Instrumental Analysis
2.5. Quality Assurance and Quality Control
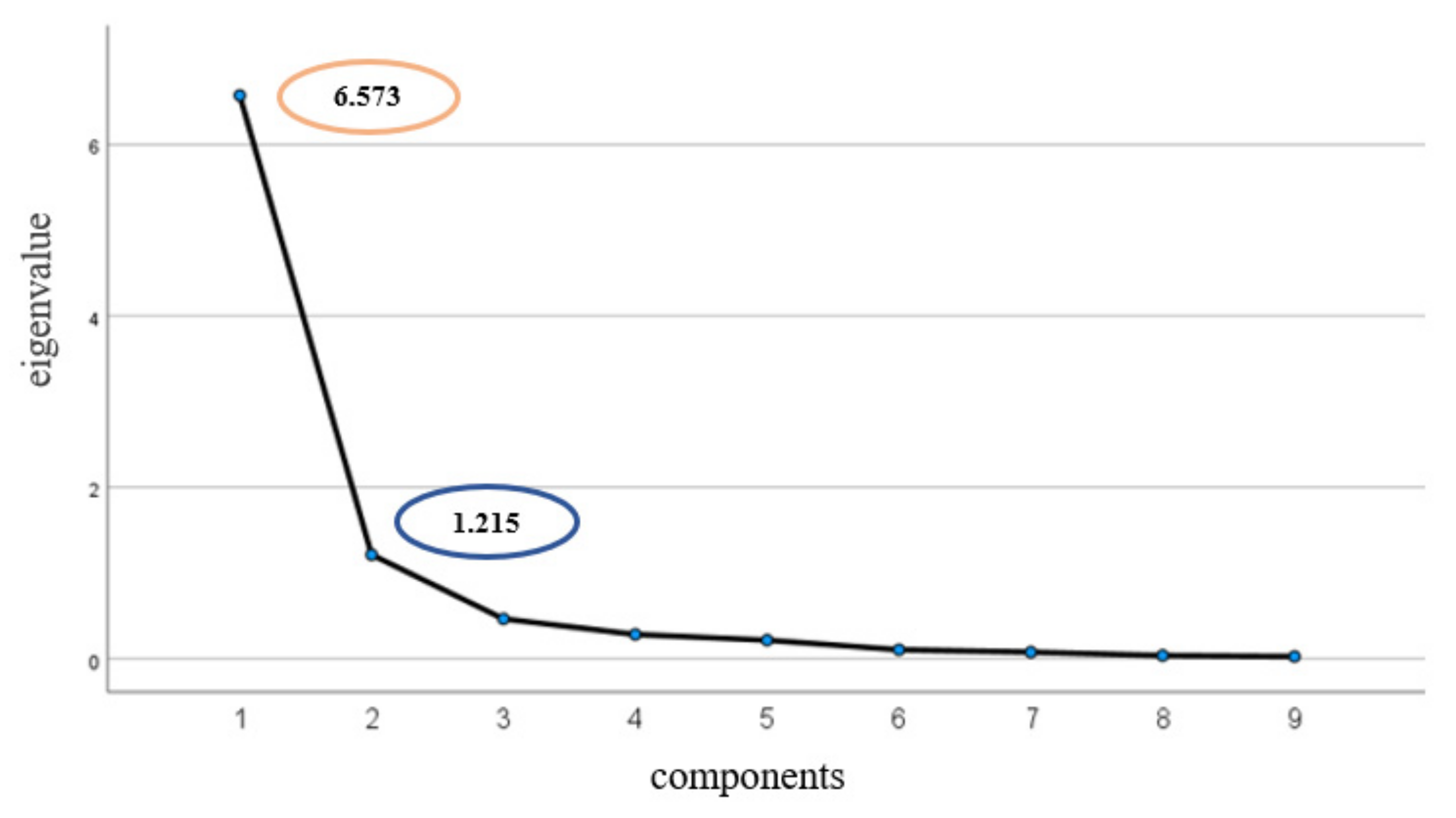
2.6. OPP Input Estimation and Statistical Analysis
2.7. Risk Assessment
3. Results and Discussions
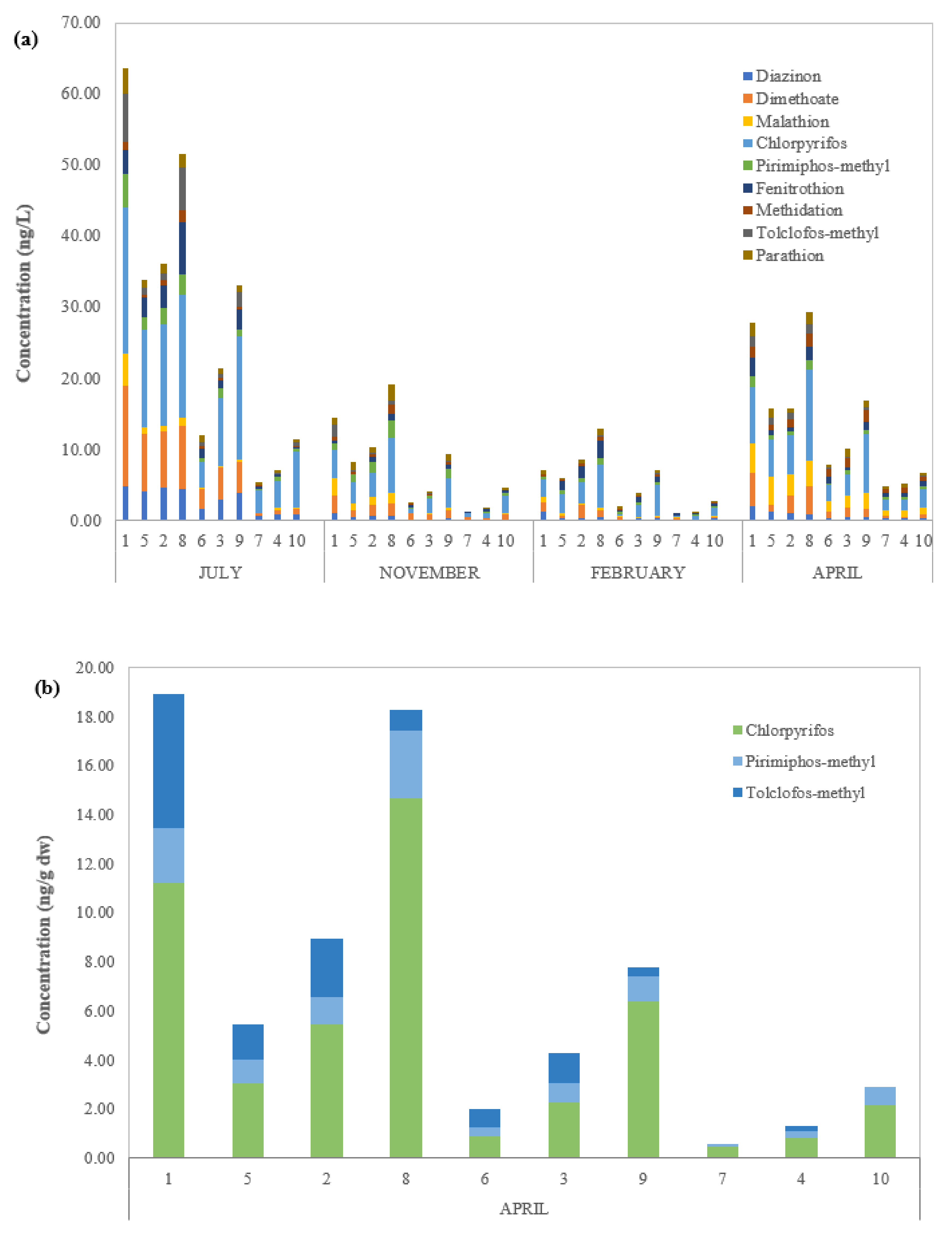
3.1. Occurrence of OPPs in WDP, SPM, and SED
3.2. OPP Distribution between WDP, SPM, and SED Samples
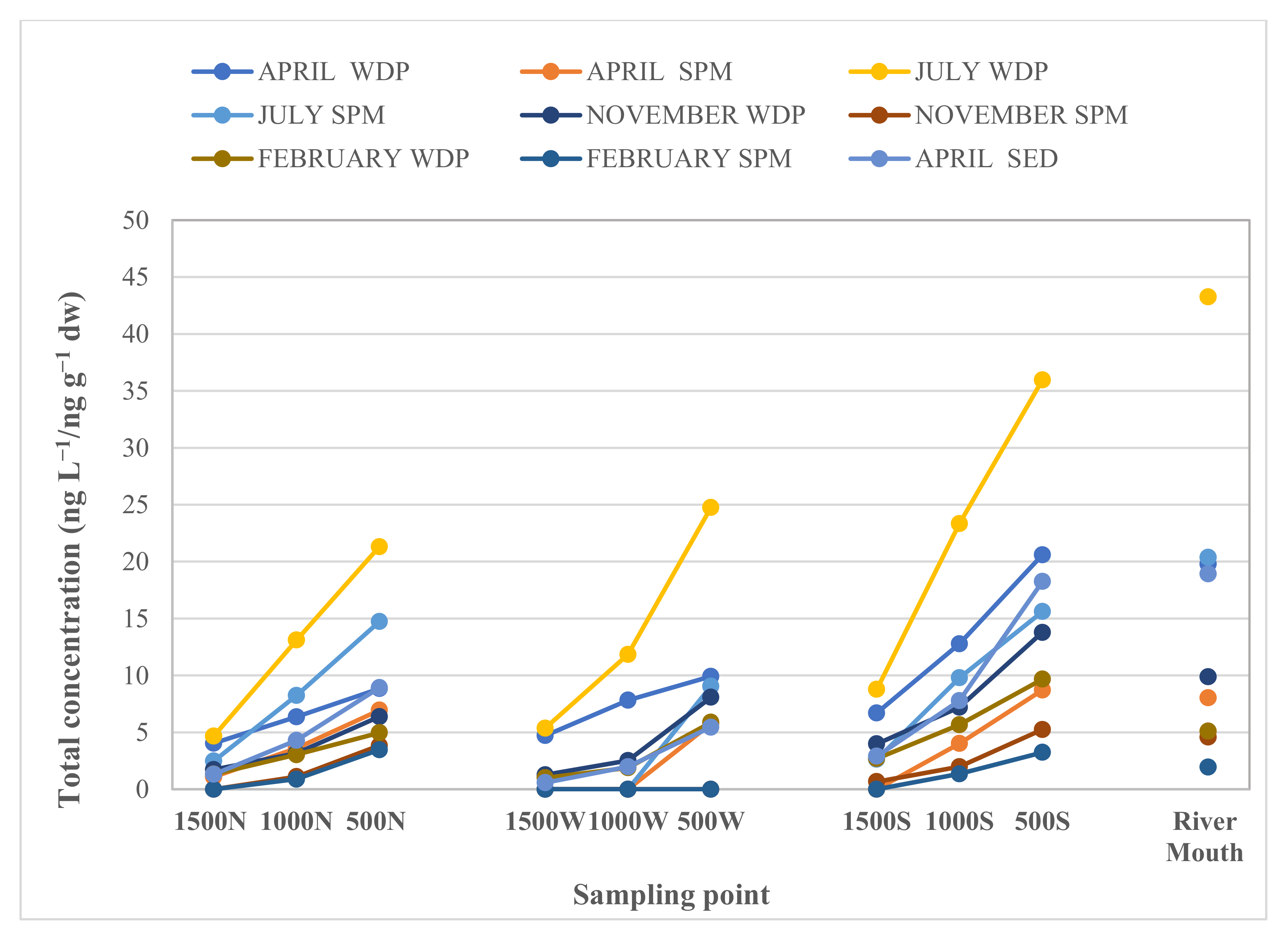
3.3. Loads and Spatial-Temporal Distribution into the Mediterranean Sea
3.4. Risk Assessment of OPPs in the Sele River and Estuary
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, Y.; Zhang, W.; Pang, S.; Chen, J.; Bhatt, P.; Mishra, S.; Chen, S. Insights into the microbial degradation and catalytic mechanisms of chlorpyrifos. Environ. Res. 2021, 194, 110660. [Google Scholar] [CrossRef] [PubMed]
- Mahugija, J.A.; Khamis, F.A.; Lugwisha, E.H. Determination of levels of organochlorine, organophosphorus, and pyrethroid pesticide residues in vegetables from markets in Dar es Salaam by GC-MS. Int. J. Anal. Chem. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yu, K.; Hassan, M.; Xu, C.; Zhang, B.; Gin, K.Y.H.; He, Y. Occurrence, distribution and risk assessment of pesticides in a river-reservoir system. Ecotoxicol. Environ. Saf. 2018, 166, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, A.; Baer-Nawrocka, A. Food and environmental function in world agriculture-Interdependence or competition? Land Use Policy 2018, 71, 578–583. [Google Scholar] [CrossRef]
- Derbalah, A.; Chidya, R.; Jadoon, W.; Sakugawa, H. Temporal trends in organophosphorus pesticides use and concentrations in river water in Japan, and risk assessment. Res. J. Environ. Sci. 2019, 79, 135–152. [Google Scholar] [CrossRef]
- de Souza, R.M.; Seibert, D.; Quesada, H.B.; de Jesus Bassetti, F.; Fagundes-Klen, M.R.; Bergamasco, R. Occurrence, impacts and general aspects of pesticides in surface water: A review. Process Saf. Environ. Prot. 2020, 135, 22–37. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Kaushal, J. An extensive review on the consequences of chemical pesticides on human health and environment. J. Clean. Prod. 2020, 283, 124657. [Google Scholar] [CrossRef]
- Cruzeiro, C.; Pardal, M.Â.; Rodrigues-Oliveira, N.; Castro, L.F.C.; Rocha, E.; Rocha, M.J. Multi-matrix quantification and risk assessment of pesticides in the longest river of the Iberian Peninsula. Sci. Total Environ. 2016, 572, 263–272. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, N.; Xing, Y.; Lian, L.; Chen, Y.; Zhang, D.; Song, Y. Microbial degradation of organophosphorus pesticides: Novel degraders, kinetics, functional genes, and genotoxicity assessment. Environ. Sci. Pollut. Res. 2019, 26, 21668–21681. [Google Scholar] [CrossRef]
- Zhou, Y.; Wu, J.; Wang, B.; Duan, L.; Zhang, Y.; Zhao, W.; Yu, G. Occurrence, source and ecotoxicological risk assessment of pesticides in surface water of Wujin District (northwest of Taihu Lake), China. Environ. Pollut. 2020, 265, 114953. [Google Scholar] [CrossRef]
- Yadav, M.; Shukla, A.K.; Srivastva, N.; Upadhyay, S.N.; Dubey, S.K. Utilization of microbial community potential for removal of chlorpyrifos: A review. Crit. Rev. Biotechnol. 2016, 36, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Kumar, G. An overview of chemical pesticides and its impact on environment and human health. Int. J. Mod. Agric. 2021, 10, 2045–2052. [Google Scholar]
