Trace Metals in Cannabis Seized by Law Enforcement in Ghana and Multivariate Analysis to Distinguish among Different Cannabis Farms
Abstract
:1. Introduction
2. Materials and Methods
- C—Concentration of final solution, B—Concentration of blank solution
- V—Final volume of solution, W—Weight of sample
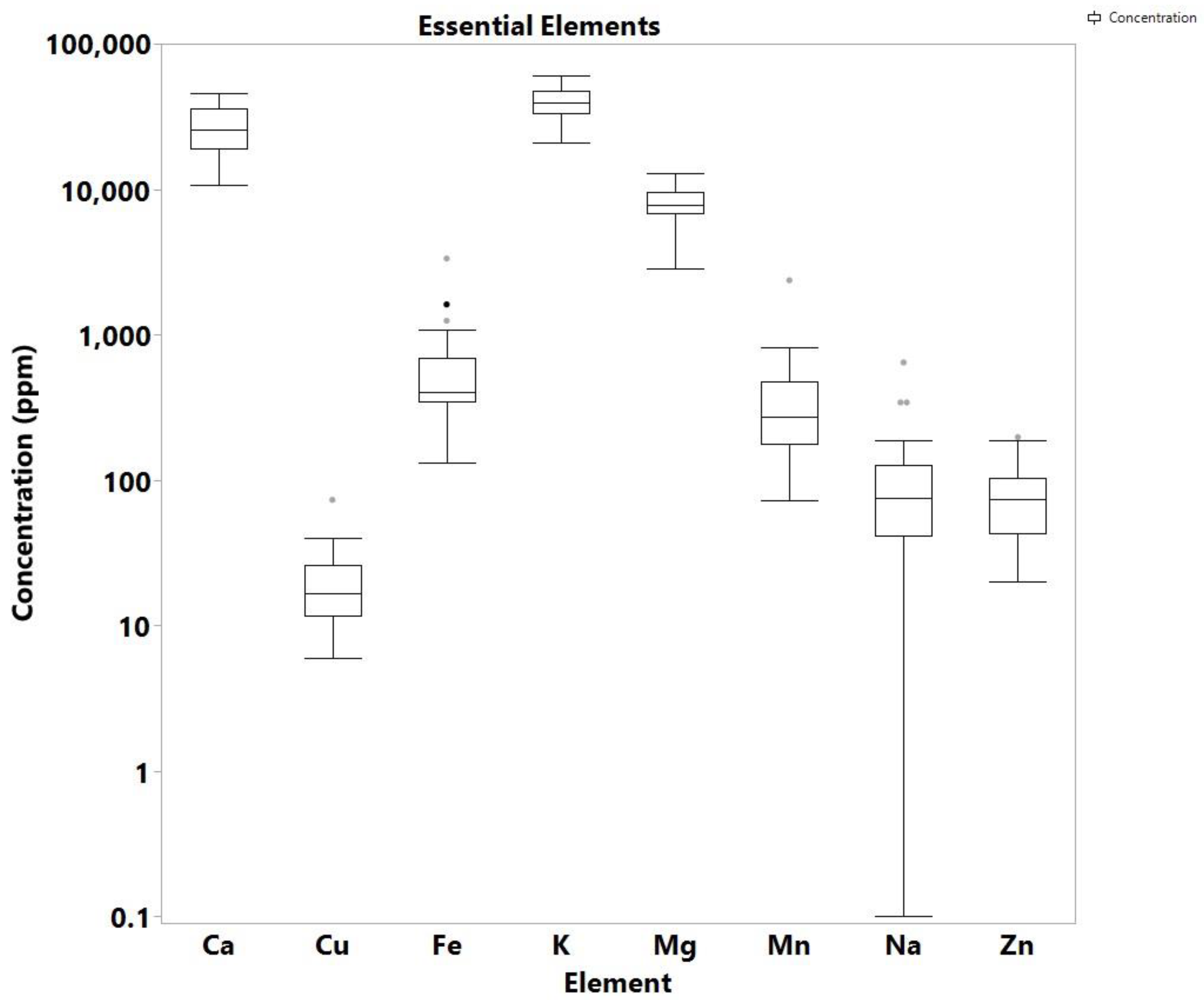
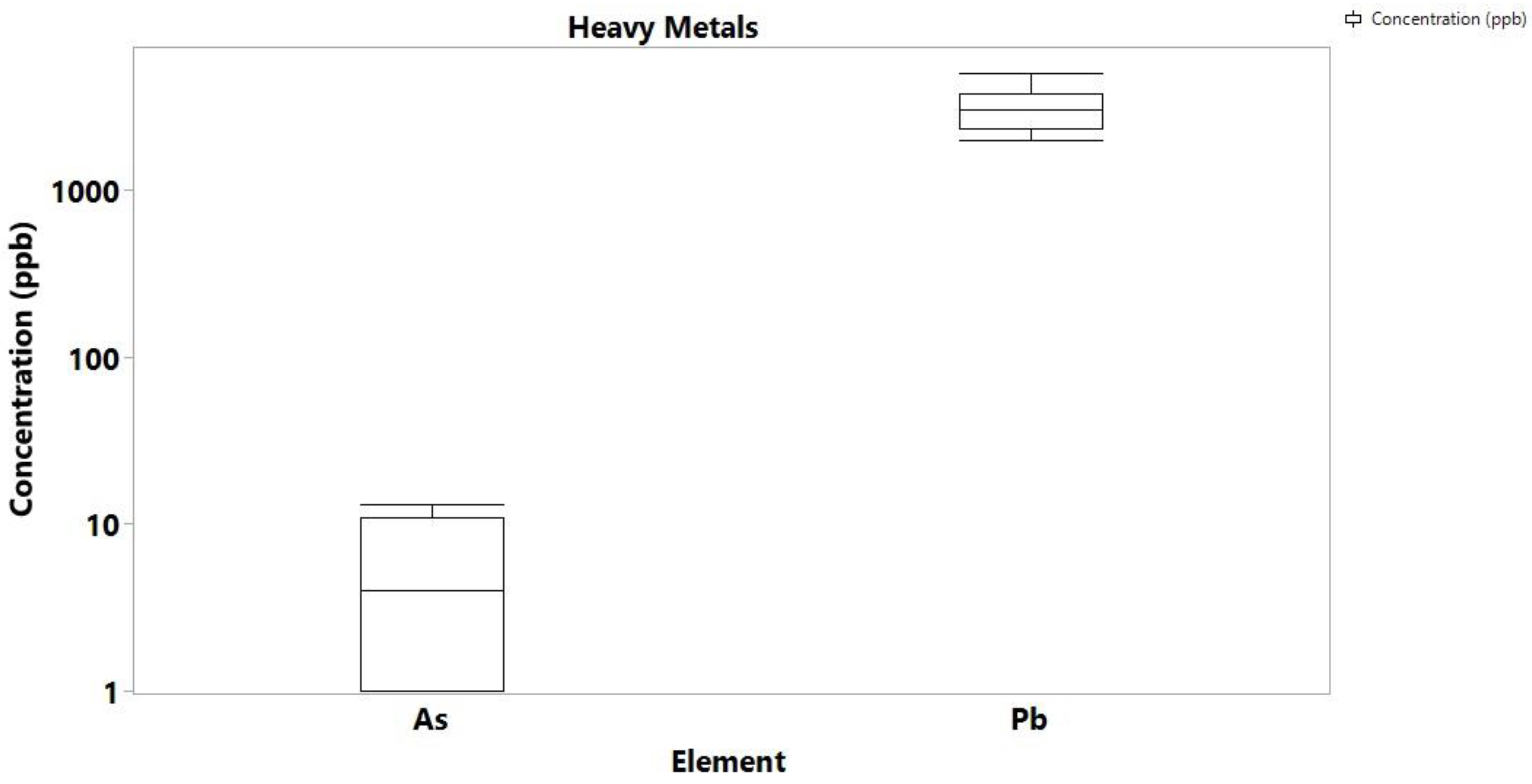
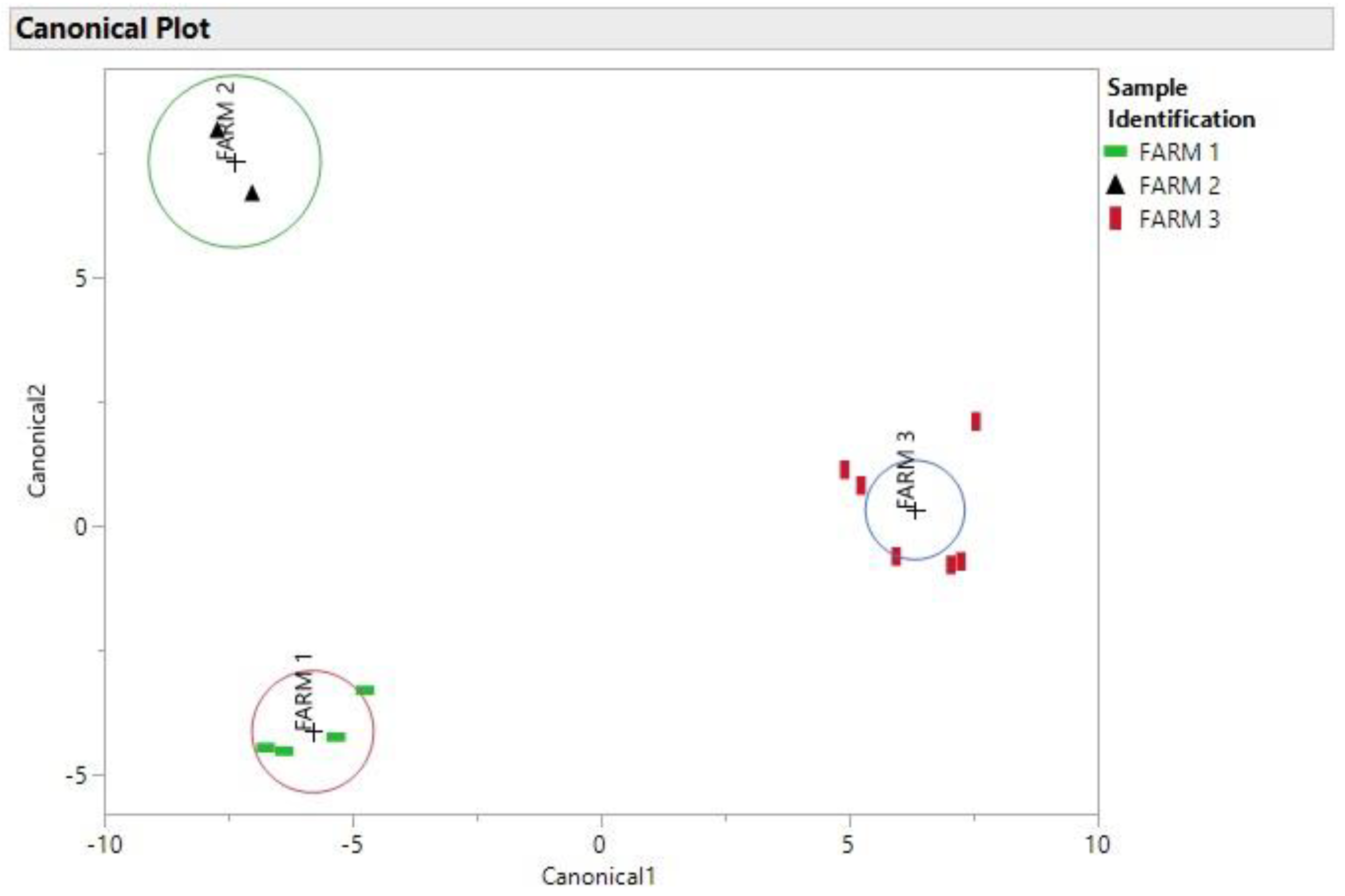
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Recommended Methods for the Identification and Analysis of Cannabis and Cannabis Products; UNODC United Nations Office on Drugs and Crime: New York, NY, USA, 2009.
