TDP-43 CSF Concentrations Increase Exponentially with Age in Metropolitan Mexico City Young Urbanites Highly Exposed to PM2.5 and Ultrafine Particles and Historically Showing Alzheimer and Parkinson’s Hallmarks. Brain TDP-43 Pathology in MMC Residents Is Associated with High Cisternal CSF TDP-43 Concentrations
Abstract
:1. Introduction
2. Materials and Methods
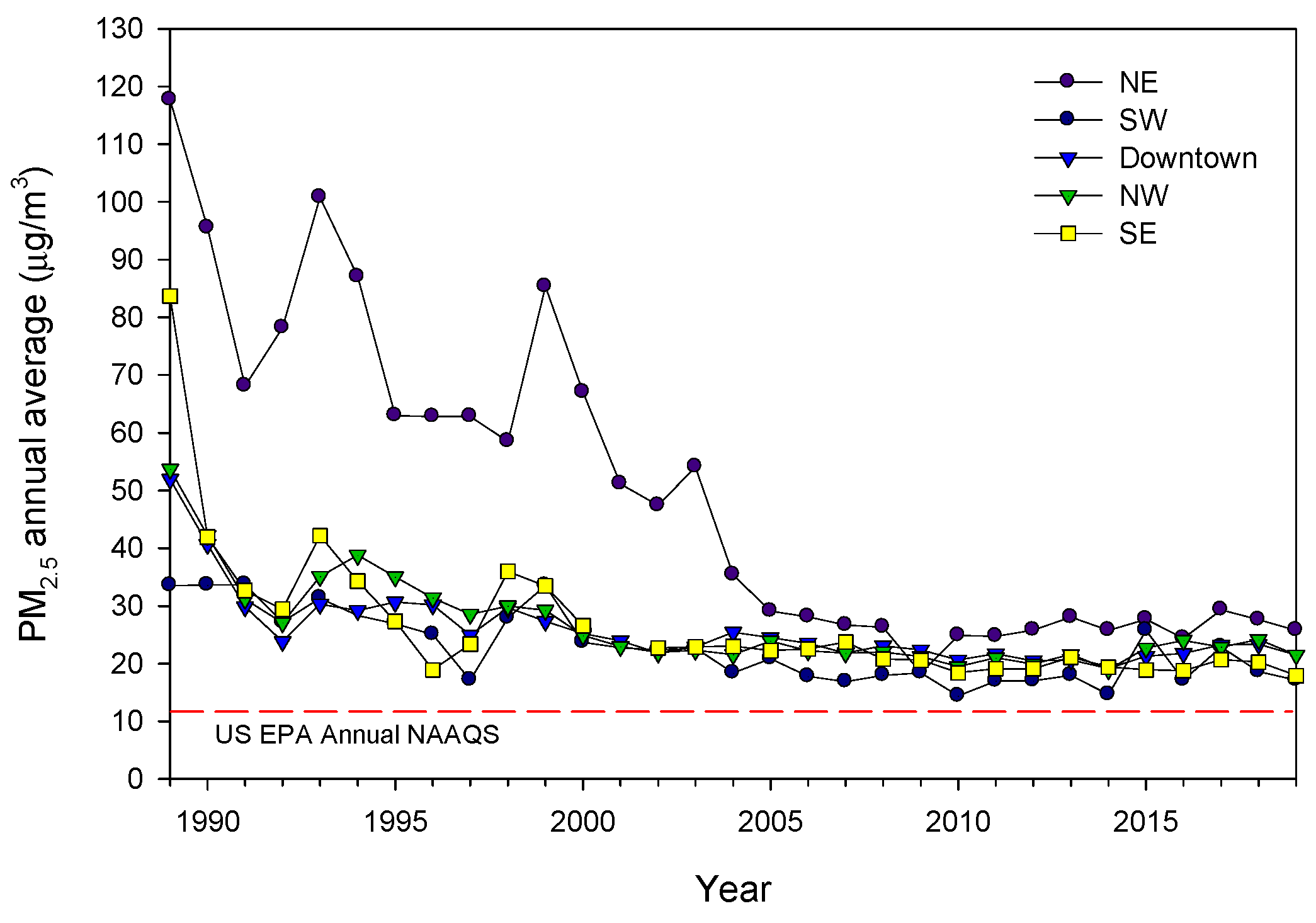
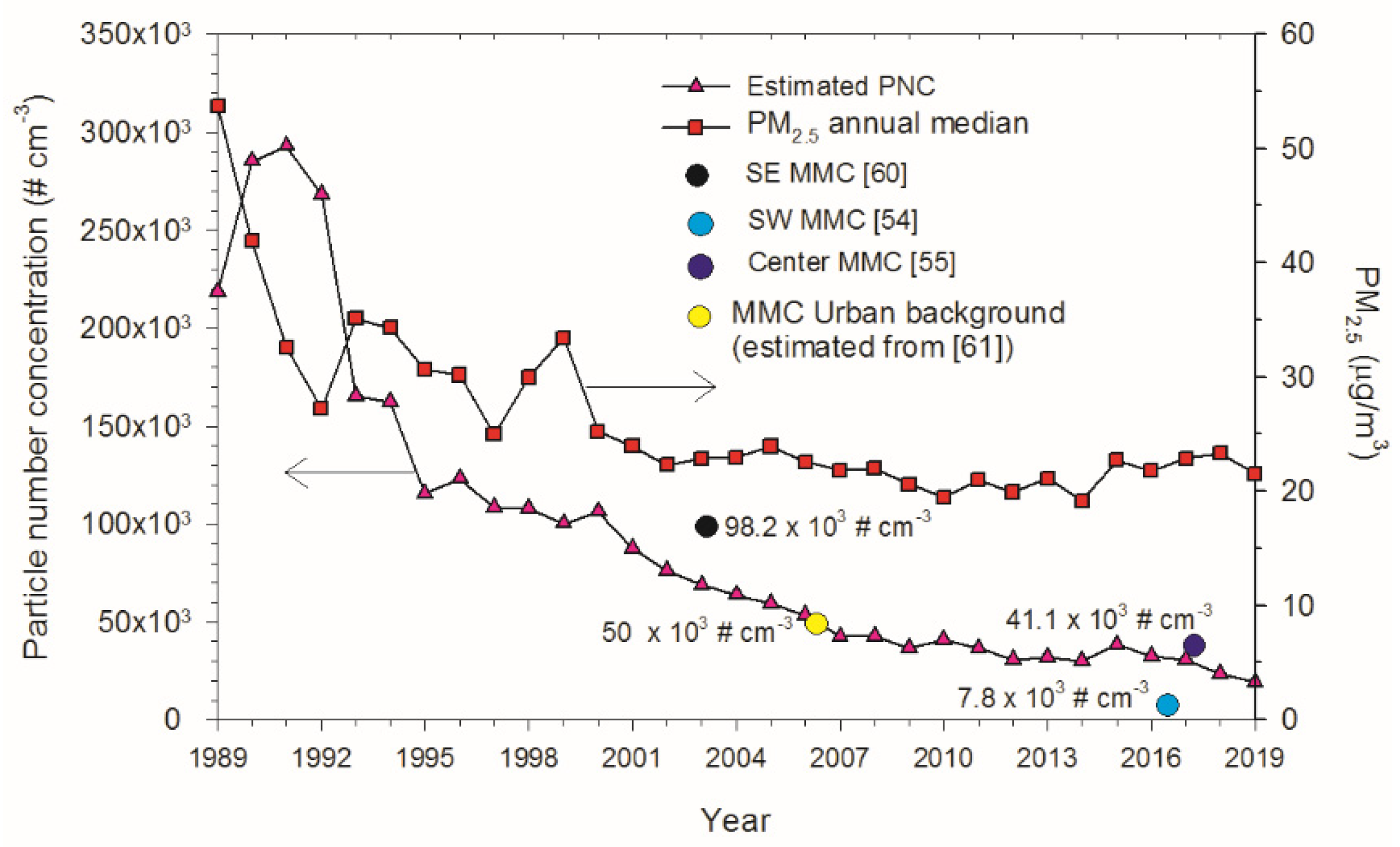
2.1. Study Cities and Air Quality
2.2. CSF and Brain Samples
2.2.1. CSF Spinal Taps
2.2.2. Pediatric Cohort for the Measurement of TDP-43 in CSF
2.2.3. Adult Cohort for the Measurement of TDP-43 in CSF
2.2.4. Children and Adult Forensic Autopsies for the Measurement of Cisternal CSF TDP-43 and Complete Brain Examination Including Immunohistochemistry for TDP-43
2.2.5. Data Analysis
3. Results
3.1. CSF TDP-43
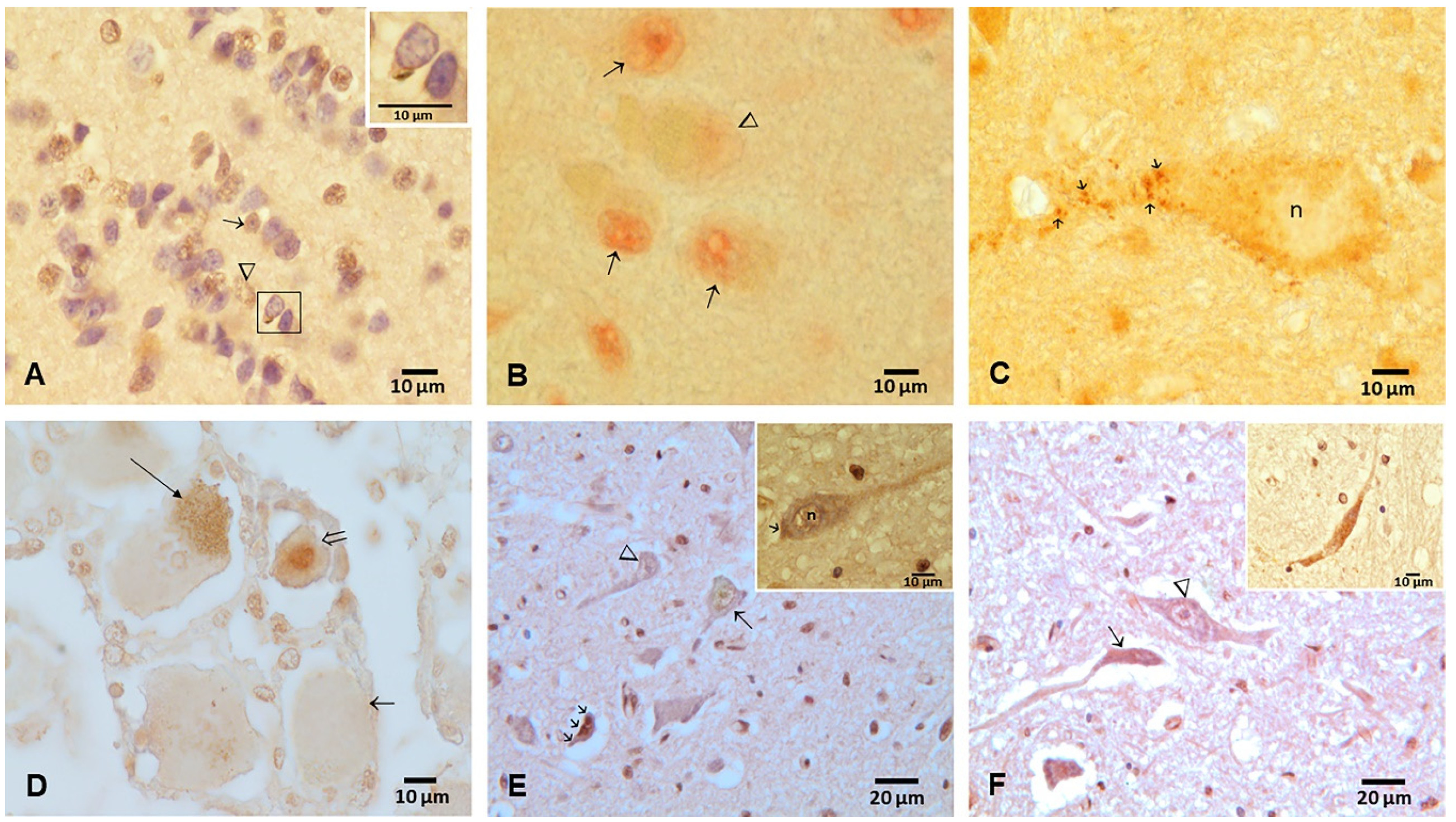
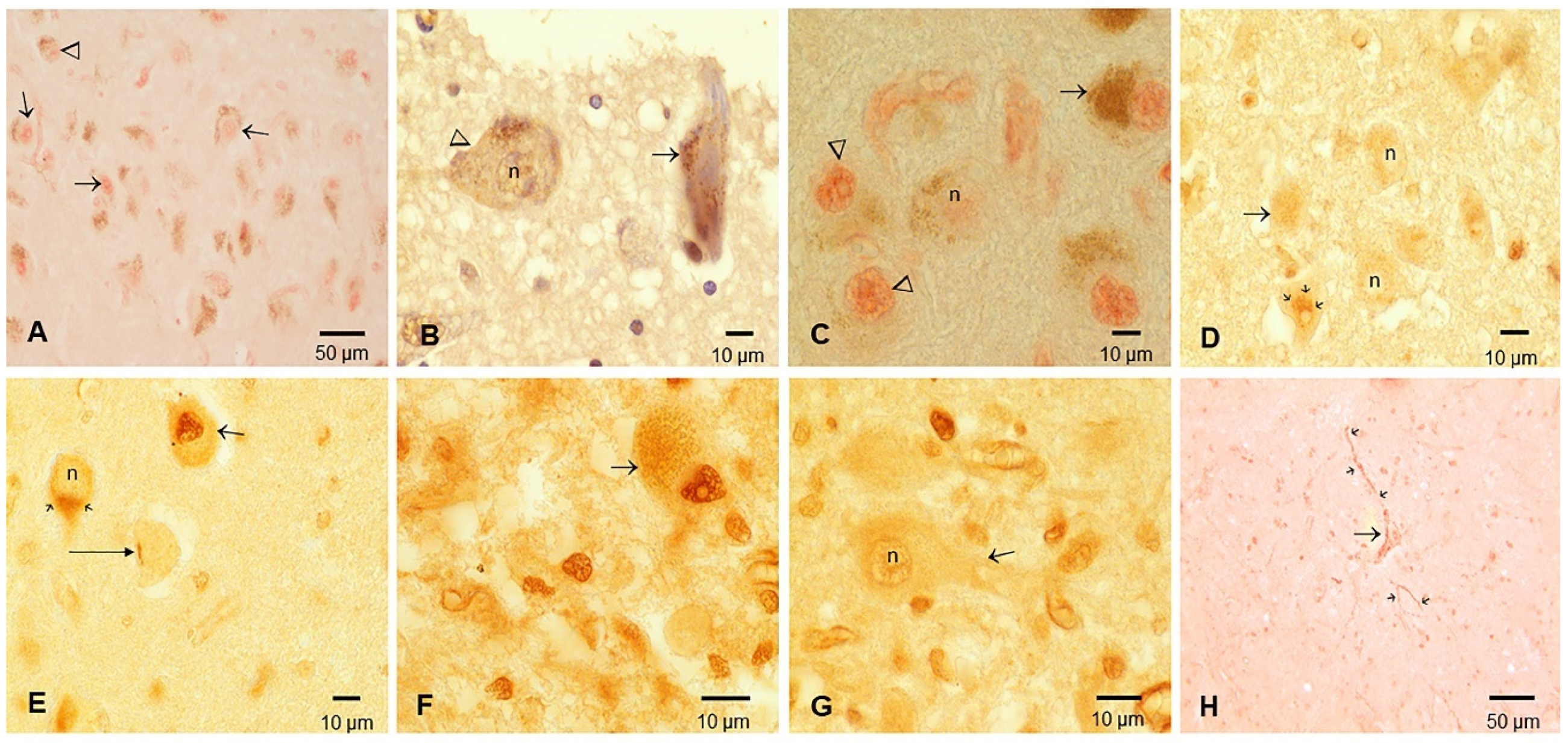
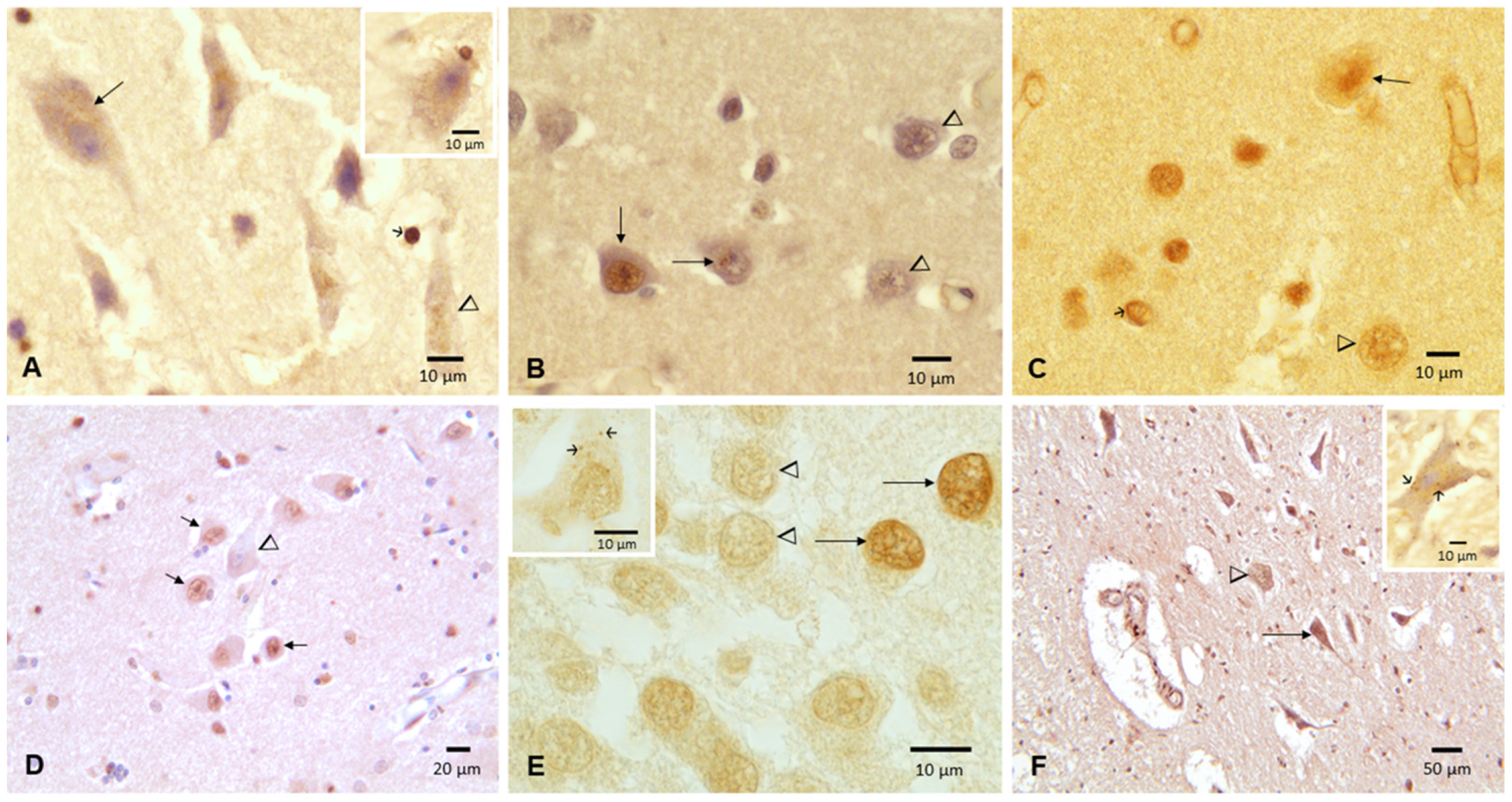
3.2. Cisternal CSF TDP-43 and Brain Pathology
4. Discussion
5. Concluding Remarks
- Particulate matter exposures—specifically, UFPM/NP sustained exposures from utero—are likely key in aberrant neural protein pathology. The CSF TDP-43 results identify logarithmic increases related to age across young megacity urbanites, crucial information in view of the 18% TDP-43 pathology