Biokinetics and Internal Dosimetry of Tritiated Steel Particles
Abstract
1. Introduction
2. Materials and Methods
2.1. Tritiated Steel Particles
2.2. In Vitro Tritium Release Study
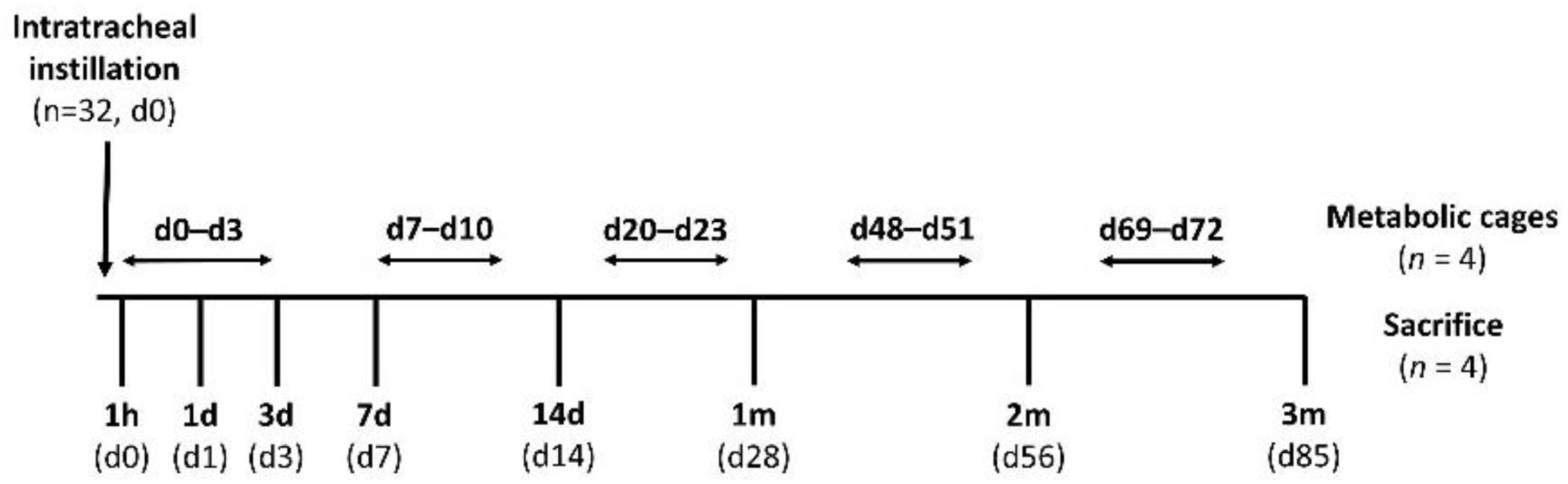
2.3. In Vivo Instillation Study
2.4. Biological Sample Analysis
2.5. Rat Biokinetic Model
2.6. Estimated Dose Coefficients for Workers Exposed to Tritiated Steel Particles
3. Results
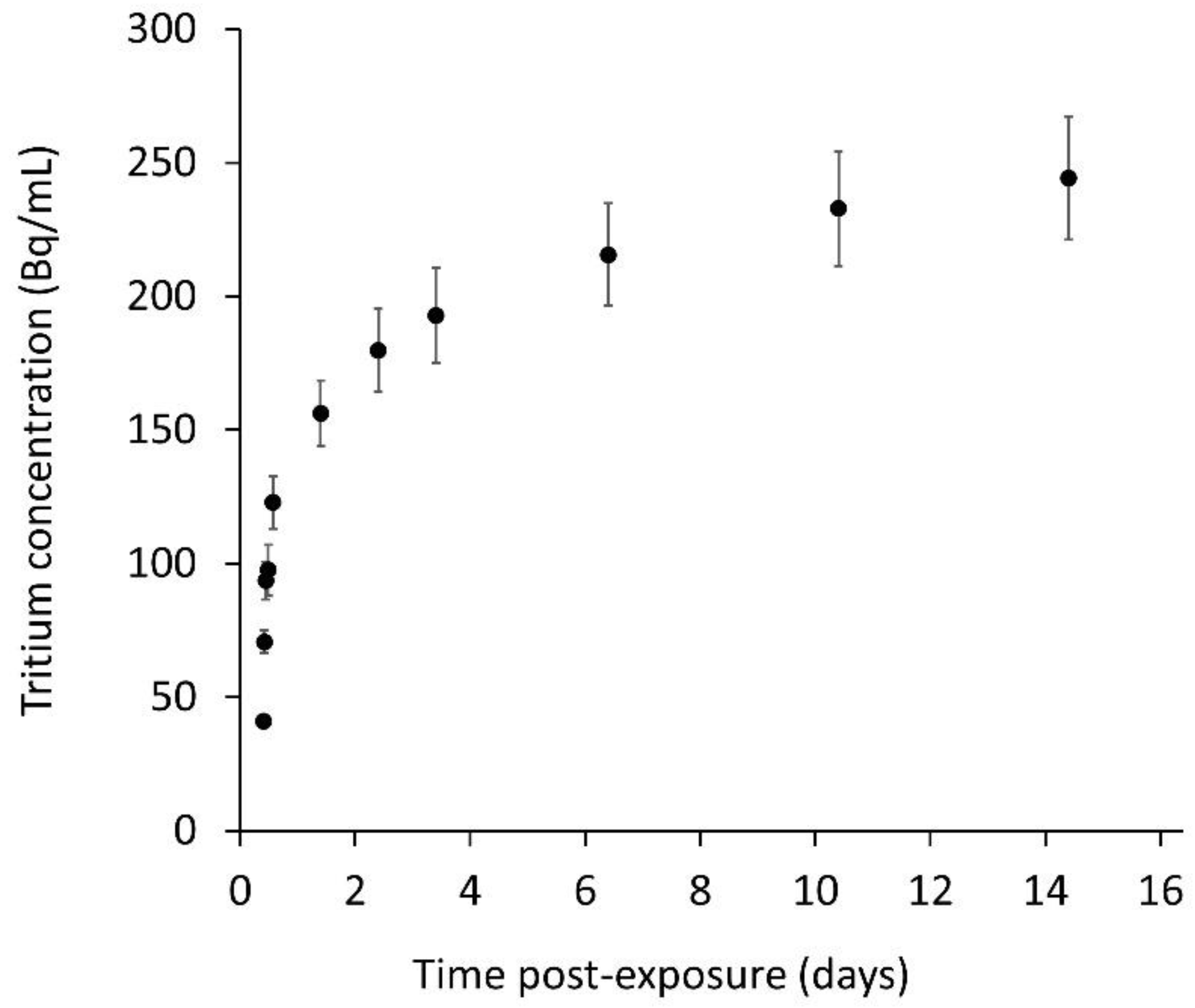
3.1. In Vitro Tritium Release Study
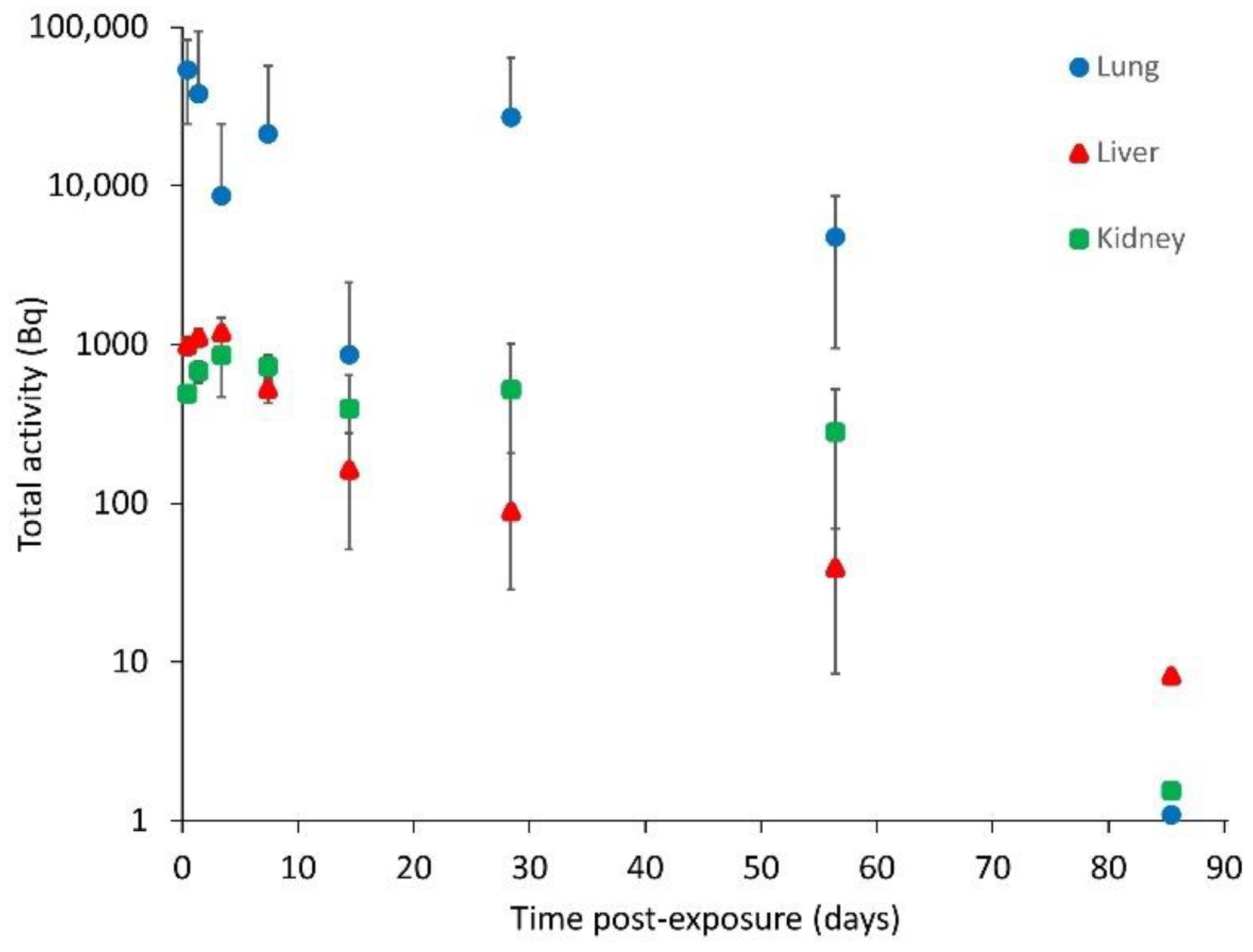
3.2. In Vivo Instillation Study
3.3. Biokinetic Model of Rat
3.4. Dose Coefficients for Workers Exposed to Tritiated Steel Particles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IAEA. Determination and Use of Scaling Factors for Waste Characterization in Nuclear Power Plants; International Atomic Energy Agency: Vienna, Austria, 2009. [Google Scholar]