- Bridgeman, M.B.; Abazia, D.T. Medicinal Cannabis: History, Pharmacology, And Implications for the Acute Care Setting. Pharmacy Ther. 2017, 42, 180–188. [Google Scholar]
- Hall, W.; Stjepanović, D.; Caulkins, J.; Lynskey, M.; Leung, J.; Campbell, G.; Degenhardt, L. Public health implications of legalising the production and sale of cannabis for medicinal and recreational use. Lancet 2019, 394, 1580–1590. [Google Scholar] [CrossRef]
- Unooda, L. World Drug Report 2021 (Set of 5 Booklets); United Nations: New York, NY, USA, 2022. [Google Scholar]
- Zerihun, A.; Chandravanshi, B.S.; Debebe, A.; Mehari, B. Levels of selected metals in leaves of Cannabis sativa L. cultivated in Ethiopia. SpringerPlus 2015, 4, 359. [Google Scholar] [CrossRef] [PubMed]
- Stambouli, H.; El Bouri, A.; Bellimam, M.A.; Bouayoun, T.; El Karni, N. Cultivation of Cannabis sativa L. in northern Morocco. Bull. Narc. 2005, 57, 79–118. [Google Scholar]
- Toonen, M.; Ribot, S.; Thissen, J. Yield of Illicit Indoor Cannabis Cultivation in The Netherlands. J. Forensic Sci. 2006, 51, 1050–1054. [Google Scholar] [CrossRef]
- Bengyella, L.; Kuddus, M.; Mukherjee, P.; Fonmboh, D.J.; Kaminski, J.E. Global Impact of Trace Non-Essential Toxic Metal Contaminants in Industrial Cannabis Bioeconomy. Toxin Rev. 2021, 1–11. [Google Scholar] [CrossRef]
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal Plant Analysis: A Historical and Regional Discussion of Emergent Complex Techniques. Front. Pharmacol. 2020, 10, 1480. [Google Scholar] [CrossRef]
- Abraham, E.J.; Kellogg, J.J. Chemometric-Guided Approaches for Profiling and Authenticating Botanical Materials. Front. Nutr. 2021, 8, 780228. [Google Scholar] [CrossRef]
- Reidy, L.; Bu, K.; Godfrey, M.; Cizdziel, J.V. Elemental fingerprinting of soils using ICP-MS and multivariate statistics: A study for and by forensic chemistry majors. Forensic Sci. Int. 2013, 233, 37–44. [Google Scholar] [CrossRef]
- Filipiak-Szok, A.; Kurzawa, M.; Szłyk, E. Determination of toxic metals by ICP-MS in Asiatic and European medicinal plants and dietary supplements. J. Trace Elem. Med. Biol. 2015, 30, 54–58. [Google Scholar] [CrossRef]
- Harris, A.; Xanthos, S.J.; Galiotos, J.K.; Douvris, C. Investigation of the Metal Content of Sediments around the Historically Polluted Potomac River Basin in Washington DC, United States by Inductively Coupled Plasma-Optical Emission Spectroscopy (ICP-OES). Microchem. J. 2018, 142, 140–143. [Google Scholar] [CrossRef]
- Bussan, D.D.; Douvris, C.; Cizdziel, J.V. Mercury Methylation Potentials in Sediments of an Ancient Cypress Wetland Using Species-Specific Isotope Dilution GC-ICP-MS. Molecules 2022, 27, 4911. [Google Scholar] [CrossRef] [PubMed]
- Bussan, D.; Harris, A.; Douvris, C. Monitoring of selected trace elements in sediments of heavily industrialized areas in Calcasieu Parish, Louisiana, United States by inductively coupled plasma-optical emission spectroscopy (ICP-OES). Microchem. J. 2018, 144, 51–55. [Google Scholar] [CrossRef]
- Snaychuk, L.; Vaughan, T.; Ullery, Z.; Douvris, C.; Bussan, D.D. Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES) Analysis of Trace Metals in Cigarette Litter Collected at McNeese State University in Lake Charles, LA, United States. Methods Objects Chem. Anal. 2022, 17, 5–9. [Google Scholar] [CrossRef]
- Thomas, R. Beyond Potency: The Importance of Measuring Elemental Contaminants in Cannabis and Hemp. Available online: http://www.scientificsolutions1.com/Cannabis%20Science%20and%20Technology%20Heavy%20Metals%20Rob%20Thomas.pdf (accessed on 24 September 2022).