- Puthur, S.; Anoopkumar, A.N.; Rebello, S.; Aneesh, E.M.; Sindhu, R.; Binod, P.; Pandey, A. Toxic Effects of Pesticides on Avifauna Inhabiting Wetlands. Sustain. Agric. Res. 2021, 47, 335–349. [Google Scholar] [CrossRef]
- UNEP/POPS/CONF/4, App. II, 2001. The Stockholm Convention on Persistent Organic Pollutants, Opened for Signature May 23, 2001, UN Doc. The Text of the Convention and Additional Information about POPs is Available Online at the United Nations Environment Programme’s (UNEP’s) POPs. Available online: http://chm.pops.int/TheConvention/Overview/TextoftheConvention/tabid/2232/Default.aspx (accessed on 12 April 2022).
- Sayed, S.S.; Abdel-Motleb, A.; Saleh, H.A.; El-Hamid, R.M.A.; Kader, A.A.; Abdel-Wareth, M.T. Pollution by organochlorine and organophosphorus pesticides residues in watercourses of some Egyptian governorates with reference to the distribution of macroinvertebrates. Int. J. Environ. Sci. 2021, 78, 914–939. [Google Scholar] [CrossRef]
- Zainuddin, A.H.; Wee, S.Y.; Aris, A.Z. Occurrence and potential risk of organophosphorus pesticides in urbanised Linggi River, Negeri Sembilan, Malaysia. Environ. Geochem. Health 2020, 42, 3703–3715. [Google Scholar] [CrossRef]
- Esposito, M.; De Roma, A.; D’Alessio, N.; Danese, A.; Gallo, P.; Galiero, G.; Santoro, M. First study on PCBs, organochlorine pesticides, and trace elements in the Eurasian otter (Lutra lutra) from southern Italy. Sci. Total Environ. 2020, 749, 141452. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Attri, K.; Kumar, M.; Kumar, P.; Suttee, A.; Singla, N. Global trends in pesticides: A looming threat and viable alternatives. Ecotoxicol. Environ. Saf. 2020, 201, 110812. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z. Ecological risk estimation of organophosphorus pesticides in riverine ecosystems. Chemosphere 2017, 188, 575–581. [Google Scholar] [CrossRef]
- Pan, H.; Lei, H.; He, X.; Xi, B.; Xu, Q. Spatial distribution of organochlorine and organophosphorus pesticides in soil-groundwater systems and their associated risks in the middle reaches of the Yangtze River Basin. Environ. Geochem. Health 2019, 41, 1833–1845. [Google Scholar] [CrossRef]
- Muckoya, V.A.; Nomngongo, P.N.; Ngila, J.C. Determination of organophosphorus pesticides in wastewater samples using vortex-assisted dispersive liquid–liquid microextraction with liquid chromatography–mass spectrometry. Int. J. Environ. Sci. Technol. 2020, 17, 2325–2336. [Google Scholar] [CrossRef]
- Picó, Y.; Campo, J.; Alfarhan, A.H.; El-Sheikh, M.A.; Barceló, D. A reconnaissance study of pharmaceuticals, pesticides, perfluoroalkyl substances and organophosphorus flame retardants in the aquatic environment, wild plants and vegetables of two Saudi Arabia urban areas: Environmental and human health risk assessment. Sci. Total Environ. 2021, 776, 145843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qin, P.; Lu, S.; Liu, X.; Zhai, J.; Xu, J.; Wan, Z. Occurrence and risk evaluation of organophosphorus pesticides in typical water bodies of Beijing, China. Environ. Sci. Pollut. Res. 2021, 28, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Gao, Y.L.; Xu, C.; Lian, Y.F. Determination of six organophosphorus pesticides in water samples by three-dimensional graphene aerogel-based solid-phase extraction combined with gas chromatography/mass spectrometry. RSC Adv. 2018, 8, 10277–10283. [Google Scholar] [CrossRef] [Green Version]
