Evaluation of Skin Irritation of Acids Commonly Used in Cleaners in 3D-Reconstructed Human Epidermis Model, KeraSkinTM
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. D-Reconstructed Human Epidermis Skin Model
2.3. In Vitro Skin Irritation Test (SIT)
2.4. Histological Analysis
2.5. Visualization of Lipid Distribution in KeraSkinTM with Nile Red Staining
2.6. Statistics
3. Results
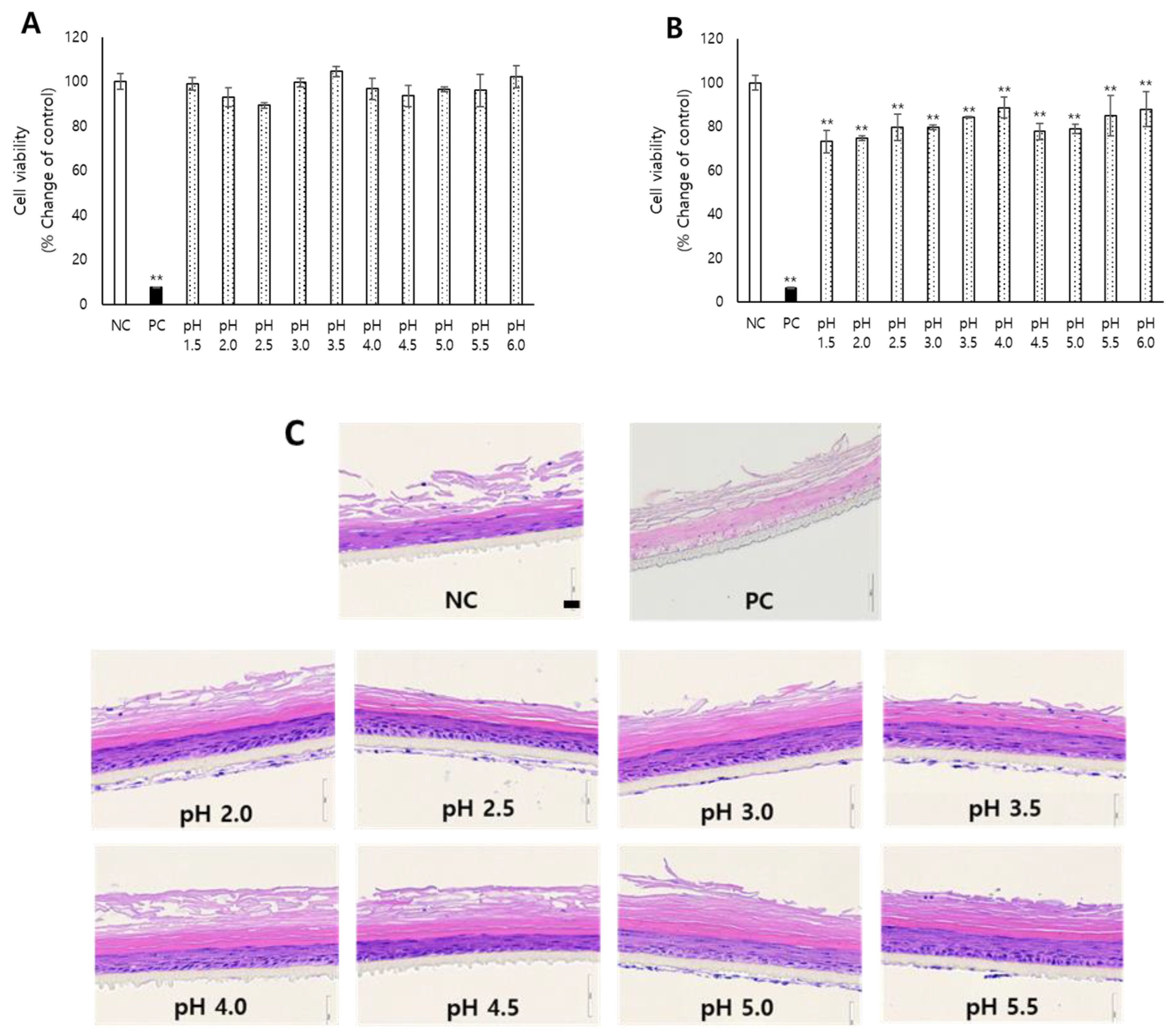
3.1. Skin Irritation of Acidic pH Determined in KeraSkin™
3.2. Evaluation of Skin Irritation of Various Organic Acids
3.3. Evaluation of Skin Irritation of Various Inorganic Acids
3.4. Evaluation of Skin Irritation of Various Organic and Inorganic Alkalis
3.5. Effects of Acid and Alkali Substances on the Lipid Composition of KeraSkinTM
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zumelzu, E.; Cabezas, C. Observations on the influence of cleaners on material corrosion in the food industry. Mater. Charact. 1996, 37, 187–194. [Google Scholar] [CrossRef]
- Lieu, V.T.; Kalbus, G.E. Potentiometric titration of acidic and basic compounds in household cleaners. J. Chem. Educ. 1988, 65, 184. [Google Scholar]
- Robinson, M.K.; Kruszewski, F.H.; Al-Atrash, J.; Blazka, M.E.; Gingell, R.; Heitfeld, F.A.; Mallon, D.; Snyder, N.K.; Swanson, J.E.; Casterton, P.L. Comparative assessment of the acute skin irritation potential of detergent formulations using a novel human 4-h patch test method. Food Chem. Toxicol. 2005, 43, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Scheel, J.; Heppenheimer, A.; Lehringer, E.; Kreutz, J.; Poth, A.; Ammann, H.; Reisinger, K.; Banduhn, N. Classification and labeling of industrial products with extreme pH by making use of in vitro methods for the assessment of skin and eye irritation and corrosion in a weight of evidence approach. Toxicol. Vitr. 2011, 25, 1435–1447. [Google Scholar] [CrossRef]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.-H.; Korting, H.C. The pH of the skin surface and its impact on the barrier function. Ski. Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef]