- Thomas, R.J. Measuring Toxic Metal Contaminants in Cannabis and Hemp; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Bentil, E.; Asiedu, N.; Ataki, J. Statistical analysis of trace metals content of cocaine using inductively coupled plasma-mass spectrometry calibrations. Cogent Chem. 2019, 5, 1671071. [Google Scholar] [CrossRef]
- Bussan, D.D.; Ochs, C.A.; Jackson, C.R.; Anumol, T.; Snyder, S.A.; Cizdziel, J.V. Concentrations of select dissolved trace elements and anthropogenic organic compounds in the Mississippi River and major tributaries during the summer of 2012 and 2013. Environ. Monit. Assess. 2017, 189, 73. [Google Scholar] [CrossRef]
- Burns, R.L.; Alexander, R.; Snaychuk, L.; Edwards, J.C.; Fitzgerald, N.; Gao, P.; Quan, D.; Douvris, C.; Vaughan, T.; Bussan, D.D. A Fast, Straightforward and Inexpensive Method for the Authentication of Baijiu Spirit Samples by Fluorescence Spectroscopy. Beverages 2021, 7, 65. [Google Scholar] [CrossRef]
- Falciani, R.; Novaro, E.; Marchesini, M.; Gucciardi, M. Multi-element analysis of soil and sediment by ICP-MS after a microwave assisted digestion method. J. Anal. At. Spectrom. 2000, 15, 561–565. [Google Scholar] [CrossRef]
- Zafeiraki, E.; Kasiotis, K.M.; Nisianakis, P.; Machera, K. Macro and Trace Elements in Hemp (Cannabis sativa L.) Cultivated in Greece: Risk Assessment of Toxic Elements. Front. Chem. 2021, 9, 654308. [Google Scholar] [CrossRef]
- Kuras, M.J.; Wachowicz, M.J. Cannabis Profiling Based on Its Elemental Composition—Is It Possible? J. Forensic Sci. 2011, 56, 1250–1255. [Google Scholar] [CrossRef]
- Shibuya, E.K.; Sarkis, J.E.S.; Negrini-Neto, O.; Ometto, J. Multivariate classification based on chemical and stable isotopic profiles in sourcing the origin of marijuana samples seized in Brazil. J. Braz. Chem. Soc. 2007, 18, 205–214. [Google Scholar] [CrossRef]