- Elfikrie, N.; Ho, Y.B.; Zaidon, S.Z.; Juahir, H.; Tan, E.S.S. Occurrence of pesticides in surface water, pesticides removal efficiency in drinking water treatment plant and potential health risk to consumers in Tengi River Basin, Malaysia. Sci. Total Environ. 2020, 712, 136540. [Google Scholar] [CrossRef]
- Sarlak, Z.; Khosravi-Darani, K.; Rouhi, M.; Garavand, F.; Mohammadi, R.; Sobhiyeh, M.R. Bioremediation of organophosphorus pesticides in contaminated foodstuffs using probiotics. Food Control. 2021, 126, 108006. [Google Scholar] [CrossRef]
- Mondal, R.; Mukherjee, A.; Biswas, S.; Kole, R.K. GC-MS/MS determination and ecological risk assessment of pesticides in aquatic system: A case study in Hooghly River basin in West Bengal, India. Chemosphere 2018, 206, 217–230. [Google Scholar] [CrossRef]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Isakovski, M.K.; Maletić, S.; Tamindžija, D.; Apostolović, T.; Petrović, J.; Tričković, J.; Agbaba, J. Impact of hydrochar and biochar amendments on sorption and biodegradation of organophosphorus pesticides during transport through Danube alluvial sediment. J. Environ. Manag. 2020, 274, 111156. [Google Scholar] [CrossRef]
- Wang, T.; Zhong, M.; Lu, M.; Xu, D.; Xue, Y.; Huang, J.; Yu, G. Occurrence, spatiotemporal distribution, and risk assessment of current-use pesticides in surface water: A case study near Taihu Lake, China. Sci. Total Environ. 2021, 782, 146826. [Google Scholar] [CrossRef]
- Mamta; Rao, R.J.; Wani, K.A. Status of organochlorine and organophosphorus pesticides in wetlands and its impact on aquatic organisms. Environ. Claims J. 2019, 31, 44–78. [Google Scholar] [CrossRef]
- Xiao, K.; Zhu, N.; Lu, Z.; Zheng, H.; Cui, C.; Gao, Y.; Cai, M. Distribution of eight organophosphorus pesticides and their oxides in surface water of the East China Sea based on high volume solid phase extraction method. Environ. Pollut. 2021, 279, 116886. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.I.; Cetinkaya, A.; Bakirhan, N.K.; Ozkan, S.A. Trends in sensitive electrochemical sensors for endocrine disruptive compounds. Trends Environ. Anal. Chem. 2020, 28, e00106. [Google Scholar] [CrossRef]
- Xu, L.; Granger, C.; Dong, H.; Mao, Y.; Duan, S.; Li, J.; Qiang, Z. Occurrences of 29 pesticides in the Huangpu River, China: Highest ecological risk identified in Shanghai metropolitan area. Chemosphere 2020, 251, 126411. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.M.; Lester, Y.; Spangler, E.K.; Von Gunten, U.; Linden, K.G. UV/H2O2 advanced oxidation for abatement of organophosphorous pesticides and the effects on various toxicity screening assays. Chemosphere 2017, 182, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Proskocil, B.J.; Grodzki, A.C.G.; Jacoby, D.B.; Lein, P.J.; Fryer, A.D. Organophosphorus pesticides induce cytokine release from differentiated human THP1 cells. Am. J. Respir. Cell Mol. Biol. 2019, 61, 620–630. [Google Scholar] [CrossRef] [PubMed]
- Karami-Mohajeri, S.; Ahmadipour, A.; Rahimi, H.R.; Abdollahi, M. Adverse effects of organophosphorus pesticides on the liver: A brief summary of four decades of research. Arh Hig Rada Toksikol. 2017, 68, 261–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Union. European Commission, Directorate-General for health and food safety. In Final Review Report for the Active Substance Dimethoate; European Union: Brussels, Belgium, 2019. [Google Scholar]