- Wohlrab, J.; Gebert, A. pH and buffer capacity of topical formulations. In pH of the Skin: Issues and Challenges, 1st ed.; Surber, C., Abels, C., Maibach, H., Eds.; Karger Publishers: Basel, Switzerland, 2018; Volume 54, pp. 123–131. [Google Scholar]
- Fluhr, J.W.; Elias, P.M. Stratum corneum pH: Formation and function of the ‘acid mantle’. Exog. Dermatol. 2002, 1, 163–175. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, K.P.; Moore, D.J.; Subramanyan, K.; Misra, M.; Meyer, F. Cleansing without compromise: The impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol. Ther. 2004, 17, 16–25. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, K.P.; Lips, A.; Vincent, C.; Meyer, F.; Caso, S.; Johnson, A.; Subramanyan, K.; Vethamuthu, M.; Rattinger, G.; Moore, D.J. pH-induced alterations in stratum corneum properties. Int. J. Cosmet. Sci. 2003, 25, 103–112. [Google Scholar] [CrossRef]
- Johnson, A.W.; Ananthapadmanabhan, K.; Hawkins, S.; Nole, G. Bar cleansers. In Cosmetic Dermatology: Products and Procedures, 3rd ed.; Draelos, Z.D., Ed.; Wiley: Hoboken, NJ, USA, 2022; pp. 115–134. [Google Scholar]
- UN. United Nations Globally Harmonized System of Classification and Labelling of Chemicals (GHS); UN: New York, NY, USA; Geneva, Switzerland, 2011.
- Craan, A.G.; Sanfacon, G.; Walker, R.H. The use of pH and acid/alkaline reserve for the classification and labelling of household cleaning products: Data from a poison control center. Int. J. Consum. Prod. Saf. 1997, 4, 191–213. [Google Scholar] [CrossRef]
- McIlvaine, T. A buffer solution for colorimetric comparison. J. Biol. Chem. 1921, 49, 183–186. [Google Scholar] [CrossRef]
- ECHA. ECHA Registration Dossier: Citric Acid. Available online: https://echa.europa.eu/el/registration-dossier/-/registered-dossier/15451 (accessed on 20 September 2022).
- ECHA. ECHA Registration Dossier: Glycollic Acid. Available online: https://echa.europa.eu/el/registration-dossier/-/registered-dossier/14561 (accessed on 20 September 2022).