- ASN. Tritium White Paper; 2010. Available online: www.asn.fr/sites/tritium (accessed on 1 September 2022). (In French).
- Gonzalez de Vicente, S.M.; Smith, N.A.; El-Guebaly, L.; Ciattaglia, S.; Di Pace, L.; Gilbert, M.; Mandoki, R.; Rosanvallon, S.; Someya, Y.; Tobita, K.; et al. Overview on the management of radioactive waste from fusion facilities: ITER, demonstration machines and power plants. Nucl. Fusion 2022, 62, 085001. [Google Scholar] [CrossRef]
- Liger, K.; Grisolia, C.; Cristescu, I.; Moreno, C.; Malard, V.; Coombs, D.; Markelj, S. Overview of the TRANSAT (TRANSversal Actions for Tritium) project. Fusion Eng. Des. 2018, 136, 168–172. [Google Scholar] [CrossRef]
- Louthan, M.R.; Derrick, R.G. Hydrogen transport in austenitic stainless steel. Corros. Sci. 1975, 15, 565–577. [Google Scholar] [CrossRef]
- Marchi, C.S.; Somerday, B.P.; Robinson, S.L. Permeability, solubility and diffusivity of hydrogen isotopes in stainless steels at high gas pressures. Int. J. Hydrog. Energy 2007, 32, 100–116. [Google Scholar] [CrossRef]
- Dickson, R.S. Tritium Interactions with Steel and Construction Materials in Fusion Devices: A Literature Review; Atomic Energy of Canada Ltd., Chalk River Laboratories: Chalk River, ON, Canada, 1990. [Google Scholar]
- Bernard, J.; Pilot, G.; Grandjean, J. Evaluation of Various Cutting Techniques Suitable for the Dismantling of Nuclear Components; EUR 17919; 1998 Office for Official Publications of the European Communities: Luxembourg; ISSN 1018-5593.