reported in 202 forensic MMC autopsies aged 27.29 ± 11.8 y and the overlap of aberrant hyperphosphorylated tau, beta amyloid, α synuclein and TDP-43. These data are striking in view of the work of Karanth et al. [71] and Carlos et al. [113]. Karanth and coworkers pointed out that quadruple misfolded proteins, including tau neurofibrillary tangles, amyloid-β [Aβ], α-synuclein and TDP-43, in the same brain are relatively common in aging. Moreover, dementia frequency was highest among those with quadruple misfolded proteins [71]. Carlos et al. [113] made a calculation of the frequency and distribution of TDP-43 pathology in 1072 cases, average age 87 years, with AD and TDP-43 pathology and antemortem cognitive studies, including 58% with dementia, 15% with mild cognitive impairment and 27% who were cognitively intact. Carlos and colleagues showed a linear increase in TDP-43 pathology with age: 30% of subjects aged 70 had TDP-43 pathology, 42% by age 80 y and 49% by age 90. Strikingly, these cases were white residents in a low-polluted area, reflecting that elderly TDP-43 increases linearly, notwithstanding the low cumulative exposures to environmental air pollutants, in sharp contrast with our logarithmic increases in pediatric and young adults with high UFPM/NP exposures already exhibiting quadruple misfolded protein pathology.