- European Commission. Commission Implementing Regulation (EU)2020/18 of 10 January 2020 Concerning the Non-Renewal of the Approvalof the Active Substance Chlorpyrifos, in Accordance with Regulation (EC)No 1107/2009 of the European Parliament and of the Council Con-Cerning the Placing of Plant Protection Products on the Market, Andamending the Annex to Commission Implementing Regulation (EU) No540/2011; European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Varghese, E.M.; MS, J. Strategies in microbial degradation enhancement of chlorpyrifos–a review based on the primary approaches in soil bioremediation. Biocatal. Biotransformation 2021, 40, 83–94. [Google Scholar] [CrossRef]
- Masiá, A.; Campo, J.; Navarro-Ortega, A.; Barceló, D.; Picó, Y. Pesticide monitoring in the basin of Llobregat River (Catalonia, Spain) and comparison with historical data. Sci. Total Environ. 2015, 503, 58–68. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, Y.; Yong, L.; Hu, B.; Zhu, L.; Wang, Y.; Sun, C. MWCNTs-solid phase extraction combined with ultra-high performance liquid chromatography-tandem mass spectrometry for the determination of eleven organophosphorus pesticides in river water. Int. J. Environ. Anal. Chem. 2018, 98, 743–757. [Google Scholar] [CrossRef]
- Ávila-Díaz, J.A.; González-Márquez, L.C.; Longoria-Espinoza, R.M.; Ahumada-Cervantes, R.; Leyva-Morales, J.B.; Rodríguez-Gallegos, H.B. Chlorpyrifos and Dimethoate in Water and Sediments of Agricultural Drainage Ditches in Northern Sinaloa, Mexico. Bull. Environ. Contam. Toxicol. 2021, 106, 839–843. [Google Scholar] [CrossRef]
- Farlin, J.; Gallé, T.; Bayerle, M.; Pittois, D.; Braun, C.; El Khabbaz, H.; Weihermueller, L. Using the long-term memory effect of pesticide and metabolite soil residues to estimate field degradation half-life and test leaching predictions. Geoderma 2013, 207, 15–24. [Google Scholar] [CrossRef]
- Ji, C.; Song, Q.; Chen, Y.; Zhou, Z.; Wang, P.; Liu, J.; Zhao, M. The potential endocrine disruption of pesticide transformation products (TPs): The blind spot of pesticide risk assessment. Environ. Int. 2020, 137, 105490. [Google Scholar] [CrossRef]
- Pappone, G.; Alberico, I.; Amato, V.; Aucelli, P.P.C.; Di Paola, G. Recent evolution and the present-day conditions of the Campanian Coastal plains (South Italy): The case history of the Sele River Coastal plain. WIT Trans. Ecol. Environ. 2011, 149, 15–27. [Google Scholar]
- Giordano, L.; Alberico, I.; Ferraro, L.; Marsella, E.; Lirer, F.; Di Fiore, V. A new tool to promote sustainability of coastal zones. The case of Sele plain, southern Italy. Rend. Lincei 2013, 24, 113–126. [Google Scholar] [CrossRef]
- Di Paola, G.; Alberico, I.; Aucelli, P.P.C.; Matano, F.; Rizzo, A.; Vilardo, G. Coastal subsidence detected by Synthetic Aperture Radar interferometry and its effects coupled with future sea--level rise: The case of the Sele Plain (Southern Italy). J. Flood Risk Manag. 2018, 11, 191–206. [Google Scholar] [CrossRef] [Green Version]
- Arienzo, M.; Bolinesi, F.; Aiello, G.; Barra, D.; Donadio, C.; Stanislao, C.; Trifuoggi, M. The Environmental Assessment of an Estuarine Transitional Environment, Southern Italy. J. Mar. Sci. Eng. 2020, 8, 628. [Google Scholar] [CrossRef]
- Database of the National Information System, SIAN. Riepiloghi Dichiarazioni di Vendita dei Prodotti Fitosanitari. Elenco dei Report Regione Campania. 2012. Available online: https://www.sian.it/farmaven/jsp/selezionaRegione.do?prompt=null;campania (accessed on 2 January 2022).