- ICRP. ICRP Publication 134: Occupational Intakes of Radionuclides: Part 2. Ann. ICRP 2016, 45, 1–352. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Snipes, M.B.; Wang, Y.; Jow, H.-N. Biokinetics and Dosimetry of Titanium Tritide Particles in the Lung. Health Phys. 1999, 76, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cheng, Y.S. Dose Assessment for Inhaling Hafnium Particles Based on Laboratory Rats Study. Health Phys. 2003, 84, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cheng, Y.-S.; Wang, Y. Dissolution Rate and Biokinetic Model of Zirconium Tritide Particles in Rat Lungs. Health Phys. 2010, 98, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Gensdarmes, F.; Payet, M.; Malard, V.; Grisolia, C. Report on Production of Steel Particles. 2019. Available online: https://transat-h2020.eu/wp-content/uploads/2020/04/TRANSAT-D3.1-Report-on-Production-of-Steel-Particles.pdfas (accessed on 1 September 2022).
- Payet, M. Report on Tritiation of Cement and Steel Particles. 2020. Available online: https://transat-h2020.eu/wp-content/uploads/2020/04/TRANSAT-D3.3-Report-on-tritiation-of-cement-and-steel-particles.pdf (accessed on 1 September 2022).
- Innes, E.; Yiu, H.H.P.; McLean, P.; Brown, W.; Boyles, M. Simulated biological fluids—A systematic review of their biological relevance and use in relation to inhalation toxicology of particles and fibres. Crit. Rev. Toxicol. 2021, 51, 217–248. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qi, Y.; Yin, C.; Wang, S.; Zhang, S.; Xu, A.; Chen, W.; Liu, S. Bio-transformation of Graphene Oxide in Lung Fluids Significantly Enhances Its Photothermal Efficacy. Nanotheranostics 2018, 2, 222–232. [Google Scholar] [CrossRef][Green Version]
- Hodgson, A.; Rance, E.R.; Pellow, P.G.D.; Stradling, G.N. In Vitro Dissolution of Tritium Loaded Carbon Particles from the JET Tokamak; NRPB-DA/1/2002; NRPB: Oxfordshire, UK, 2002. [Google Scholar]
- Hodgson, S.A.; Scott, J.E.; Hodgson, A. In Vitro Dissolution of Tritium Loaded Particles from the JET Fusion Machine; RPD-DAR-02-2006; HPA: Oxfordshire, UK, 2006. [Google Scholar]