- Bing, H.; Wu, Y.; Sun, Z.; Yao, S. Historical Trends of Toxic Metal Contamination and Their Sources in Lacustrine Sediment from Xijiu Lake, Taihu Lake Catchment, China. J. Environ. Sci. 2011, 23, 1671–1678. [Google Scholar] [CrossRef]
- Benipal, G.; Harris, A.; Srirajayatsayai, C.; Tate, A.; Topalidis, V.; Eswani, Z.; Qureshi, M.; Hardaway, C.J.; Galiotos, J.; Douvris, C. Examination of Al, As, Cd, Cr, Cu, Fe, Mg, Mn, Ni, Pb, Sb, Se, V, and Zn in sediments collected around the downtown Houston, Texas area, using inductively coupled plasma-optical emission spectroscopy. Microchem. J. 2017, 130, 255–262. [Google Scholar] [CrossRef]
- Mensah, E.; Allen, H.; Shoji, R.; Odai, S.; Kyei-Baffo, N.; Ofori, E.; Mezler, D. Cadmium (Cd) and Lead (Pb) Concentrations Effects on Yields of Some Vegetables Due to Uptake from Irrigation Water in Ghana. Int. J. Agric. Res. 2008, 3, 243–251. [Google Scholar] [CrossRef]
- Bussan, D.D.; Snaychuk, L.; Bartzas, G.; Douvris, C. Quantification of trace elements in surgical and KN95 face masks widely used during the SARS-COVID-19 pandemic. Sci. Total Environ. 2021, 814, 151924. [Google Scholar] [CrossRef] [PubMed]
- Citterio, S.; Santagostino, A.; Fumagalli, P.; Prato, N.; Ranalli, P.; Sgorbati, S. Toxic Metal Tolerance and Accumulation of Cd, Cr and Ni by Cannabis sativa L. Plant Soil 2003, 256, 243–252. [Google Scholar] [CrossRef]
- Abernathy, C.O.; Liu, Y.P.; Longfellow, D.; Aposhian, H.V.; Beck, B.; Fowler, B.; Goyer, R.; Menzer, R.; Rossman, T.; Thompson, C.; et al. Arsenic: Health, Mechanisms of Actions, and Research Issues. Environ. Health Perspect. 1999, 107, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Q.; Young, M.R.; Diwan, B.A. Association of Arsenic-Induced Malignant Transformation with DNA Hypmethylation and Aberrant Gene Expression. Proc. Natl. Acad. Sci. USA 1997, 94, 10907–10912. [Google Scholar] [CrossRef]