- ECHA. ECHA Registration Dossier: Lactic Acid. Available online: https://echa.europa.eu/el/registration-dossier/-/registered-dossier/5165 (accessed on 20 September 2022).
- ECHA. ECHA Registration Dossier: Malic Acid. Available online: https://echa.europa.eu/el/registration-dossier/-/registered-dossier/11511 (accessed on 20 September 2022).
- ECHA. ECHA Registration Dossier: Succinic Acid. Available online: https://echa.europa.eu/el/registration-dossier/-/registered-dossier/15265 (accessed on 20 September 2022).
- ECHA. ECHA Registration Dossier: Hydrogen Bromide. Available online: https://echa.europa.eu/el/registration-dossier/-/registered-dossier/14814 (accessed on 20 September 2022).
- ECHA. ECHA Registration Dossier: Hydrogen Chloride. Available online: https://echa.europa.eu/el/registration-dossier/-/registered-dossier/15859 (accessed on 20 September 2022).
- ECHA. ECHA Registration Dossier: Nitric Acid. Available online: https://echa.europa.eu/el/registration-dossier/-/registered-dossier/15881 (accessed on 20 September 2022).
- ECHA. ECHA Registration Dossier: Sulphuric Acid. Available online: https://echa.europa.eu/el/registration-dossier/-/registered-dossier/16122 (accessed on 20 September 2022).
- ECHA. ECHA Registration Dossier: 2-Aminoethanol. Available online: https://echa.europa.eu/el/registration-dossier/-/registered-dossier/15808 (accessed on 20 September 2022).
- ECHA. ECHA Registration Dossier: 2,2′,2″-Nitrilotriethanol. Available online: https://echa.europa.eu/el/registration-dossier/-/registered-dossier/15134 (accessed on 20 September 2022).
- ECHA. ECHA Registration Dossier: Sodium Hydroxide. Available online: https://echa.europa.eu/el/registration-dossier/-/registered-dossier/15566 (accessed on 20 September 2022).
- ECHA. ECHA Registration Dossier: Sodium Carbonate. Available online: https://echa.europa.eu/el/registration-dossier/-/registered-dossier/15432 (accessed on 20 September 2022).
- Jung, K.M.; Lee, S.H.; Jang, W.H.; Jung, H.S.; Heo, Y.; Park, Y.H.; Bae, S.; Lim, K.M.; Seok, S.H. KeraSkin™-VM: A novel reconstructed human epidermis model for skin irritation tests. Toxicol. Vitr. 2014, 28, 742–750. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 439: In Vitro Skin Irritation: Reconstructed Human Epidermis Test Method; OECD: Paris, France, 2021. [Google Scholar]
- Lee, N.; Jang, D.Y.; Lee, D.H.; Jeong, H.; Nam, K.T.; Choi, D.W.; Lim, K.M. Local Toxicity of Biocides after Direct and Aerosol Exposure on the Human Skin Epidermis and Airway Tissue Models. Toxics 2021, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Jeong, H.; Jung, Y.O.; Nam, K.T.; Lim, K.M. Skin irritation and inhalation toxicity of biocides evaluated with reconstructed human epidermis and airway models. Food Chem. Toxicol. 2021, 150, 112064. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.H.; Jeong, H.; Hur, S.; Nam, K.T.; Lim, K.M. Employment of cytology for in vitro skin irritation test using a reconstructed human epidermis model, Keraskin. Toxicol. Vitr. 2020, 69, 104962. [Google Scholar] [CrossRef]
- Jung, Y.O.; Jeong, H.; Cho, Y.; Lee, E.O.; Jang, H.W.; Kim, J.; Nam, K.; Lim, K.M. Lysates of a Probiotic, Lactobacillus rhamnosus, Can Improve Skin Barrier Function in a Reconstructed Human Epidermis Model. Int. J. Mol. Sci. 2019, 20, 4289. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, H.E.; Yang, G.; Kim, K.B.; Kwack, S.J.; Lee, J.Y. Para-phenylenediamine, an oxidative hair dye ingredient, increases thymic stromal lymphopoietin and proinflammatory cytokines causing acute dermatitis. Toxicol. Res. 2020, 36, 329–336. [Google Scholar] [CrossRef]
- Lee, K.J.; Park, K.H.; Hahn, J.H. Alleviation of Ultraviolet-B Radiation-Induced Photoaging by a TNFR Antagonistic Peptide, TNFR2-SKE. Mol. Cells 2019, 42, 151–160. [Google Scholar]
- Fowler, S.D.; Greenspan, P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: Comparison with oil red O. J. Histochem. Cytochem. 1985, 33, 833–836. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Azambuja, D.S.; Martini, E.M. Semiconductive properties of titanium anodic oxide films in McIlvaine buffer solution. Corros. Sci. 2006, 48, 2901–2912. [Google Scholar] [CrossRef]
- Agency, U.S.E.P. 40 CFR 180.940. Title 40—Protection of Environment, Part 180—Tolerances and Exemptions for Pesticide Chemical Residues In Food, Sec. 180.940—Tolerance Exemptions for Active and Inert Ingredients for Use in Antimicrobial Formulations (Food-Contact Surface Sanitizing Solutions). 2004. Available online: www.law.cornell.edu/cfr/text/40/180.940 (accessed on 20 September 2022).