- Rico, A.; Dafouz, R.; Vighi, M.; Rodríguez--Gil, J.L.; Daam, M.A. Use of postregistration monitoring data to evaluate the ecotoxicological risks of pesticides to surface waters: A case study with chlorpyrifos in the Iberian Peninsula. Environ. Toxicol. Chem. 2021, 40, 500–512. [Google Scholar] [CrossRef]
- Paíga, P.; Sousa, S.; Vera, J.; Bitencourt, L.; Vieira, J.; Jorge, S.; Delerue-Matos, C. Multi-residue analysis of fifty pesticides in river waters and in wastewaters. Environ. Sci. Pollut. Res. 2021, 28, 66787–66803. [Google Scholar] [CrossRef] [PubMed]
- Montuori, P.; Aurino, S.; Nardone, A.; Cirillo, T.; Triassi, M. Spatial distribution and partitioning of organophosphates pesticide in water and sediment from Sarno River and Estuary, Southern Italy. Environ. Sci. Pollut. Res. 2015, 22, 8629–8642. [Google Scholar] [CrossRef] [Green Version]
- Montuori, P.; Aurino, S.; Garzonio, F.; Sarnacchiaro, P.; Polichetti, S.; Nardone, A.; Triassi, M. Estimates of Tiber River organophosphate pesticide loads to the Tyrrhenian Sea and ecological risk. Sci. Total Environ. 2016, 559, 218–231. [Google Scholar] [CrossRef] [Green Version]
- Triassi, M.; Nardone, A.; Giovinetti, M.C.; De Rosa, E.; Canzanella, S.; Sarnacchiaro, P.; Montuori, P. Ecological risk and estimates of organophosphate pesticides loads into the Central Mediterranean Sea from Volturno River, the river of the “Land of Fires” area, southern Italy. Sci. Total Environ. 2019, 678, 741–754. [Google Scholar] [CrossRef]
- Kuzmanovic, M.; Dolédec, S.; de Castro-Catala, N.; Ginebreda, A.; Sabater, S.; Muñoz, I.; Barceló, D. Environmental stressors as a driver of the trait composition of benthic macroinvertebrate assemblages in polluted Iberian rivers. Environ. Res. 2017, 156, 485–493. [Google Scholar] [CrossRef]
- Arenas-Sánchez, A.; Rico, A.; Rivas-Tabares, D.; Blanco, A.; Garcia-Doncel, P.; Romero-Salas, A.; Vighi, M. Identification of contaminants of concern in the upper Tagus River basin (central Spain). Part 2: Spatio-temporal analysis and ecological risk assessment. Sci. Total Environ. 2019, 667, 222–233. [Google Scholar] [CrossRef]
- WFD, Water Framework Directive. Directive of European Parliament and of the Council 2000/06/EC—Establishing a Framework for Community Action in the Field of Water Policy. J. Eur. Comm. 2012, 327, 1–73. [Google Scholar]
- Qiu, W.; Shao, H.; Jin, W.; Xiong, Y.; Xu, B.; Chen, B. Determination of OCPs, OPPs, and 21 SVOCs in water and sediment samples in five rivers of Shenzhen, China, during the period of 2017 and 2018. Environ. Sci. Pollut. Res. 2021, 28, 42444–42457. [Google Scholar] [CrossRef] [PubMed]
- Hazardous Substances Data Bank (HSDB). Available online: https://www.nlm.nih.gov/toxnet/index.html (accessed on 5 January 2022).
- ILO International Chemical Safety Cards (ICSC). Available online: https://www.ilo.org/global/copyright/request-for-permission/lang--en/index.htm (accessed on 5 January 2022).
- PPDB (Pesticide Properties DataBase). 2021. Available online: http://sitem.herts.ac.uk/aeru/ppdb/en/index.htm (accessed on 12 January 2022).
- Budillon, F.; Senatore, M.R.; Insinga, D.D.; Iorio, M.; Lubritto, C.; Roca, M.; Rumolo, P. Late Holocene sedimentary changes in shallow water settings: The case of the Sele river offshore in the Salerno Gulf (south-eastern Tyrrhenian Sea, Italy). Rend. Lincei 2012, 23, 25–43. [Google Scholar] [CrossRef]
- Amato, V.; Aucelli, P.P.C.; Ciampo, G.; Cinque, A.; Di Donato, V.; Pappone, G.; Ermolli, E.R. Relative sea level changes and paleogeographical evolution of the southern Sele plain (Italy) during the Holocene. Quat. Int. 2013, 288, 112–128. [Google Scholar] [CrossRef]
- Gómez-Gutiérrez, A.I.; Jover, E.; Bodineau, L.; Albaigés, J.; Bayona, J.M. Organic contaminant loads into the Western Mediterranean Sea: Estimate of Ebro river inputs. Chemosphere 2006, 65, 224–236. [Google Scholar] [CrossRef]
- UNEP; MAP. Guidelines for River (Including Estuary) Pollution Monitoring Programme for the Mediterranean Region MAP; Technical Reports Series No 151; UNEP/MAP: Athens, Greece, 2004. [Google Scholar]
- Montuori, P.; Triassi, M. Polycyclic aromatic hydrocarbons loads into the Mediterranean Sea: Estimate of Sarno River inputs. Mar. Pollut. Bull. 2012, 64, 512–520. [Google Scholar] [CrossRef] [Green Version]
- Palma, P.; Köck-Schulmeyer, M.; Alvarenga, P.; Ledo, L.; Barbosa, I.R.; De Alda, M.L.; Barceló, D. Risk assessment of pesticides detected in surface water of the Alqueva reservoir (Guadiana basin, southern of Portugal). Sci. Total Environ. 2014, 488, 208–219. [Google Scholar] [CrossRef]
- TGD, E. Technical Guidance Document on Risk Assessment in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances, Commission Regulation (EC) No 1488/94 on Risk Assessment for Existing Substances, and Directive 98/8/EC of the European Parliament and of the Council Concerning the Placing of Biocidal Products on the Market. Part I–IV; European Chemicals Bureau (ECB): Helsinki, Finland; JRC-Ispra (VA): Varese, Italy, 2003. [Google Scholar]
- Owojori, O.J.; Ademosu, O.T.; Jegede, O.O.; Fajana, H.O.; Kehinde, T.O.; Badejo, M.A. Tropical oribatid mites in soil toxicity testing: Optimization of test protocol and the effect of two model chemicals (cadmium and dimethoate) on Muliercula inexpectata. Chemosphere 2019, 218, 948e954. [Google Scholar] [CrossRef] [PubMed]
- Onwona-Kwakye, M.; Hogarh, J.N.; Van den Brink, P.J. Environmental risk assessment of pesticides currently applied in Ghana. Chemosphere 2020, 254, 126845. [Google Scholar] [CrossRef] [PubMed]
- Mit, C.; Tebby, C.; Gueganno, T.; Bado-Nilles, A.; Beaudouin, R. Modeling acetylcholine esterase inhibition resulting from exposure to a mixture of atrazine and chlorpyrifos using a physiologically-based kinetic model in fish. Sci. Total Environ. 2021, 773, 144734. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.; Theobald, N.; Lammel, G.; Hühnerfuss, H. Spatial, seasonal and vertical distributions of currently-used pesticides in the marine boundary layer of the North Sea. Atmos. Environ. 2013, 75, 92–102. [Google Scholar] [CrossRef]
- U.S. EPA Distributed Structure-Searchable Toxicity (DSSTox). Available online: https://comptox.epa.gov/dashboard/ (accessed on 8 March 2022).