- We identified a significant relationship between cisternal CSF TDP-43, an average of 572 ± 208 pg/mL, and TDP-43 brain pathology. This is particularly serious for highly exposed children, as with the 16 y old boy (1013 pg/mL) who had extensive nonmotor and motor TDP43 pathology. As toxicologists working with forensic colleagues, we suggest that forensic cases are very helpful to explore the extent of brain pathology in a population at large, and taking a cisternal sample is simple and quick.
- Defining early markers of the quadruple aberrant neurodegenerative diseases, including TDP-43 pathology, ought to be the core of our future efforts. MMC residents are showing early clinical symptomatology—including gait and balance alterations, cognitive deficits and MRI volumetric cortical and subcortical abnormalities, all of which may help identify young subjects at higher risk.
- Exposed children and young adults in highly polluted areas need early neuroprotection and multidisciplinary prevention efforts. Control of combustion and friction UFPM sources and engineered NPs (food products, cosmetics, toothpaste, sun protectors, surface disinfectants, paints, e-waste) is becoming increasingly important and urgent to diminish the human and economic costs of a global neurodegenerative epidemic.
- UFPM/NP exposure should be included in any assessment of the neurodegenerative risk profile of exposed individuals. No matter the portal of entry, chronic delivery of exogenous particles to the brain induces oxidative stress and neuroinflammation.
- We have described the overlap of multiple neurodegenerative pathologies; the presence of anthropogenic UFPM in fetal brains; and the early development of AD, PD and TDP-43 pathology, along with their progression and their neuropsychiatric consequences: this body of knowledge resulting from multidisciplinary studies cannot be disregarded by those concerned with public health.
- We urgently need to practice preventive medicine and develop tools to identify children at risk in order to implement neuroprotective strategies. As neurotoxicologists, we ought to define the mechanistic pathways involving complex NPs containing metalloids, metals, organic compounds, plastics, etc., that can cause extensive brain pathology. As physicians, our focus should be protecting the brains of our future citizens and our younger generations, identifying neurotoxic emission sources and being active players in multidisciplinary teams to prevent, ameliorate or halt neurodegenerative diseases.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Calderón-Garcidueñas, L.; Gónzalez-Maciel, A.; Reynoso-Robles, R.; Delgado-Chávez, R.; Mukherjee, P.S.; Kulesza, R.J.; Torres-Jardón, R.; Ávila-Ramírez, J.; Villarreal-Ríos, R. Hallmarks of Alzheimer disease are evolving relentlessly in Metropolitan Mexico City infants, children and young adults. APOE4 carriers have higher suicide risk and higher odds of reaching NFT stage V at ≤40 years of age. Environ. Res. 2018, 164, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Reynoso-Robles, R.; Hammond, J.; Kulesza, R.; Lachmann, I.; Torres-Jardón, R.; Mukherjee, P.S.; Maher, B.A. Quadruple abnormal protein aggregates in brainstem pathology and exogenous metal-rich magnetic nanoparticles (and engineered Tirich nanorods). The substantia nigrae is a very early target in young urbanites and the gastrointestinal tract a key brainstem portal. Environ. Res. 2020, 191, 110139. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Pérez-Calatayud, A.; González-Maciel, A.; Reynoso-Robles, R.; Silva-Pereyra, H.G.; Ramos-Morales, A.; Torres-Jardón, R.; Soberanes-Cerino, C.D.J.; Carrillo-Esper, R.; Briones-Garduño, J.C.; et al. Environmental Nanoparticles Reach Human Fetal Brains. Biomedicines 2022, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Ayala, A. Air Pollution, Ultrafine Particles, and Your Brain: Are Combustion Nanoparticle Emissions and Engineered Nanoparticles Causing Preventable Fatal Neurodegenerative Diseases and Common Neuropsychiatric Outcomes? Environ. Sci. Technol. 2022, 56, 6847–6856. [Google Scholar] [CrossRef]
- Ehsanifar, M.; Yavari, Z.; Rafati, M. Exposure to urban air pollution particulate matter: Neurobehavioral alteration and hippocampal inflammation. Environ. Sci. Pollut. Res. 2022, 29, 50856–50866. [Google Scholar] [CrossRef]
- Jung, C.R.; Lin, Y.T.; Hwang, B. Ozone, particulate matter, and newly diagnosed Alzheimer’s disease: A population-based cohort study in Taiwan. J. Alzheimers Dis. 2015, 44, 573–584. [Google Scholar] [CrossRef]
- Lee, P.-C.; Liu, L.-L.; Sun, Y.; Chen, Y.-A.; Liu, C.-C.; Li, C.-Y.; Yu, H.-L.; Ritz, B. Traffic-related air pollution increased the risk of Parkinson’s disease in Taiwan: A nationwide study. Environ. Int. 2016, 96, 75–81. [Google Scholar] [CrossRef]
- Chen, H.; Kwong, J.C.; Copes, R.; Tu, K.; Villeneuve, P.J.; van Donkelaar, A.; Hystad, P.; Martin, R.V.; Murray, B.J.; Jessiman, B.; et al. Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: A population-based cohort study. Lancet 2017, 389, 718–726. [Google Scholar] [CrossRef]
- Shi, L.; Steenland, K.; Li, H.; Liu, P.; Zhang, Y.; Lyles, R.H.; Requia, W.J.; Ilango, S.D.; Chang, H.H.; Wingo, T.; et al. A national cohort study (2000–2018) of long-term air pollution exposure and incident dementia in older adults in the United States. Nat. Commun. 2021, 12, 6754. [Google Scholar] [CrossRef]
- Mortamais, M.; Gutierrez, L.-A.; de Hoogh, K.; Chen, J.; Vienneau, D.; Carrière, I.; Letellier, N.; Helmer, C.; Gabelle, A.; Mura, T.; et al. Long-term exposure to ambient air pollution and risk of dementia: Results of the prospective Three-City Study. Environ. Int. 2021, 148, 106376. [Google Scholar] [CrossRef]
- Russ, T.C.; Cherrie, M.P.; Dibben, C.; Tomlinson, S.; Reis, S.; Dragosits, U.; Vieno, M.; Beck, R.; Carnell, E.; Shortt, N.K.; et al. Life Course Air Pollution Exposure and Cognitive Decline: Modelled Historical Air Pollution Data and the Lothian Birth Cohort 1936. J. Alzheimer’s Dis. 2021, 79, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Gładka, A.; Rymaszewska, J.; Zatoński, T. Impact of air pollution on depression and suicide. Int. J. Occup. Med. Environ. Health 2018, 31, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Petkus, A.J.; Resnick, S.M.; Wang, X.; Beavers, D.P.; Espeland, M.A.; Gatz, M.; Gruenewald, T.; Millstein, J.; Chui, H.C.; Kaufman, J.D.; et al. Ambient air pollution exposure and increasing depressive symptoms in older women: The mediating role of the prefrontal cortex and insula. Sci. Total Environ. 2022, 823, 153642. [Google Scholar] [CrossRef]
- Nguyen, A.-M.; Malig, B.J.; Basu, R. The association between ozone and fine particles and mental health-related emergency department visits in California, 2005–2013. PLoS ONE 2021, 16, e0249675. [Google Scholar] [CrossRef] [PubMed]
- Hautekiet, P.; Saenen, N.D.; Demarest, S.; Keune, H.; Pelgrims, I.; Van der Heyden, J.; De Clercq, E.M.; Nawrot, T.S. Air pollution in association with mental and self-rated health and the mediating effect of physical activity. Environ. Health 2022, 21, 29. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Mora-Tiscareño, A.; Ontiveros, E.; Gómez-Garza, G.; Barragán-Mejía, G.; Broadway, J. Air pollution, cognitive deficits and brain abnormalities: A pilot study with children and dogs. Brain Cogn. 2008, 68, 117–127. [Google Scholar] [CrossRef]