- Seweryn, A. Interactions between surfactants and the skin–Theory and practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Danby, S.G.; Andrew, P.V.; Kay, L.J.; Pinnock, A.; Chittock, J.; Brown, K.; Williams, S.F.; Cork, M.J. Enhancement of stratum corneum lipid structure improves skin barrier function and protects against irritation in adults with dry, eczema-prone skin. Br. J. Dermatol. 2022, 186, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Bouwstra, J.A.; Gooris, G.S.; van der Spek, J.A.; Lavrijsen, S.; Bras, W. The lipid and protein structure of mouse stratum corneum: A wide and small angle diffraction study. Biochim. Biophys. Acta-Lipids Lipid Metab. 1994, 1212, 183–192. [Google Scholar] [CrossRef]
- Kim, E.; Kim, S.; Nam, G.; Lee, H.; Moon, S.; Chang, I. The alkaline pH-adapted skin barrier is disrupted severely by SLS-induced irritation. Int. J. Cosmet. Sci. 2009, 31, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Kuehl, B.; Fyfe, K.; Shear, N. Cutaneous cleansers. Ski. Ther. Lett. 2003, 8, 1–4. [Google Scholar]
- Pham, Q.D.; Bjorklund, S.; Engblom, J.; Topgaard, D.; Sparr, E. Chemical penetration enhancers in stratum corneum - Relation between molecular effects and barrier function. J. Control Release 2016, 232, 175–187. [Google Scholar] [CrossRef]
- Worth, A.P.; Cronin, M.T. The use of pH measurements to predict the potential of chemicals to cause acute dermal and ocular toxicity. Toxicology 2001, 169, 119–131. [Google Scholar] [CrossRef]
- Worth, A.P.; Balls, M. The importance of the prediction model in the validation of alternative tests. Altern. Lab. Anim. 2001, 29, 135–143. [Google Scholar] [CrossRef]
- OECD. Test No. 404: Acute Dermal Irritation/Corrosion; OECD: Paris, France, 2015. [Google Scholar]




| Chemical | IUPAC Name | CAS No. | Molecular Weight | Molecular Formula | Purity | Water Solubility b | pH (Skin Irritation a) | ECHAb | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 M | 1.0 M | Skin hazard | Source | |||||||
| Organic acid | ||||||||||
| Citric acid | 2-Hydroxypropane-1,2,3-tricarboxylic acid | 77-92-9 | 192.12 | C6H8O7 | ≥97% | 540 g/L | 1.89 (NI) | 1.13 (I) | Mildly irritating (0.5 g powder) | [15] |
| Glycolic acid | 2-Hydroxyacetic acid | 79-14-1 | 76.05 | C2H4O3 | 99% | > 300 g/L | 2.41 (NI) | 1.61 (I) | Skin Corr. 1B (99% liquid) | [16] |
| Lactic acid | 2-Hydroxypropanoic acid | 50-21-5 | 90.08 | C3H6O3 | 88–92% | 861 g/L | 2.43 (NI) | 1.60 (NI) | Mildly irritating (88% aqueous solution) | [17] |