- Stradling, G.N.; Gray, S.A.; Moody, J.C.; Ellender, M. Efficacy of tiron for enhancing the excretion of uranium from the rat. Hum. Exp. Toxicol. 1991, 10, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.H.R.; Bell, B.M.; Cobelli, C.; Golde, H.; Schumitzky, A.; Vicini, P.; Foster, D.M. SAAM II: Simulation, analysis, and modeling software for tracer and pharmacokinetic studies. Metabolism 1998, 47, 484–492. [Google Scholar] [CrossRef]
- ICRP. ICRP Publication 130: Occupational Intakes of Radionuclides: Part 1. Ann. ICRP 2015, 44, 5–188. [Google Scholar] [CrossRef]
- ICRP. Human alimentary tract model for radiological protection. ICRP Publication 100. A report of The International Commission on Radiological Protection. Ann. ICRP 2006, 36, 25–327. [Google Scholar] [CrossRef]
- Blanchardon, E.; Davesne, E.; Bohand, S.; Laroche, P. ICARE software for calculation of dose coefficients and retained/excreted fractions of intake. BIO Web Conf. 2019, 14, 03016. [Google Scholar] [CrossRef]
- ICRP. ICRP Publication 119: Compendium of Dose Coefficients based on ICRP Publication 60. Ann. ICRP 2012, 41, 1–130. [Google Scholar] [CrossRef] [PubMed]
- Piao, Y.; Liu, Y.; Xie, X. Change trends of organ weight background data in sprague dawley rats at different ages. J. Toxicol. Pathol. 2013, 26, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Granton, P.V.; Norley, C.J.; Umoh, J.; Turley, E.A.; Frier, B.C.; Noble, E.G.; Holdsworth, D.W. Rapid in vivo whole body composition of rats using cone beam μCT. J. Appl. Physiol. 2010, 109, 1162–1169. [Google Scholar] [CrossRef]
- ICRU. Tissue Substitutes in Radiation Dosimetry and Measurements; International Commission on Radiation Units and Measurements: Bethesda, MA, USA, 1989. [Google Scholar]
- Schmidt, F.; Yoshimura, Y.; Ni, R.X.; Kneesel, S.; Constantinou, C.E. Influence of gender on the diurnal variation of urine production and micturition characteristics of the rat. Neurourol. Urodyn. 2001, 20, 287–295. [Google Scholar] [CrossRef] [PubMed]
- ICRP. Guide for the practical application of the ICRP Human Respiratory Tract Model. ICRP supporting guidance 3. Ann. ICRP 2002, 32, 13–306. [Google Scholar] [CrossRef]
- ICRP. Basic anatomical and physiological data for use in radiological protection: Reference values. A report of age- and gender-related differences in the anatomical and physiological characteristics of reference individuals. ICRP Publication 89. Ann. ICRP 2002, 32, 5–265. [Google Scholar]