- Meftaul, I.M.; Venkateswarlu, K.; Dharmarajan, R.; Annamalai, P.; Megharaj, M. Sorption–desorption of dimethoate in urban soils and potential environmental impacts. Environ. Sci. Process. Impacts 2020, 22, 2256–2265. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EC) No 2009/1107 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. Off. J. Eur. Union. 2009, 309, 1–50.
- Charalampous, N.; Kindou, A.; Vlastos, D.; Tsarpali, V.; Antonopoulou, M.; Konstantinou, I.; Dailianis, S. A multidisciplinary assessment of river surface water quality in areas heavily influenced by human activities. Arch. Environ. Contam. Toxicol. 2015, 69, 208–222. [Google Scholar] [CrossRef]
- Aguilar, J.A.P.; Campo, V.A.J.; Masiá, Y.P.A. Pesticide occurrence in the waters of Júcar River, Spain from different farming landscapes. Sci. Total Environ. 2007, 607–608, 752–760. [Google Scholar] [CrossRef]
- Mawussi, G.; Scorza, J.R.P.; Dossa, E.L.; Alaté, K.K. Insecticide residues in soil and water in coastal areas of vegetable production in Togo. Environ. Monit. Assess. 2014, 186, 7379–7385. [Google Scholar] [CrossRef]
- Kanzari, F.; Asia, L.; Syakti, A.D.; Piram, A.; Malleret, L.; Mille, G.; Doumenq, P. Distribution and risk assessment of hydrocarbons (aliphatic and PAHs), polychlorinated biphenyls (PCBs), and pesticides in surface sediments from an agricultural river (Durance) and an industrialized urban lagoon (Berre lagoon), France. Environ. Monit. Assess. 2015, 187, 591. [Google Scholar] [CrossRef]
- Ccanccapa, A.; Masiá, A.; Andreu, V.; Picó, Y. Spatio-temporal patterns of pesticide residues in the Turia and Júcar Rivers (Spain). Sci. Total Environ. 2016, 540, 200–210. [Google Scholar] [CrossRef] [PubMed]
- USEPA. National Recommended Water Quality Criteria. United States Environmental Protection Agency. 2010. Available online: http://water.epa.gov/scitech/swguidance/standards/criteria/current/index.cfm (accessed on 5 January 2022).




| Pesticide Name | Molecular Formula | Molecular Weight | Chemical Structure | LogKow | Solubility in Water at 20 °C (mg L−1) | Uses |
|---|---|---|---|---|---|---|
| Parathion | C10H14NO5PS | 291.26 |  | 3.83 a | 12.4 c | Insecticide Acaricide Avicide |
| Malathion | C10H19O6PS2 | 330.40 |  | 2.36 a | 148 c | Insecticide |
| Chlorpyrifos | C9H11Cl3NO3PS | 350.60 | 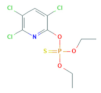 | 4.96 a,b | 1.05 c | Insecticide Acaricide |
| Diazinon | C12H21N2O3PS | 304.35 | 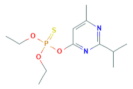 | 3.81 a | 60 c | Acaricide Nematicide |
| Fenitrothion | C9H12NO5PS | 277.24 | 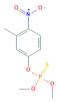 | 3.30 a | 19 c | Insecticide Acaricide |
| Methidathion | C6H11N2O4PS3 | 302.30 |  | 2.20 b | 240 c | Insecticide Acaricide |
| Pirimiphos-methyl | C11H20N3O3PS | 305.34 | 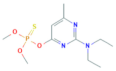 | 4.12 a | 11 c | Insecticide Acaricide |
| Tolclofos-methyl | C9H11Cl2O3PS | 301.10 | 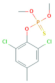 | 4.56 a | 0.71 c | Fungicide |
| Dimethoate | C5H12NO3PS2 | 229.3 | 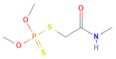 | 0.78 a | 25,900 c | Insecticide Acaricide |
| Sampling Location | Organophosphate Pesticide Concentration Range (ng L−1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site Number Identification | Site Characteristics | Site Location | Diazinon | Dimethoate | Malathion | Clorpyrifos | Pirimiphos-Methyl | Fenitrothion | Methidathion | Tolclofos-Methyl | Parathion | Total |
| 1 (river water) | Sele River mouth | 40°28′55″ N 14°56′33″ E | 0.64–3.17 | 1.01–10.08 | 0.53–3.24 | 1.61–14.08 | 0.38–3.03 | n.d.–2.19 | 0.28–0.83 | 0.14–4.03 | 0.38–2.83 | 5.10–43.24 |
| 2 (sea water) | River mouth 500 m north | 40°29′04″ N 14°56′14″ E | 0.26–2.84 | 0.38–5.04 | 0.21–2.26 | 1.65–8.79 | 0.31–1.00 | 0.52–1.47 | 0.22–0.39 | 0.14–0.45 | 0.29–1.01 | 4.96–21.32 |
| 3 (sea water) | River mouth 1000 m north | 40°29′12″ N 14°55′56″ E | n.d.–1.50 | 0.13–2.41 | 0.14–0.96 | 1.14–6.75 | 0.21–0.51 | 0.20–0.55 | n.d–0.90 | n.d.–0.31 | 0.31–0.71 | 3.04–13.12 |
| 4 (sea water) | River mouth 1500 m north | 40°29′20″ N 14°55′38″ E | n.d–0.42 | n.d–0.37 | n.d–0.69 | 0.41–2.21 | 0.20–0.42 | 0.21–0.56 | n.d.–0.44 | n.d.–0.17 | 0.20–0.42 | 1.32–4.68 |