| Malic acid | 2-Hydroxybutanedioic acid | 6915-15-7 | 134.09 | C4H6O5 | ≥99% | 647 g/L | 2.25 (NI) | 1.43 (I) | Mildly irritating (0.5 g wetted with paraffin oil) | [18] |
| Succinic acid | Butanedioic acid | 110-15-6 | 118.09 | C4H6O4 | ≥99% | 83 g/L | 2.60 (NI) | 1.82 (I) | Not irritating (0.5 g moistened with water) | [19] |
| Inorganic acid | ||||||||||
| Hydrogen bromide | Bromane | 10035-10-6 | 80.91 | HBr | 30–32% in acetic acid | 665 g/L | 0.55 (NI) | −0.66 (I) | Skin Corr. 1A | [20] |
| Hydrogen chloride | Chlorane | 7647-01-0 | 36.46 | HCl | 37% aq. solution | 500 g/L | 0.69 (NI) | −0.29 (I) | Skin Corr. 1A (25~30%) | [21] |
| Nitric acid | Nitric acid | 7697-37-2 | 63.01 | HNO3 | 70% aq. solution | >500 g/L | 0.58 (NI) | −0.39 (I) | Skin Corr. 1A (≥28%) | [22] |
| Sulfuric acid | Sulfuric acid | 7664-93-9 | 98.08 | H2SO4 | 95–98% | 1000 g/L | 0.47 (NI) | −0.55 (I) | Skin Corr. 1A (Predicted) | [23] |
| Organic alkali | ||||||||||
| Ethanolamine | 2-Aminoethanol | 141-43-5 | 61.08 | C2H7NO | ≥99% | 1000 g/L | 11.40 (NI) | 12.00 (I) | Skin Corr. 1B (Neat liquid, 20% solution) | [24] |
| Triethanolamine | 2-[Bis(2-hydroxyethyl)amino]ethanol | 102-71-6 | 149.19 | C6H15NO3 | ≥99% | 1000 g/L | 10.36 (NI) | 10.90 (NI) | Not irritating (0.5 mL neat) | [25] |
| Inorganic alkali | ||||||||||
| Sodium hydroxide | Sodium hydroxide | 1310-73-2 | 39.997 | HNaO | ≥97% | 1000 g/L | 13.07 (NI) | 13.68 (I) | Skin Corr. 1A (>2%) | [26] |
| Sodium carbonate | Disodium carbonate | 497-19-8 | 105.988 | Na2CO3 | ≥99.5% | 212.5 g/L | 11.49 (NI) | 11.60 (NI) | Not irritating (0.5 g) | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, J.-h.; Lee, S.; Lee, H.G.; Choi, D.; Lim, K.-M. Evaluation of Skin Irritation of Acids Commonly Used in Cleaners in 3D-Reconstructed Human Epidermis Model, KeraSkinTM. Toxics 2022, 10, 558. https://doi.org/10.3390/toxics10100558
Hwang J-h, Lee S, Lee HG, Choi D, Lim K-M. Evaluation of Skin Irritation of Acids Commonly Used in Cleaners in 3D-Reconstructed Human Epidermis Model, KeraSkinTM. Toxics. 2022; 10(10):558. https://doi.org/10.3390/toxics10100558
Chicago/Turabian StyleHwang, Jee-hyun, Seungmi Lee, Ho Geon Lee, Dalwoong Choi, and Kyung-Min Lim. 2022. "Evaluation of Skin Irritation of Acids Commonly Used in Cleaners in 3D-Reconstructed Human Epidermis Model, KeraSkinTM" Toxics 10, no. 10: 558. https://doi.org/10.3390/toxics10100558
APA StyleHwang, J.-h., Lee, S., Lee, H. G., Choi, D., & Lim, K.-M. (2022). Evaluation of Skin Irritation of Acids Commonly Used in Cleaners in 3D-Reconstructed Human Epidermis Model, KeraSkinTM. Toxics, 10(10), 558. https://doi.org/10.3390/toxics10100558