- Zhao, Y. Comparision of the Trace Elements and Active Components of Lonicera Japonica Flos and Lonicera Flos Using ICP-MS and HPLC-PDA. Biol. Trace Elem. Res. 2018, 183, 379–388. [Google Scholar] [CrossRef] [PubMed]


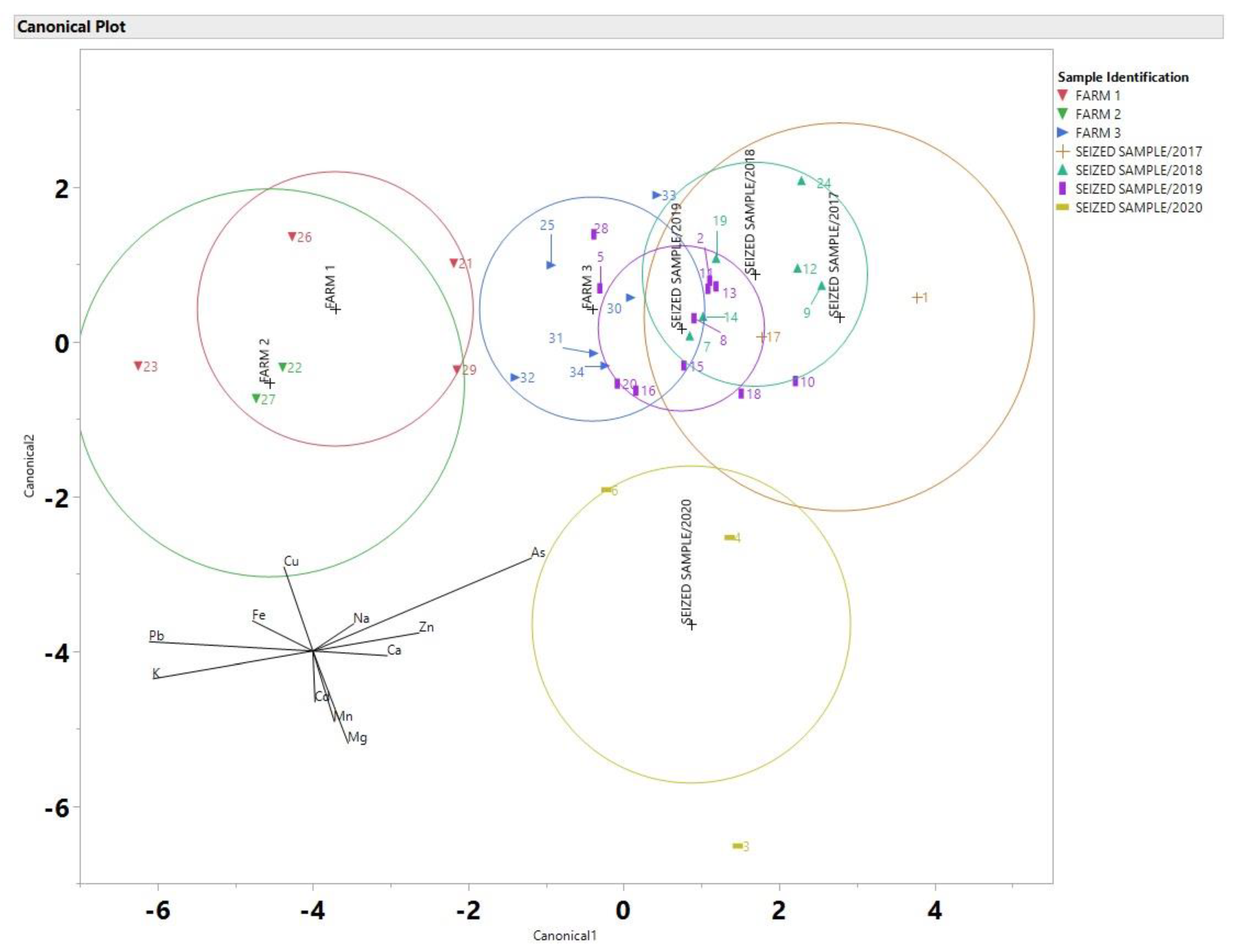
| Na | Mg | K | Ca | Mn | Fe | Cu | Zn | As | Cd | Pb | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| percent Δ (samples 34 and 20) | 104.8 | 0.1 | 16.0 | 54.6 | 77.0 | 54.6 | 20.0 | 10.5 | 1.7 | 0.0 | 37.5 |
| percent Δ (samples 8 and 14) | 42.1 | 3.6 | 0.2 | 40.4 | 33.4 | 128.5 | 28.6 | 4.8 | 131.3 | 147.2 | 124.4 |
| percent Δ (samples 31 and 34) | 161.0 | 0.3 | 0.6 | 5.9 | 20.7 | 2.8 | 25.0 | 42.7 | 58.0 | 28.6 | 27.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Douvris, C.; Bentil, E.; Ayensu, I.; Osei Akoto, C.; Amponsah, I.K.; Adu, J.; Bussan, D. Trace Metals in Cannabis Seized by Law Enforcement in Ghana and Multivariate Analysis to Distinguish among Different Cannabis Farms. Toxics 2022, 10, 567. https://doi.org/10.3390/toxics10100567
Douvris C, Bentil E, Ayensu I, Osei Akoto C, Amponsah IK, Adu J, Bussan D. Trace Metals in Cannabis Seized by Law Enforcement in Ghana and Multivariate Analysis to Distinguish among Different Cannabis Farms. Toxics. 2022; 10(10):567. https://doi.org/10.3390/toxics10100567
Chicago/Turabian StyleDouvris, Chris, Edward Bentil, Isaac Ayensu, Clement Osei Akoto, Isaac Kingsley Amponsah, Joseph Adu, and Derek Bussan. 2022. "Trace Metals in Cannabis Seized by Law Enforcement in Ghana and Multivariate Analysis to Distinguish among Different Cannabis Farms" Toxics 10, no. 10: 567. https://doi.org/10.3390/toxics10100567
APA StyleDouvris, C., Bentil, E., Ayensu, I., Osei Akoto, C., Amponsah, I. K., Adu, J., & Bussan, D. (2022). Trace Metals in Cannabis Seized by Law Enforcement in Ghana and Multivariate Analysis to Distinguish among Different Cannabis Farms. Toxics, 10(10), 567. https://doi.org/10.3390/toxics10100567