- Cheng, Y.-S.; Zhou, Y.; Wang, Y.-S.; Inkret, W.C.; Wermer, J.R. Dose Estimate of Inhaled Hafnium Tritide using the ICRP 66 Lung Model. Health Phys. 2002, 82, 817–824. [Google Scholar] [CrossRef]
- Inkret, W.C.T.; Schillaci, M.E.; Boyce, M.K.; Cheng, Y.S.; Efurd, D.W.; Little, T.T.; Miller, G.; Musgrave, J.A.; Wermer, J.R. Internal Dosimetry for Inhalation of Hafnium Tritide Aerosols. Radiat. Prot. Dosim. 2001, 93, 55–60. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Dahl, A.R.; Jow, H.N. Dissolution of Metal Tritides in a Simulated Lung Fluid. Health Phys. 1997, 73, 633–638. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, Y.S. Dosimetry of Metal Tritide Particles as Evaluated by the ICRP 66 Model and a Biokinetic Model from Laboratory Rats. Health Phys. 2004, 86, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Cool, D.A.; Maillie, H.D. Dissolution of tritiated glass microballoon fragments: Implications for inhalation exposure. Health Phys. 1983, 45, 791–794. [Google Scholar] [PubMed]
- Hodgson, S.A.; Scott, J.E.; Hodgson, A. In vitro dissolution of tritium-loaded particles from the JET fusion machine. Radiat. Prot. Dosim. 2007, 127, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, C.S.; Choi, S.I.; Kim, R.-K.; Kim, J.Y.; Nam, S.Y.; Jin, Y.W.; Kim, I.G. Tissue distribution, excretion and effects on genotoxicity of tritium following oral administration to rats. Nucl. Eng. Technol. 2019, 51, 303–309. [Google Scholar] [CrossRef]
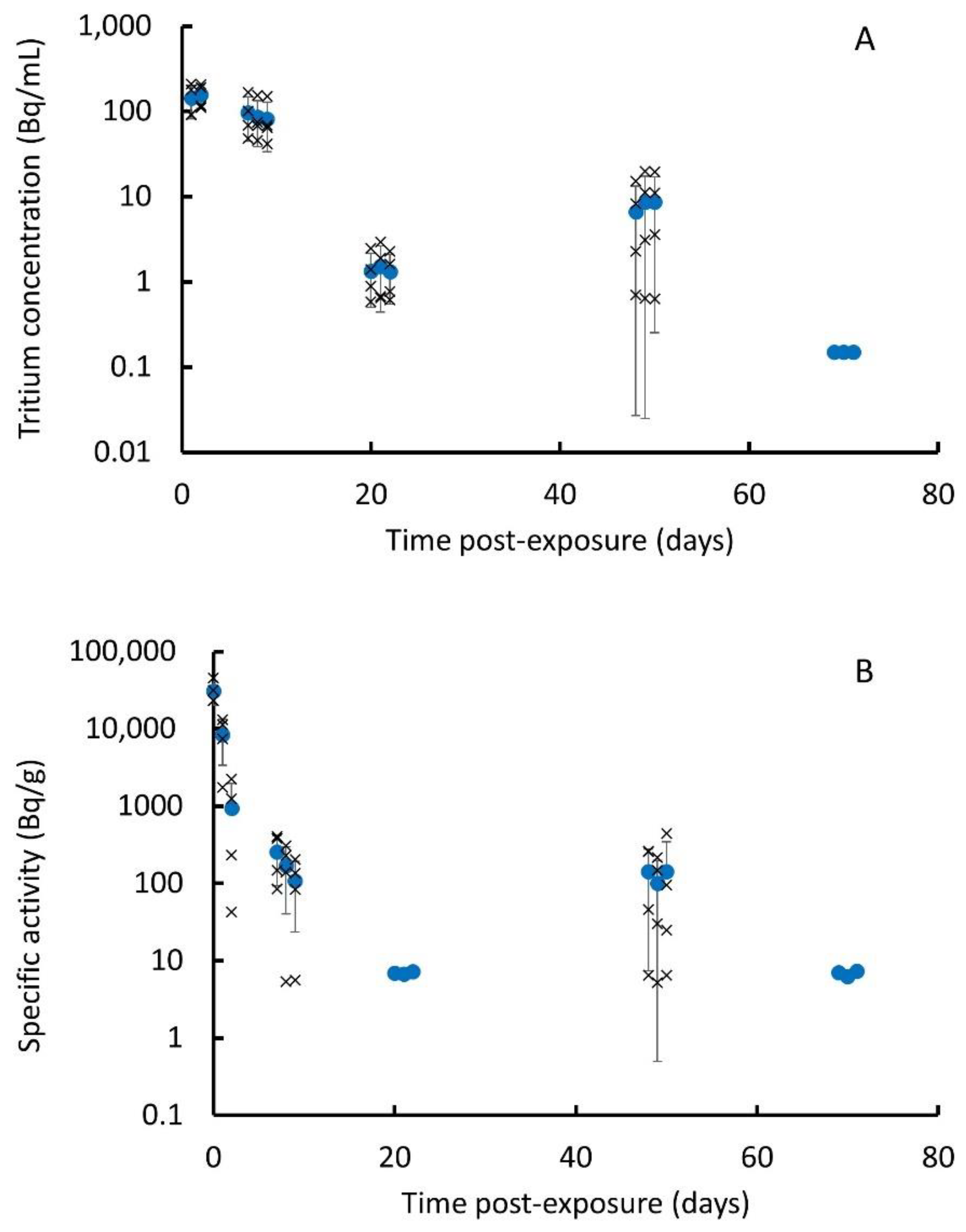


| Post-Exposure Time (d) | Specific Activity (Bq/g) * | |||
|---|---|---|---|---|
| Muscle | Liver | Kidney | Lung | |
| 0.04 | 78 ± 7 | 128 ± 16 | 214 ± 14 | 34,880 ± 18,990 |
| 1 | 96 ± 9 | 144 ± 18 | 296 ± 46 | 24,660 ± 36,360 |
| 3 | 137 ± 20 | 153 ± 37 | 373 ± 170 | 5613 ± 10,322 |
| 7 | 68 ± 11 | 68 ± 12 | 316 ± 59 | 13,710 ± 22,810 |
| 14 | 20 ± 7 | 21 ± 14 | 170 ± 107 | 555 ± 1028 |
| 28 | 14 ± 10 | 11 ± 15 | 219 ± 207 | 17,290 ± 23,350 |
| 56 | 22 ± 14 | 4.9 ± 3.9 | 115 ± 100 | 3002 ± 2403 |
| 85 | <LOD # | <LOD | <LOD | <LOD |
| Post-Exposure Time (d) | Total Activity (Bq) * | |||
|---|---|---|---|---|
| Lung | Liver | Kidney | Total Soft Tissue $ | |
| 0.04 | 53,890 ± 29,330 | 988 ± 124 | 491 ± 32 | 20,860 ± 1948 |
| 1 | 38,100 ± 56,180 | 1114 ± 138 | 680 ± 106 | 25,480 ± 2472 |