- Porta, D.; Narduzzi, S.; Badaloni, C.; Bucci, S.; Cesaroni, G.; Colelli, V.; Davoli, M.; Sunyer, J.; Zirro, E.; Schwartz, J.; et al. Air pollution and cognitive development at age seven in a prospective Italian birth cohort. Epidemiology 2015, 27, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Kicinski, M.; Vermeir, G.; Van Larebeke, N.; Hond, E.D.; Schoeters, G.; Bruckers, L.; Sioen, I.; Bijnens, E.; Roels, H.A.; Baeyens, W.; et al. Neurobehavioral performance in adolescents is inversely associated with traffic exposure. Environ. Int. 2014, 75, 136–143. [Google Scholar] [CrossRef]
- Beckwith, T.; Cecil, K.; Altaye, M.; Severs, R.; Wolfe, C.; Percy, Z.; Maloney, T.; Yolton, K.; Lemasters, G.; Brunst, K.; et al. Reduced gray matter volume and cortical thickness associated with traffic-related air pollution in a longitudinally studied pediatric cohort. PLoS ONE 2020, 15, e0228092. [Google Scholar] [CrossRef]
- Carter, S.A.; Rahman, M.; Lin, J.C.; Shu, Y.-H.; Chow, T.; Yu, X.; Martinez, M.P.; Eckel, S.P.; Chen, J.-C.; Chen, Z.; et al. In utero exposure to near-roadway air pollution and autism spectrum disorder in children. Environ. Int. 2021, 158, 106898. [Google Scholar] [CrossRef]
- Gartland, N.; Aljofi, H.E.; Dienes, K.; Munford, L.A.; Theakston, A.L.; van Tongeren, M. The Effects of Traffic Air Pollution in and around Schools on Executive Function and Academic Performance in Children: A Rapid Review. Int. J. Environ. Res. Public Health 2022, 19, 749. [Google Scholar] [CrossRef] [PubMed]
- Maitre, L.; Julvez, J.; López-Vicente, M.; Warembourg, C.; Tamayo-Uria, I.; Philippat, C.; Gützkow, K.B.; Guxens, M.; Andrusaityte, S.; Basagaña, X.; et al. Early-life environmental exposure determinants of child behavior in Europe: A longitudinal, population-based study. Environ. Int. 2021, 153, 106523. [Google Scholar] [CrossRef] [PubMed]
- Ritz, B.; Yan, Q.; He, D.; Wu, J.; Walker, D.I.; Uppal, K.; Jones, D.P.; Heck, J.E. Child serum metabolome and traffic-related air pollution exposure in pregnancy. Environ. Res. 2021, 203, 111907. [Google Scholar] [CrossRef] [PubMed]
- Stone, V.; Miller, M.R.; Clift, M.J.D.; Elder, A.; Mills, N.L.; Møller, P.; Schins, R.P.F.; Vogel, U.; Kreyling, W.G.; Jensen, K.A.; et al. Nanomaterials versus ambient ultrafine particles: An op-portunity to exchange toxicology knowledge. Environ. Health Perspect. 2017, 125, 106002. [Google Scholar] [CrossRef] [PubMed]
- Maher, B.A.; Ahmed, I.A.M.; Karloukovski, V.; MacLaren, D.A.; Foulds, P.G.; Allsop, D.; Mann, D.M.A.; Torres-Jardón, R.; Calderon-Garciduenas, L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. USA 2016, 113, 10797–10801. [Google Scholar] [CrossRef]
- Ayala, A. Ultrafine Particles and Air Pollution Policy. In Ambient Combustion Ultrafine Particles and Health; Brugge, D., Fuller, C.H., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2021; ISBN 978-1-53618-831-8. [Google Scholar]
- Javdani, N.; Rahpeyma, S.S.; Ghasemi, Y.; Raheb, J. Effect of superparamagnetic nanoparticles coated with various electric charges on α-synuclein and β-amyloid proteins fibrillation process. Int. J. Nanomed. 2019, 14, 799–808. [Google Scholar] [CrossRef]
- Mohammadi, S.; Nikkhah, M. TiO2 Nanoparticles as Potential Promoting Agents of Fibrillation of α-Synuclein, a Parkinson’s Disease-Related Protein. Iran. J. Biotechnol. 2017, 15, 87–94. [Google Scholar] [CrossRef]
- Yarjanli, Z.; Ghaedi, K.; Esmaeili, A.; Rahgozar, S.; Zarrabi, A. Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neurosci. 2017, 18, 51. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Cross, J.V.; Franco-Lira, M.; Aragón-Flores, M.; Kavanaugh, M.; Torres-Jardón, R.; Chao, C.K.; Thompson, C.; Chang, J.; Zhu, H.; et al. Brain immune interactions and air pollution: Macrophage inhibitory factor (MIF), prion cellular protein (PrPc), interleukin-6 (IL6), interleukin 1 receptor antagonist (IL-1Ra), and interleukin-2 (IL-2) in cerebrospinal fluid and MIF in serum differentiate urban children exposed to severe vs. low air pollution. Front Neurosci. 2013, 7, 183–194. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Chao, C.; Thompson, C.; Rodríguez-Díaz, J.; Franco-Lira, M.; Mukherjee, P.S.; Perry, G. CSF biomarkers: Low amyloid β 1-42 and BDNF and High IFNϒ differentiate children exposed to Mexico City High air pollution v controls. Alzheimer’s disease uncertainties. Alzheimer’s Dis. Parkinsonism 2015, 5, 2. [Google Scholar]
- Calderón-Garcidueñas, L.; Avila-Ramírez, J.; González-Heredia, T.; Acuña-Ayala, H.; Chao, C.-K.; Thompson, C.; Ruiz-Ramos, R.; Cortés-González, V.; Martínez-Martínez, L.; García-Pérez, M.A.; et al. Cerebrospinal Fluid Biomarkers in Highly Exposed PM2.5 Urbanites: The Risk of Alzheimer’s and Parkinson’s Diseases in Young Mexico City Residents. J. Alzheimer’s Dis. 2016, 54, 597–613. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Mukherjee, P.S.; Waniek, K.; Holzer, M.; Chao, C.-K.; Thompson, C.; Ruiz-Ramos, R.; Franco-Lira, M.; Reynoso-Robles, R.; Gónzalez-Maciel, A.; et al. Non-Phosphorylated Tau in Cerebrospinal Fluid is a Marker of Alzheimer’s Disease Continuum in Young Urbanites Exposed to Air Pollution. J. Alzheimer’s Dis. 2018, 66, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Vojdani, A.; Blaurock-Busch, E.; Busch, Y.; Friedle, A.; Franco-Lira, M.; Sarathi-Mukherjee, P.; Martínez-Aguirre, X.; Park, S.; Torres-Jardón, R.; et al. Air pollution and children: Neural and tight junction antibodies and combustion metals, the role of barrier breakdown and brain immunity in neurodegeneration. J. Alzheimer. Dis. 2015, 43, 1039–1058. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Serrano-Sierra, A.; Torres-Jardón, R.; Zhu, H.; Yuan, Y.; Smith, D.; Delgado-Chávez, R.; Cross, J.V.; Medina-Cortina, H.; Kavanaugh, M.; et al. The impact of environmental metals in young urbanites’ brains. Exp. Toxicol. Pathol. 2013, 65, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Reiber, H. Non-linear ventriculo–Lumbar protein gradients validate the diffusion-flow model for the blood-CSF barrier. Clin. Chim. Acta 2020, 513, 64–67. [Google Scholar] [CrossRef]
- Reiber, H. Blood-cerebrospinal fluid (CSF) barrier dysfunction means reduced CSF flow not barrier leakage-conclusions from CSF protein data. Arq. De Neuro-Psiquiatr. 2021, 79, 56–67. [Google Scholar] [CrossRef]
- Peyron, P.-A.; Hirtz, C.; Baccino, E.; Ginestet, N.; Tiers, L.; Martinez, A.Y.; Lehmann, S.; Delaby, C. Tau protein in cerebrospinal fluid: A novel biomarker of the time of death? Int. J. Legal. Med. 2021, 135, 2081–2089. [Google Scholar] [CrossRef]
- Brettschneider, J.; Del Tredici, K.; Toledo, J.B.; Robinson, J.L.; Irwin, D.J.; Grossman, M.; Suh, E.R.; Van Deerlin, V.M.; Wood, E.M.; Baek, Y.; et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 2013, 74, 20–38. [Google Scholar] [CrossRef]