| 5 (sea water) | River mouth 500 m west | 40°28′55″ N 14°56′12″ E | 0.30–2.97 | 0.38–5.85 | 0.25–2.69 | 2.62–9.59 | 0.41–1.20 | n.d.–1.96 | 0.25–0.53 | n.d.–0.65 | 0.20–1.20 | 5.90–24.76 |
| 6 (sea water) | River mouth 1000 m west | 40°28′55″ N 14°55′50″ E | 0.13–1.57 | 0.23–2.78 | n.d.–1.37 | 0.17–3.41 | n.d.–0.62 | 0.25–1.27 | n.d.–1.03 | n.d.–0.59 | 0.17–0.82 | 1.88–11.85 |
| 7 (sea water) | River mouth 1500 m west | 40°28′55″ N 14°55′28″ E | 0.12–0.53 | 0.10–0.42 | n.d.–0.78 | 0.32–3.21 | n.d.–0.26 | 0.27–0.65 | n.d.–0.45 | n.d.–0.16 | n.d.–0.26 | 1.02–5.37 |
| 8 (sea water) | River mouth 500 m south | 40°28′47″ N 14°56′16″ E | 0.37–3.05 | 0.95–6.02 | 0.52–2.40 | 3.56–11.78 | 0.92–1.95 | 0.73–4.92 | 0.52–1.21 | 0.20–3.89 | 0.92–1.95 | 9.67–35.96 |
| 9 (sea water) | River mouth 1000 m south | 40°28′39″ N 14°55′56″ E | 0.16–2.61 | 0.29–3.24 | 0.22–1.77 | 2.88–11.33 | 0.34–0.99 | 0.57–1.86 | 0.36–1.17 | 0.12–1.42 | 0.43–0.99 | 5.67–23.35 |
| 10 (sea water) | River mouth 1500 m south | 40°28′30″ N 14°55′38″ E | 0.14–0.80 | 0.29–0.77 | 0.11–0.89 | 1.11–5.30 | 0.19–0.41 | 0.27–0.82 | n.d.–0.61 | n.d.–0.58 | 0.22–0.41 | 2.71–8.78 |
| Sampling Location | Organophosphate Pesticides Concentration Ranges (ng L−1) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site Number Identification | Site Characteristics | Site Location | Diazinon | Dimethoate | Malathion | Clorpyrifos | Pirimiphos-Methyl | Fenitrothion | Methidathion | Tolclofos-Methyl | Parathion | Total |
| 1 (river water) | Sele River mouth | 40°28′55″ N 14°56′33″ E | 0.33–1.63 | 0.43–4.11 | 0.26–1.40 | 0.81–6.58 | n.d.—1.54 | n.d.—1.23 | n.d.—0.46 | n.d.—2.64 | 0.14–0.92 | 1.96–20.37 |
| 2 (sea water) | River mouth 500 m north | 40°29′04″ N 14°56′14″ E | n.d.—1.75 | 0.85–2.74 | n.d.—0.67 | 1.27–5.47 | n.d.—1.24 | n.d.—1.76 | n.d.—0.72 | n.d.—0.43 | n.d.—0.46 | 3.48–14.63 |
| 3 (sea water) | River mouth 1000 m north | 40°29′12″ N 14°55′56″ E | n.d.—1.33 | n.d.—2.08 | n.d.—0.83 | 0.60–3.01 | n.d.—0.78 | n.d.—0.60 | n.d.—0.36 | n.d.—0.21 | n.d.—0.74 | 0.89–8.25 |
| 4 (sea water) | River mouth 1500 m north | 40°29′20″ N 14°55′38″ E | n.d.—0.34 | n.d.—0.30 | n.d.—0.23 | n.d.—1.59 | n.d.—0.25 | n.d. | n.d.—0.23 | n.d. | n.d.—0.13 | n.d.—2.48 |
| 5 (sea water) | River mouth 500 m west | 40°28′55″ N 14°56′12″ E | n.d.—1.06 | n.d.—2.26 | n.d.—1.17 | n.d.—4.14 | n.d.—0.49 | n.d.—0.83 | n.d.—0.26 | n.d.—0.30 | n.d.—0.97 | n.d.—9.06 |
| 6 (sea water) | River mouth 1000 m west | 40°28′55″ N 14°55′50″ E | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 7 (sea water) | River mouth 1500 m west | 40°28′55″ N 14°55′28″ E | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| 8 (sea water) | River mouth 500 m south | 40°28′47″ N 14°56′16″ E | n.d.—1.32 | n.d.—2.81 | n.d.—1.08 | 2.44–5.57 | n.d.—0.78 | n.d.—2.38 | n.d.—0.63 | n.d.—2.18 | n.d.—0.88 | 3.24–15.62 |
| 9 (sea water) | River mouth 1000 m south | 40°28′39″ N 14°55′56″ E | n.d.—1.26 | n.d.—0.84 | n.d.—0.50 | 1.20–5.98 | n.d.—0.40 | n.d.—0.93 | n.d.—0.62 | n.d.—0.62 | n.d.- 0.40 | 1.36–9.78 |
| 10 (sea water) | River mouth 1500 m south | 40°28′30″ N 14°55′38″ E | n.d | n.d. | n.d. | n.d.—2.64 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d.—2.64 |
| Sampling Location | Sampling Season | Organophosphate Pesticides Concentration (ng g−1 dw) ± Standard Deviations (SD) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Site Number Identification | Site Characteristics | Site Location | Campaigns | Diazinon | Dimethoate | Malathion | Clorpyrifos | Pirimiphos -Methyl | Fenitrothion | Methidathion | Tolclofos-Methyl | Parathion | Total |
| 1 (river water) | Sele River mouth | 40°28′55″ N 14°56′33″ E | April | n.d. | n.d. | n.d. | 11.21 ± 0.72 | 2.23 ± 0.27 | n.d. | n.d. | 5.49 ± 0.49 | n.d. | 18.93 ± 0.91 |
| 2 (sea water) | River mouth 500 m north | 40°29′04″ N 14°56′14″ E | April | n.d. | n.d. | n.d. | 5.43 ± 0.47 | 1.11 ± 0.14 | n.d. | n.d. | 2.38 ± 0.21 | n.d. | 8.93 ± 0.46 |
| 3 (sea water) | River mouth 1000 m north | 40°29′12″ N 14°55′56″ E | April | n.d. | n.d. | n.d. | 2.25 ± 0.33 | 0.83 ± 0.19 | n.d. | n.d. | 1.22 ± 0.21 | n.d. | 4.30 ± 0.30 |
| 4 (sea water) | River mouth 1500 m north | 40°29′20″ N 14°55′38″ E | April | n.d. | n.d. | n.d. | 0.85 ± 0.18 | 0.26 ± 0.08 | n.d. | n.d. | 0.21 ± 0.08 | n.d. | 1.32 ± 0.07 |