| 3 | 8701 ± 16,000 | 1188 ± 288 | 862 ± 392 | 36,700 ± 5412 |
| 7 | 21,250 ± 35,350 | 524 ± 95 | 729 ± 136 | 18,250 ± 2834 |
| 14 | 863 ± 1599 | 163 ± 112 | 395 ± 248 | 5524 ± 1840 |
| 28 | 27,150 ± 36,660 | 89 ± 117 | 520 ± 491 | 3857 ± 2674 |
| 56 | 4773 ± 3821 | 39 ± 31 | 281 ± 245 | 6087 ± 3987 |
| 85 | <LOD # | <LOD | <LOD | <LOD |
| From | To | Transfer Coefficient (d−1) |
|---|---|---|
| Blood | Liver HTO | 0.45 |
| Blood | Kidney HTO | 0.18 |
| Blood | RoB * HTO | 17 |
| Blood | Urine | 0.56 |
| Blood | Faeces | 0.04 |
| Blood | Other water loss | 0.41 |
| Liver HTO | Blood | 2.7 |
| Kidney HTO | Blood | 0.10 |
| RoB HTO | Blood | 4.0 |
| Liver HTO | Liver OBT | 0.025 |
| Kidney HTO | Kidney OBT | 0.01 |
| RoB HTO | RoB OBT | 0.03 |
| Liver OBT | Liver HTO | 0.10 |
| Kidney OBT | Kidney HTO | 0.20 |
| RoB OBT | RoB HTO | 0.05 |
| Kidney HTO | Urine | 1.0 |
| GIT # | Faeces | 2.0 |
| GIT | Blood | 0.10 |
| Parameter | Value |
|---|---|
| Rapidly released fraction (fr) | 0.30 |
| Rapid release rate (sr) | 100 d−1 |
| Slow release rate (ss) | 0.064 d−1 |
| Absorbed fraction of activity entering GIT (fA) | 0.052 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, R.; Ellender, M.; Guo, C.; Hammond, D.; Laycock, A.; Leonard, M.O.; Wright, M.; Davidson, M.; Malard, V.; Payet, M.; et al. Biokinetics and Internal Dosimetry of Tritiated Steel Particles. Toxics 2022, 10, 602. https://doi.org/10.3390/toxics10100602
Smith R, Ellender M, Guo C, Hammond D, Laycock A, Leonard MO, Wright M, Davidson M, Malard V, Payet M, et al. Biokinetics and Internal Dosimetry of Tritiated Steel Particles. Toxics. 2022; 10(10):602. https://doi.org/10.3390/toxics10100602
Chicago/Turabian StyleSmith, Rachel, Michele Ellender, Chang Guo, Derek Hammond, Adam Laycock, Martin O. Leonard, Matthew Wright, Michael Davidson, Véronique Malard, Mickaël Payet, and et al. 2022. "Biokinetics and Internal Dosimetry of Tritiated Steel Particles" Toxics 10, no. 10: 602. https://doi.org/10.3390/toxics10100602
APA StyleSmith, R., Ellender, M., Guo, C., Hammond, D., Laycock, A., Leonard, M. O., Wright, M., Davidson, M., Malard, V., Payet, M., Grisolia, C., & Blanchardon, E. (2022). Biokinetics and Internal Dosimetry of Tritiated Steel Particles. Toxics, 10(10), 602. https://doi.org/10.3390/toxics10100602