- Braak, H.; Del Tredici, K. Anterior Cingulate Cortex TDP-43 Pathology in Sporadic Amyotrophic Lateral Sclerosis. J. Neuropathol. Exp. Neurol. 2017, 77, 74–83. [Google Scholar] [CrossRef]
- James, B.D.; Wilson, R.S.; Boyle, P.A.; Trojanowski, J.Q.; Bennett, D.A.; Schneider, J.A. TDP-43 stage, mixed pathol-ogies, and clinical Alzheimer’s-type dementia. Brain 2016, 139, 2983–2993. [Google Scholar] [CrossRef]
- Nelson, P.T.; Dickson, D.W.; Trojanowski, J.Q.; Jack, C.R.; Boyle, P.A.; Arfanakis, K.; Rademakers, R.; Alafuzoff, I.; Attems, J.; Brayne, C.; et al. Limbic-predominant age-related TDP-43 encephalopathy (LATE): Consensus working group report. Brain 2019, 142, 1503–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamshidi, P.; Kim, G.; Shahidehpour, R.K.; Bolbolan, K.; Gefen, T.; Bigio, E.H.; Mesulam, M.-M.; Geula, C. Distribution of TDP-43 Pathology in Hippocampal Synaptic Relays Suggests Transsynaptic Propagation in Frontotemporal Lobar Degeneration. J. Neuropathol. Exp. Neurol. 2020, 79, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Geula, C.; Keszycki, R.; Jamshidi, P.; Kawles, A.; Minogue, G.; Flanagan, M.E.; Zaccard, C.R.; Mesulam, M.M.; Gefen, T. Propagation of TDP-43 proteinopathy in neurodegenerative disorders. Neural Regen. Res. 2022, 17, 1498. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Rajkumar, R.P.; Stommel, E.W.; Kulesza, R.; Mansour, Y.; Rico-Villanueva, A.; Flores-Vázquez, J.O.; Brito-Aguilar, R.; Ramírez-Sánchez, S.; García-Alonso, G.; et al. Brain-stem Quadruple Aberrant Hyperphosphorylated Tau, Beta-Amyloid, Alpha-Synuclein and TDP-43 Pathology, Stress and Sleep Behavior Disorders. Int. J. Environ. Res. Public Health 2021, 18, 6689. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; González-Maciel, A.; Reynoso-Robles, R.; Silva-Pereyra, H.G.; Torres-Jardón, R.; Brito-Aguilar, R.; Ayala, A.; Stommel, E.W.; Delgado-Chávez, R. Environmentally Toxic Solid Nanoparticles in Noradrenergic and Dopaminergic Nuclei and Cerebellum of Metropolitan Mexico City Children and Young Adults with Neural Quadruple Misfolded Protein Pathologies and High Exposures to Nano Particulate Matter. Toxics 2022, 10, 164. [Google Scholar] [CrossRef]
- Garcia-Gomar, M.G.; Videnovic, A.; Singh, K.; Stauder, M.; Lewis, L.D.; Wald, L.L.; Rosen, B.R.; Bianciardi, M. Disruption of Brain-stem Structural Connectivity in REM Sleep Behavior Disorder Using 7 Tesla Magnetic Resonance Imaging. Mov. Disord. 2022, 37, 847–853. [Google Scholar] [CrossRef]
- Singh, K.; Cauzzo, S.; García-Gomar, M.G.; Stauder, M.; Vanello, N.; Passino, C.; Bianciardi, M. Functional connectome of arousal and motor brainstem nuclei in living humans by 7 Tesla resting-state fMRI. NeuroImage 2022, 249, 118865. [Google Scholar] [CrossRef]
- Llibre-Guerra, J.J.; Behrens, M.I.; Hosogi, M.L.; Montero, L.; Torralva, T.; Custodio, N.; Longoria-Ibarrola, E.M.; Giraldo-Chica, M.; Aguillón, D.; Hardi, A.; et al. Frontotemporal Dementias in Latin America: History, Epidemiology, Genetics, and Clinical Research. Front. Neurol. 2021, 12, 710332. [Google Scholar] [CrossRef]
- Cervantes-Aragón, I.; Ramírez-García, S.A.; Baltazar-Rodríguez, L.M.; García-Cruz, D.; Castañeda-Cisneros, G. Genetic approach in amyotrophic lateral sclerosis. Gac. Med. Mex. 2020, 155, 475–482. [Google Scholar] [CrossRef]
- Bos, M.V.D.; Geevasinga, N.; Higashihara, M.; Menon, P.; Vucic, S. Pathophysiology and Diagnosis of ALS: Insights from Advances in Neurophysiological Techniques. Int. J. Mol. Sci. 2019, 20, 2818. [Google Scholar] [CrossRef]
- Barker, M.S.; Gottesman, R.T.; Manoochehri, M.; Chapman, S.; Appleby, B.S.; Brushaber, D.; Devick, K.L.; Dickerson, B.C.; Domoto-Reilly, K.; Fields, J.A.; et al. Proposed research criteria for prodromal behavioural variant frontotemporal dementia. Brain 2022, 145, 1079–1097. [Google Scholar] [CrossRef] [PubMed]
- Caudillo, L.; Salcedo, D.; Peralta, O.; Castro, T.; Alvarez-Ospina, H. Nanoparticle size distributions in Mexico city. Atmos. Pollut. Res. 2019, 11, 78–84. [Google Scholar] [CrossRef]
- Velasco, E.; Retama, A.; Segovia, E.; Ramos, R. Particle exposure and inhaled dose while commuting by public transport in Mexico City. Atmos. Environ. 2019, 219, 117044. [Google Scholar] [CrossRef]
- Mugica-Álvarez, V.; Figueroa-Lara, J.; Romero-Romo, M.; Sepúlveda-Sánchez, J.; López-Moreno, T. Concentrations and properties of airborne particles in the Mexico City subway system. Atmos. Environ. 2012, 49, 284–293. [Google Scholar] [CrossRef]
- Hernández-López, A.E.; Miranda Martín del Campo, J.; Mugica Álvarez, V.; Valle-Hernández, B.L.; Mejía-Ponce, L.V.; Pine-da-Santamaría, J.C.; Reynoso-Cru, S.; Mendoza-Flores1, J.A.; Rozanes-Valenz, D. A study of PM2.5 elemental composition in southwest Mexico City and development of receptor models with positive matrix factorization. Rev. Int. Contam. Ambie. 2021, 37, 67–88. [Google Scholar] [CrossRef]
- Molina, L.T.; Velasco, E.; Retama, A.; Zavala, M. Experience from Integrated Air Quality Management in the Mexico City Metropolitan Area and Singapore. Atmosphere 2019, 10, 512. [Google Scholar] [CrossRef]
- Zavala, M.; Brune, W.H.; Velasco, E.; Retama, A.; Cruz-Alavez, L.A.; Molina, L.T. Changes in ozone production and VOC reactivity in the atmosphere of the Mexico City Metropolitan Area. Atmos. Environ. 2020, 238, 117747. [Google Scholar] [CrossRef]
- Velasco, E.; Retama, A. Ozone’s threat hits back Mexico City. Sustain. Cities Soc. 2017, 31, 260–263. [Google Scholar] [CrossRef]
- Dunn, M.J.; Jiménez, J.-L.; Baumgardner, D.; Castro, T.; McMurry, P.H.; Smith, J.N. Measurements of Mexico City nanoparticle size distributions: Observations of new particle formation and growth. Geophys. Res. Lett. 2004, 31. [Google Scholar] [CrossRef]
- Kleinman, L.I.; Springston, S.R.; Wang, J.; Daum, P.H.; Lee, Y.N.; Nunnermacker, L.J.; Senum, G.I.; Weinstein-Lloyd, J.; Alexander, M.L.; Hubbe, J.; et al. The time evolution of aerosol size distribution over the Mexico City plateau. Atmos. Chem. Phys. 2009, 9, 4261–4278. [Google Scholar] [CrossRef]
- Morton-Bermea, O.; Garza-Galindo, R.; Hernández-Álvarez, E.; Ordoñez-Godínez, S.L.; Amador-Muñoz, O.; Beramendi-Orosco, L.; Miranda, J.; Rosas-Pérez, I. Atmospheric PM2.5 Mercury in the Metropolitan Area of Mexico City. Bull. Environ. Contam. Toxicol. 2018, 100, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Morawska, L.; Birmili, W.; Paasonen, P.; Hu, M.; Kulmala, M.; Harrison, R.M.; Norford, L.; Britter, R. Ultrafine particles in cities. Environ. Int. 2014, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mugica, V.; Hernández, S.; Torres, M.; García, R. Seasonal Variation of Polycyclic Aromatic Hydrocarbon Exposure Levels in Mexico City. J. Air Waste Manag. Assoc. 2010, 60, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Cárdenas, B.; Alberto, A.; Benitez, S.; González, E.H.; Jaimes, M.; Retama, A. Ultrafine Particles in Mexico City Metropolitan Area: A review. In Proceedings of the 7th International Symposium on Ultrafine Particles, Air Quality and Climate, Brüssel, Belgien, 15–16 May 2019. [Google Scholar]