| 5 (sea water) | River mouth 500 m west | 40°28′55″ N 14°56′12″ E | April | n.d. | n.d. | n.d. | 3.04 ± 0.20 | 0.97 ± 0.12 | n.d. | n.d. | 1.45 ± 0.21 | n.d. | 5.46 ± 0.53 |
| 6 (sea water) | River mouth 1000 m west | 40°28′55″ N 14°55′50″ E | April | n.d. | n.d. | n.d. | 0.90 ± 0.21 | 0.35 ± 0.11 | n.d. | n.d. | 0.74 ± 0.14 | n.d. | 1.99 ± 0.20 |
| 7 (sea water) | River mouth 1500 m west | 40°28′55″ N 14°55′28″ E | April | n.d. | n.d. | n.d. | 0.45 ± 0.14 | 0.13 ± 0.07 | n.d. | n.d. | nd | n.d. | 0.58 ± 0.21 |
| 8 (sea water) | River mouth 500 m south | 40°28′47″ N 14°56′16″ E | April | n.d. | n.d. | n.d. | 14.70 ± 3.18 | 2.72 ± 0.41 | n.d. | n.d. | 0.83 ± 0.19 | n.d. | 18.26 ± 3.76 |
| 9 (sea water) | River mouth 1000 m south | 40°28′39″ N 14°55′56″ E | April | n.d. | n.d. | n.d. | 6.38 ± 0.41 | 1.01 ± 0.19 | n.d. | n.d. | 0.39 ± 0.13 | n.d. | 7.79 ± 0.09 |
| 10 (sea water) | River mouth 1500 m south | 40°28′30″ N 14°55′38″ E | April | n.d. | n.d. | n.d. | 2.16 ± 0.18 | 0.74 ± 0.18 | n.d. | n.d. | n.d. | n.d. | 2.91 ± 0.36 |
| Pesticide Name | Koc a | Soil Degradation DT50 (Days) b | Water-Sediment DT₅₀ (Days) b | Water Phase Only DT₅₀ (Days) b | |||
|---|---|---|---|---|---|---|---|
| Parathion | 1580 | 49 | moderately persistent | 4.3 | fast | 3.5 | fast |
| Malathion | 229 | 0.17 | non-persistent | 0.4 | fast | 0.4 | fast |
| Chlorpyrifos | 5010 | 386 | very persistent | 36.5 | moderately fast | 5 | moderately fast |
| Diazinon | 562 | 9.1 | non-persistent | 10.4 | moderately fast | 4.3 | fast |
| Fenitrothion | 427 | 2.7 | non-persistent | 1.57 | fast | 1.1 | fast |
| Methidathion | 33.9 | 10 | non-persistent | 70 | moderately fast | 6 | moderately fast |
| Pirimiphos-methyl | 1000 | 39 | moderately persistent | 4.73 a | fast | 4.73 a | fast |
| Tolclofos-methyl | 761–1540 c | 7.6 | non-persistent | 15 | moderately fast | 1.25 | fast |
| Dimethoate | 15.8 | 2.5 | non-persistent | 15.5 | moderately fast | 12.6 | moderately fast |
| Compound | Ecotoxicology Endpoints Trophic Levels (µg/L) | Critical Concentration (µg/L) | AF | PNEC | RQm | RQex | ||
|---|---|---|---|---|---|---|---|---|
| Algae | Aquatic Invertebrates | Fish | ||||||
| Diazinon | >10,000 | 0.56 | 700 | 0.56 | 10 | 0.0560 | 0.0132 | 0.0567 |
| Dimethoate | 32,000 | 40 | 400 | 40 | 10 | 4.0000 | 0.0004 | 0.0025 |
| Malathion | 13,000 (EC50) | 0.06 | 91 | 0.06 | 10 | 0.0060 | 0.1332 | 0.5407 |
| Chlorpyrifos | 43 | 4.6 | 0.14 | 0.14 | 10 | 0.0140 | 0.2668 | 1.0056 |
| Pirimiphos-methyl | 1000 (EC50) | 0.08 | 23 | 0.08 | 50 | 0.0016 | 0.4020 | 1.8952 |
| Fenitrothion | 100 | 0.087 | 88 | 0.087 | 10 | 0.0087 | 0.0995 | 0.5658 |
| Methidathion | >200 | 0.64 | 10 (LC50) | 0.64 | 50 | 0.0128 | 0.0340 | 0.0948 |
| Tolclofos-methyl | 32 | 26 | 12 | 12 | 10 | 1.2000 | 0.0004 | 0.0034 |
| Parathion | 10,000 (EC50) | 0.1 | >98 | 0.1 | 50 | 0.0020 | 0.3095 | 1.4161 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montuori, P.; De Rosa, E.; Di Duca, F.; De Simone, B.; Scippa, S.; Russo, I.; Sorrentino, M.; Sarnacchiaro, P.; Triassi, M. Occurrence, Distribution, and Risk Assessment of Organophosphorus Pesticides in the Aquatic Environment of the Sele River Estuary, Southern Italy. Toxics 2022, 10, 377. https://doi.org/10.3390/toxics10070377
Montuori P, De Rosa E, Di Duca F, De Simone B, Scippa S, Russo I, Sorrentino M, Sarnacchiaro P, Triassi M. Occurrence, Distribution, and Risk Assessment of Organophosphorus Pesticides in the Aquatic Environment of the Sele River Estuary, Southern Italy. Toxics. 2022; 10(7):377. https://doi.org/10.3390/toxics10070377
Chicago/Turabian StyleMontuori, Paolo, Elvira De Rosa, Fabiana Di Duca, Bruna De Simone, Stefano Scippa, Immacolata Russo, Michele Sorrentino, Pasquale Sarnacchiaro, and Maria Triassi. 2022. "Occurrence, Distribution, and Risk Assessment of Organophosphorus Pesticides in the Aquatic Environment of the Sele River Estuary, Southern Italy" Toxics 10, no. 7: 377. https://doi.org/10.3390/toxics10070377
APA StyleMontuori, P., De Rosa, E., Di Duca, F., De Simone, B., Scippa, S., Russo, I., Sorrentino, M., Sarnacchiaro, P., & Triassi, M. (2022). Occurrence, Distribution, and Risk Assessment of Organophosphorus Pesticides in the Aquatic Environment of the Sele River Estuary, Southern Italy. Toxics, 10(7), 377. https://doi.org/10.3390/toxics10070377