- Steinacker, P.; Hendrich, C.; Sperfeld, A.D.; Jesse, S.; von Arnim, C.A.F.; Lehnert, S.; Pabst, A.; Uttner, I.; Tumani, H.; Lee, V.M.-Y.; et al. TDP-43 in Cerebrospinal Fluid of Patients With Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis. Arch. Neurol. 2008, 65, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Pandya, S.; Maia, P.D.; Freeze, B.; Menke, R.A.L.; Talbot, K.; Turner, M.R.; Raj, A. Modeling seeding and neuroanatomic spread of pathology in amyotrophic lateral sclerosis. NeuroImage 2022, 251, 118968. [Google Scholar] [CrossRef]
- Hasan, R.; Humphrey, J.; Bettencourt, C.; Newcombe, J.; NYGC ALS Consortium; Lashley, T.; Fratta, P.; Raj, T. Transcriptomic analysis of frontotemporal lobar degeneration with TDP-43 pathology reveals cellular alterations across multiple brain regions. Acta Neuropathol. 2022, 143, 383–401. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Garcidueñas, L.; Hernández-Luna, J.; Mukherjee, P.S.; Styner, M.; Chávez-Franco, D.A.; Luévano-Castro, S.C.; Crespo-Cortés, C.N.; Stommel, E.W.; Torres-Jardón, R. Hemispheric Cortical, Cerebellar and Caudate Atrophy Associated to Cognitive Impairment in Metropolitan Mexico City Young Adults Exposed to Fine Particulate Matter Air Pollution. Toxics 2022, 10, 156. [Google Scholar] [CrossRef]
- Shafiei, G.; Bazinet, V.; Dadar, M.; Manera, A.L.; Collins, D.L.; Dagher, A.; Borroni, B.; Sanchez-Valle, R.; Moreno, F.; Laforce, R.; et al. Network structure and transcriptomic vulnerability shape atrophy in frontotemporal dementia. Brain 2022, awac069. [Google Scholar] [CrossRef]
- Karanth, S.; Nelson, P.T.; Katsumata, Y.; Kryscio, R.J.; Schmitt, F.A.; Fardo, D.W.; Cykowski, M.D.; Jicha, G.A.; Van Eldik, L.J.; Abner, E.L. Prevalence and Clinical Phenotype of Quadruple Misfolded Proteins in Older Adults. JAMA Neurol. 2020, 77, 1299–1307. [Google Scholar] [CrossRef]
- Scarioni, M.; Gami-Patel, P.; Peeters, C.F.W.; de Koning, F.; Seelaar, H.; Mol, M.O.; van Swieten, J.C.; Rozemuller, A.J.M.; Hoozemans, J.J.M.; Pijnenburg, Y.A.L.; et al. Psychiatric symptoms of frontotemporal dementia and subcortical (co-)pathology burden: New insights. Brain 2022, awac043. [Google Scholar] [CrossRef]
- Ohm, D.T.; Cousins, K.A.Q.; Xie, S.X.; Peterson, C.; McMillan, C.T.; Massimo, L.; Raskovsky, K.; Wolk, D.A.; Van Deerlin, V.M.; Elman, L.; et al. Signature laminar distributions of pathology in frontotemporal lobar degeneration. Acta Neuropathol. 2022, 143, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Tisdall, M.D.; Ohm, D.T.; Lobrovich, R.; Das, S.R.; Mizsei, G.; Prabhakaran, K.; Ittyerah, R.; Lim, S.; McMillan, C.T.; Wolk, D.A.; et al. Ex vivo MRI and histopathology detect novel iron-rich cortical inflammation in frontotemporal lobar degeneration with tau versus TDP-43 pathology. NeuroImage: Clin. 2021, 33, 102913. [Google Scholar] [CrossRef] [PubMed]
- Bayram, E.; Shan, G.; Cummings, J.L. Associations between Comorbid TDP-43, Lewy Body Pathology, and Neuropsychiatric Symptoms in Alzheimer’s Disease. J. Alzheimers Dis. 2019, 69, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Sennik, S.; Schweizer, T.A.; Fischer, C.E.; Munoz, D.G. Risk Factors and Pathological Substrates Associated with Agita-tion/Aggression in Alzheimer’s Disease: A Preliminary Study using NACC Data. J. Alzheimers Dis. 2017, 55, 1519–1528. [Google Scholar] [CrossRef] [PubMed]
- Kon, T.; Mori, F.; Tanji, K.; Miki, Y.; Nishijima, H.; Nakamura, T.; Kinoshita, I.; Suzuki, C.; Kurotaki, H.; Tomiyama, M.; et al. Accumulation of Nonfibrillar TDP-43 in the Rough En-doplasmic Reticulum Is the Early-Stage Pathology in Amyotrophic Lateral Sclerosis. J. Neuropathol. Exp. Neurol. 2022, 81, 271–281. [Google Scholar] [CrossRef]
- Andrew, A.; Zhou, J.; Gui, J.; Harrison, A.; Shi, X.; Li, M.; Guetti, B.; Nathan, R.; Tischbein, M.; Pioro, E.; et al. Airborne lead and polychlorinated biphenyls (PCBs) are associated with amyotrophic lateral sclerosis (ALS) risk in the U.S. Sci. Total Environ. 2022, 819, 153096. [Google Scholar] [CrossRef] [PubMed]
- Vandenbulcke, M.; Van de Vliet, L.; Sun, J.; Huang, Y.-A.; Bossche, M.J.A.V.D.; Sunaert, S.; Peeters, R.; Zhu, Q.; Vanduffel, W.; de Gelder, B.; et al. A paleo-neurologic investigation of the social brain hypothesis in frontotemporal dementia. Cereb. Cortex 2022, bhac089. [Google Scholar] [CrossRef]
- Lulé, D.; Michels, S.; Finsel, J.; Braak, H.; Del Tredici, K.; Strobel, J.; Beer, A.J.; Uttner, I.; Müller, H.-P.; Kassubek, J.; et al. Clinicoanatomical substrates of selfish behaviour in amyotrophic lateral sclerosis—An observational cohort study. Cortex 2021, 146, 261–270. [Google Scholar] [CrossRef]
- Mendez, M.F. Behavioral Variant Frontotemporal Dementia and Social and Criminal Transgressions. J. Neuropsychiatry Clin. Neurosci. 2022, 21080224. [Google Scholar] [CrossRef]
- Fondevila, G.; Meneses-Reyes, R. Lethal Violence, Childhood, and Gender in Mexico City. Int. Crim. Justice Rev. 2017, 29, 33–47. [Google Scholar] [CrossRef]
- Violencia en México. Available online: http://www.seguridadjusticiaypaz.org.mx/ (accessed on 10 May 2022).
- Mangold, S.A.; Das, J.M. Neuroanatomy, Reticular Formation. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Cauzzo, S.; Singh, K.; Stauder, M.; García-Gomar, M.G.; Vanello, N.; Passino, C.; Staab, J.; Indovina, I.; Bianciardi, M. Functional connectome of brainstem nuclei involved in autonomic, limbic, pain and sensory processing in living humans from 7 Tesla resting state fMRI. NeuroImage 2022, 250, 118925. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Perkins, E.; Zhou, L.; Warren, S.; May, P.J. Reticular Formation Connections Underlying Horizontal Gaze: The Central Mesencephalic Reticular Formation (cMRF) as a Conduit for the Collicular Saccade Signal. Front. Neuroanat. 2017, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Leisman, G.; Melillo, R. Front and center: Maturational dysregulation of frontal lobe functional neuroanatomic connections in attention deficit hyperactivity disorder. Front. Neuroanat. 2022, 16, 936025. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.E.; Spratt, T.J.; Kaplan, G.B. Translational approaches to influence sleep and arousal. Brain Res. Bull. 2022, 185, 140–161. [Google Scholar] [CrossRef]
- Liu, S.; Ye, M.; Pao, G.M.; Song, S.M.; Jhang, J.; Jiang, H.; Kim, J.-H.; Kang, S.J.; Kim, D.-I.; Han, S. Divergent brainstem opioidergic pathways that coordinate breathing with pain and emotions. Neuron 2021, 110, 857–873.e9. [Google Scholar] [CrossRef]
- Bourilhon, J.; Mullie, Y.; Olivier, C.; Cherif, S.; Belaid, H.; Grabli, D.; Czernecki, V.; Karachi, C.; Welter, M.L. Stimulation of the pe-dunculopontine and cuneiform nuclei for freezing of gait and falls in Parkinson disease: Cross-over single-blinded study and long-term follow-up. Parkinsonism Relat Disord 2022, 96, 13–17. [Google Scholar] [CrossRef]
- Burdge, J.; Jhumka, Z.A.; Bravo, I.M.; Abdus-Saboor, I. Taking a deep breath: How a brainstem pathway integrates pain and breathing. Neuron 2022, 110, 739–741. [Google Scholar] [CrossRef]
- Özkan, M.; Köse, B.; Algın, O.; Oğuz, S.; Erden, M.E.; Çavdar, S. Non-motor connections of the pedunculopontine nucleus of the rat and human brain. Neurosci. Lett. 2021, 767, 136308. [Google Scholar] [CrossRef]
- Robinson, D.A. Neurophysiology of the saccadic system: The reticular formation. Prog. Brain Res. 2022, 267, 355–378. [Google Scholar] [CrossRef]
- He, S.; Deli, A.; Fischer, P.; Wiest, C.; Huang, Y.; Martin, S.; Khawaldeh, S.; Aziz, T.Z.; Green, A.L.; Brown, P.; et al. Gait-Phase Modulates Alpha and Beta Oscillations in the Pedunculopontine Nucleus. J. Neurosci. 2021, 41, 8390–8402. [Google Scholar] [CrossRef]
- Singh, K.; Garcia-Gomar, M.G.; Bianciardi, M. Probabilistic Atlas of the Mesencephalic Reticular Formation, Isthmic Reticular Formation, Microcellular Tegmental Nucleus, Ventral Tegmental Area Nucleus Complex, and Caudal-Rostral Linear Raphe Nucleus Complex in Living Humans from 7 Tesla Magnetic Resonance Imaging. Brain Connect 2021, 11, 613–623. [Google Scholar] [PubMed]
- Coulombe, V.; Saikali, S.; Goetz, L.; Takech, M.A.; Philippe, E.; Parent, A.; Parent, M. A Topographic Atlas of the Human Brainstem in the Ponto-Mesencephalic Junction Plane. Front. Neuroanat. 2021, 15, 627656. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Torres-Solorio, A.K.; Kulesza, R.J.; Torres-Jardón, R.; González-González, L.O.; García-Arreola, B.; Chávez-Franco, D.A.; Luévano-Castro, S.C.; Hernández-Castillo, A.; Carlos-Hernández, E.; et al. Gait and balance disturbances are common in young urbanites and associated with cognitive impairment. Air pollution and the historical development of Alzheimer’s disease in the young. Environ. Res. 2020, 191, 110087. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Ludoph, A.; Thal, D.R.; Del Tredeci, K. Amyotrophic lateral sclerosis: Dash-like accumulation of phosphorylated TDP-43 in somatodendritic and axonal compartments of somatomotor neurons of the lower brainstem and spinal cord. Acta Neuropathol. 2010, 120, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Del Tredici, K.; Ludolph, A.C.; Hoozemans, J.; Rozemuller, A.J.; Braak, H.; Knippschild, U. Stages of granulovacuolar degeneration: Their relation to Alzheimer’s disease and chronic stress response. Acta Neuropathol. 2011, 122, 577–589. [Google Scholar] [CrossRef]
- Brettschneider, J.; Arai, K.; Del Tredici, K.; Toledo, J.B.; Robinson, J.L.; Lee, E.B.; Kuwabara, S.; Shibuya, K.; Irwin, D.J.; Fang, L.; et al. TDP-43 pathology and neuronal loss in amyotrophic lateral sclerosis spinal cord. Acta Neuropathol. 2014, 128, 423–437. [Google Scholar] [CrossRef]
- Eisen, A.; Braak, H.; Del Tredici, K.; Lemon, R.; Ludolph, A.C.; Kiernan, M.C. Cortical influences drive amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2017, 88, 917–924. [Google Scholar] [CrossRef]
- Del Tredeci, K.; Braal, H. Neuropathology and neuroanatomy of TDP-43 amyotrophic lateral sclerosis. Curr. Opin. Neurol. 2022, 35, 660–671. [Google Scholar] [CrossRef]
- Šušnjar, U.; Škrabar, N.; Brown, A.-L.; Abbassi, Y.; Phatnani, H.; Fratta, P.; Kwan, J.; Sareen, D.; Broach, J.R.; Simmons, Z.; et al. Cell environment shapes TDP-43 function with implications in neuronal and muscle disease. Commun. Biol. 2022, 5, 314. [Google Scholar] [CrossRef]
- Koper, M.J.; Tomé, S.O.; Gawor, K.; Belet, A.; Van Schoor, E.; Schaeverbeke, J.; Vandenberghe, R.; Vandenbulcke, M.; Ghebremedhin, E.; Otto, M.; et al. LATE-NC aggravates GVD-mediated necroptosis in Alzheimer’s disease. Acta Neuropathol. Commun. 2022, 10, 128. [Google Scholar] [CrossRef]
- Kawles, A.; Nishihira, Y.; Feldman, A.; Gill, N.; Minogue, G.; Keszycki, R.; Coventry, C.; Spencer, C.; Lilek, J.; Ajroud, K.; et al. Cortical and subcortical pathological burden and neuronal loss in an autopsy series of FTLD-TDP-type C. Brain 2021, 145, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Sainouchi, M.; Tada, M.; Fitrah, Y.A.; Hara, N.; Tanaka, K.; Idezuka, J.; Aida, I.; Nakajima, T.; Miyashita, A.; Akazawa, K.; et al. Brain TDP-43 pathology in corticobasal degeneration: Topographical correlation with neuronal loss. Neuropathol. Appl. Neurobiol. 2021, 48, e12786. [Google Scholar] [CrossRef]
- Aizawa, H.; Teramoto, S.; Hideyama, T.; Kato, H.; Terashi, H.; Suzuki, Y.; Kimura, T.; Kwak, S. Nuclear pore destruction and loss of nuclear TDP-43 in FUS mutation-related amyotrophic lateral sclerosis motor neurons. J. Neurol. Sci. 2022, 436, 120187. [Google Scholar] [CrossRef]
- Keating, S.S.; Gil, R.S.; Swanson, M.E.; Scotter, E.L.; Walker, A.K. TDP-43 pathology: From noxious assembly to therapeutic removal. Prog. Neurobiol. 2022, 211, 102229. [Google Scholar] [CrossRef] [PubMed]
- Altman, T.; Ionescu, A.; Ibraheem, A.; Priesmann, D.; Gradus-Pery, T.; Farberov, L.; Alexandra, G.; Shelestovich, N.; Dafinca, R.; Shomron, N.; et al. Axonal TDP-43 condensates drive neuromuscular junction disruption through inhibition of local synthesis of nuclear encoded mitochondrial proteins. Nat. Commun. 2021, 12, 6914. [Google Scholar] [CrossRef] [PubMed]
- Homma, H.; Tanaka, H.; Jin, M.; Jin, X.; Huang, Y.; Yoshioka, Y.; Bertens, C.J.; Tsumaki, K.; Kondo, K.; Shiwaku, H.; et al. DNA damage in embryonic neural stem cell determines FTLDs’ fate via early-stage neuronal necrosis. Life Sci. Alliance 2021, 4, e202101022. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.M.; Halliday, G.; Hodges, J.R. Hypothalamic symptoms of frontotemporal dementia disorders. Handb. Clin. Neurol. 2021, 182, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, E.; De Marchi, F.; Contaldi, E.; Dianzani, U.; Cantello, R.; Mazzini, L.; Comi, C. The Role of Tau beyond Alzheimer’s Disease: A Narrative Review. Biomedicines 2022, 10, 760. [Google Scholar] [CrossRef]
- Carlos, A.F.; Tosakulwong, N.; Weigand, S.D.; Boeve, B.F.; Knopman, D.S.; Petersen, R.C.; Nguyen, A.; Reichard, R.R.; Murray, M.E.; Dickson, D.W.; et al. Frequency and distribution of TAR DNA-binding protein 43 (TDP-43) pathology increase linearly with age in a large cohort of older adults with and without dementia. Acta Neuropathol. 2022, 144, 159–160. [Google Scholar] [CrossRef]
- Esteban-García, N.; Fernández-Beltrán, L.C.; Godoy-Corchuelo, J.M.; Ayala, J.L.; Matias-Guiu, J.A.; Corrochano, S. Body Complexion and Circulating Lipids in the Risk of TDP-43 Related Disorders. Front. Aging Neurosci. 2022, 14, 838141. [Google Scholar] [CrossRef]
- Zetterberg, H. Biofluid-based biomarkers for Alzheimer’s disease-related pathologies: An update and synthesis of the literature. Alzheimers Dement 2022, 18, 1687–1693. [Google Scholar] [CrossRef] [PubMed]
- Körtvelyessy, P.; Heinze, H.J.; Prudlo, J.; Bittner, D. CSF Biomarkers of Neurodegeneration in Progressive Non-fluent Aphasia and Other Forms of Frontotemporal Dementia: Clues for Pathomechanisms? Front. Neurol. 2018, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, M.; Galimberti, D.; Elias, N.; Boonkamp, L.; Pijnenburg, Y.A.; van Swieten, J.C.; Watts, K.; Paciotti, S.; Beccari, T.; Hu, W.; et al. Novel CSF biomarkers to discriminate FTLD and its pathological subtypes. Ann. Clin. Transl. Neurol. 2018, 5, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Molinuevo, J.L.; Ayton, S.; Batrla, R.; Bednar, M.M.; Bittner, T.; Cummings, J.; Fagan, A.M.; Hampel, H.; Mielke, M.M.; Mikulskis, A.; et al. Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol. 2018, 136, 821–853. [Google Scholar] [CrossRef] [PubMed]
- Foiani, M.S.; Cicognola, C.; Ermann, N.; Woollacott, I.O.C.; Heller, C.; Heslegrave, A.J.; Keshavan, A.; Paterson, R.W.; Ye, K.; Kornhuber, J.; et al. Searching for novel cerebrospinal fluid biomarkers of tau pathology in frontotemporal dementia: An elusive quest. J. Neurol. Neurosurg. Psychiatry 2019, 90, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Khosla, R.; Rain, M.; Sharma, S.; Anand, A. Amyotrophic Lateral Sclerosis (ALS) prediction model derived from plasma and CSF biomarkers. PLoS ONE 2021, 16, e0247025. [Google Scholar] [CrossRef] [PubMed]
- Escal, J.; Fourier, A.; Formaglio, M.; Zimmer, L.; Bernard, E.; Mollion, H.; Bost, M.; Herrmann, M.; Ollagnon-Roman, E.; Quadrio, I.; et al. Comparative diagnosis interest of NfL and pNfH in CSF and plasma in a context of FTD–ALS spectrum. J. Neurol. 2021, 269, 1522–1529. [Google Scholar] [CrossRef]
- Chouliaras, L.; Thomas, A.; Malpetti, M.; Donaghy, P.; Kane, J.; Mak, E.; Savulich, G.; Prats-Sedano, M.A.; Heslegrave, A.J.; Zetterberg, H.; et al. Differential levels of plasma biomarkers of neurodegeneration in Lewy body dementia, Alzheimer’s disease, frontotemporal dementia and progressive supranuclear palsy. J. Neurol. Neurosurg. Psychiatry 2022, 93, 651–658. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Mukherjee, P.S.; Kulesza, R.J.; Torres-Jardón, R.; Hernández-Luna, J.; Ávila-Cervantes, R.; Macías-Escobedo, E.; González-González, O.; González-Maciel, A.; García-Hernández, K.; et al. Mild Cognitive Impairment and Dementia Involving Multiple Cognitive Domains in Mexican Urbanites. J. Alzheimers Dis. 2019, 68, 1113–1123. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; Chávez-Franco, D.A.; Luévano-Castro, S.C.; Macías-Escobedo, E.; Hernández-Castillo, A.; Carlos-Hernández, E.; Franco-Ortíz, A.; Castro-Romero, S.P.; Cortés-Flores, M.; Crespo-Cortés, C.N.; et al. Metals, Nanoparticles, Particulate Matter, and Cognitive Decline. Front. Neurol. 2022, 12, 794071. [Google Scholar] [CrossRef]
- Calderón-Garcidueñas, L.; González-González, L.O.; Kulesza, R.J.; Fech, T.M.; Pérez-Guillé, G.; Luna, M.A.J.-B.; Soriano-Rosales, R.E.; Solorio, E.; Miramontes-Higuera, J.D.J.; Chew, A.G.-M.; et al. Exposures to fine particulate matter (PM2.5) and ozone above USA standards are associated with auditory brainstem dysmorphology and abnormal auditory brainstem evoked potentials in healthy young dogs. Environ. Res. 2017, 158, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Milà-Alomà, M.; Suárez-Calvet, M.; Molinuevo, J.L. Latest advances in cerebrospinal fluid and blood biomarkers of Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419888819. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, D.S.; Ashton, N.J.; Blennow, K.; Zetterberg, H.; Simrén, J.; Lantero-Rodriguez, J.; Karikari, T.K.; Hiniker, A.; Rissman, R.A.; Salmon, D.P.; et al. Plasma biomarkers for Alz-heimer’s Disease in relation to neuropathology and cognitive change. Acta Neuropathol. 2022, 143, 487–503. [Google Scholar] [CrossRef] [PubMed]
- González, A.C.; Irwin, D.J.; Alcolea, D.; McMillan, C.T.; Chen-Plotkin, A.; Wolk, D.; Sirisi, S.; Dols-Icardo, O.; Querol-Vilaseca, M.; Illán-Gala, I.; et al. Multimarker synaptic protein cerebrospinal fluid panels reflect TDP-43 pathology and cognitive performance in a pathological cohort of frontotemporal lobar degeneration. Mol. Neurodegener. 2022, 17, 29. [Google Scholar] [CrossRef]
- Irwin, D.J.; Lleó, A.; Xie, S.X.; McMillan, C.T.; Wolk, D.A.; Lee, E.B.; Van Deerlin, V.M.; Shaw, L.M.; Trojanowski, J.Q.; Grossman, M. Ante mortem cerebrospinal fluid tau levels correlate with postmortem tau pathology in frontotemporal lobar degeneration. Ann. Neurol. 2017, 82, 247–258. [Google Scholar] [CrossRef]
- Re, D.B.; Yan, B.; Calderón-Garcidueñas, L.; Andrew, A.S.; Tischbein, M.; Stommel, E.W. A perspective on persistent toxicants in veterans and amyotrophic lateral sclerosis: Identifying exposures determining higher ALS risk. J. Neurol. 2022, 269, 2359–2377. [Google Scholar] [CrossRef]

| CSF Samples | Age and Gender | TDP-43 pg/mL |
|---|---|---|
| Control children n: 26 | 11.5 ± 4.4 y (8.25, 12.50, 15.00) y 11F/15M | 102 ± 59 (58.14, 88.65, 129.77) |
| MMC children n: 92 | 10.27 ± 4.7 y (7.00, 11.00, 15.00) y 33F/59M | 239 ± 152 (130.28, 229.39, 299.47) |
| ALS patients n: 19 | 52.4 ± 14.1 y (56.50, 61.00, 64.00) y 9F/10M | 902 ± 269 (683.30, 906.07, 1085.79) |
| MMC adults n: 43 | 43.2 ± 15.9 y (28.50, 45.00, 54.00) y 16F/27M | 373 ± 358 (159.86, 275.10, 473.55) |
| Control adults n: 14 | 33.14 ± 12.0 y (27.50, 32.00, 33.75) y 5F/9M | 108 ± 67 (56.49, 81.75, 150.75) |
| ID | Age | Gender | APOE | AD pτ * | AD Aβ ** | SN pτ § | SN αS §§ | TDP-43 Brain ‡ | TDP-43 Cisternal pg/mL |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | 1 | 0 | 2 | 2 | 1 | 0 | 1 | 1013 |
| 2 | 21 | 1 | 0 | 5 | 3 | 0 | 0 | 0 | 392 |
| 3 | 24 | 1 | 0 | 2 | 2 | 1 | 1 | 1 | 375 |
| 4 | 25 | 1 | 0 | 1 | 2 | 0 | 0 | 1 | 565 |
| 5 | 27 | 1 | 0 | 2 | 2 | 1 | 1 | 0 | 218 |
| 6 | 37 | 0 | 0 | 5 | 4 | 1 | 1 | 0 | 42 |
| 7 | 38 | 1 | 0 | 4 | 3 | 1 | 0 | 0 | 306 |
| 8 | 39 | 1 | 0 | 3 | 2 | 1 | 1 | 0 | 167 |
| 9 | 40 | 1 | 0 | 4 | 3 | 1 | 1 | 1 | 562 |
| 10 | 42 | 1 | 0 | 3 | 2 | 1 | 0 | 1 | 496 |
| 11 | 47 | 1 | 0 | 3 | 2 | 0 | 0 | 0 | 194 |
| 12 | 48 | 1 | 0 | 4 | 3 | 1 | 1 | 0 | 293 |
| 13 | 55 | 1 | 0 | 5 | 3 | 0 | 1 | 0 | 150 |
| 14 | 75 | 0 | 0 | 4 | 3 | 1 | 1 | 1 | 424 |
| 15 | 83 | 0 | 0 | 4 | 3 | 1 | 0 | 0 | 66 |
| 1 CTL | 29 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 364 |
| 2 CTL | 34 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 191 |
| 3CTL | 43 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 435 |
| 4 CTL | 56 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 226 |
| 5 CTL | 68 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 110 |
| Anatomical Areas | 16 1013 pg/mL | 24 375 pg/mL | 25 565 pg/mL | 40 562 pg/mL | 42 496 pg/mL | 75 424 pg/mL |
| Frontal motor | 1 | 1 | 1 | 1 | 1, 2 | 1, 2 |
| Frontal non-motor | 1 | 1 | 1 | 1 | 1 | 1 |
| Parietal | 1 | 0 | 0 | 1 | 1 | 1 |
| Temporal | 1 | 1 | 0 | 1 | 1, 2 | 1 |
| Hippocampus | 1 | 1 | 1, 2, 4 | 1, 2 | 1, 2 | 1, 2, 4 |
| Caudate | 1 | 0 | 1 | 0 | 0 | 0 |
| Putamen | 0 | 0 | 1 | 0 | 0 | 1 |
| Globus pallidus | 0 | 0 | 1 | 0 | 1 | 0 |
| XII * | 1, 2 | 1, 2 | 1 | 1 | 1,2 | 1 |
| X * | 1 | 1, 2 | 1 | 1 | 1 | 1 |
| IX * | 0 | 0 | 1 | 1 | 0 | 0 |
| VIII * | 1 | 1 | 1 | 0 | 0 | 1 |
| VII * | 1 | 0 | 1, 2 | 1,2 | 1 | 1 |
| VI * | 0 | 1 | 1 | 0 | 0 | 0 |
| V * | 1, 2 | 1 | 1, 2 | 1 | 1 | 1 |
| IV * | 1 | 1 | 1 | 0 | 1 | 1 |
| III * | 1 | 1 | 1, 2 | 1 | 1 | 1 |
| II * | 0 | 0 | 0 | 0 | 0 | 0 |
| I * | 1 | 1 | 1, 2, 3, 4 | 1 | 1, 2 | 1 |
| Substantia nigrae pc | 1, 2 | 1, 2 | 1 | 1, 2 | 1, 2 | 0 |
| Locus coeruleus | 1, 2 | 1 | 1 | 1, 2 | 1, 2 | 1, 2 |
| Pons neurons | 1, 2, 4 | 1, 2, 4 | 1, 2 | 1, 2 | 1, 2 | 1, 2 |
| Mesencephalic reticular formation | 1, 2 | 1 | 1, 2 | 1, 2 | 1, 2 | 1 |
| Area postrema | 1 | 1 | 1, 2 | 1, 2 | 1 | 1 |
| Trigeminal ganglia | 0 | 1 | 1 | 1, 2 | 1, 2 | 1, 2 |
| Inferior olivary complex | 1 | 1 | 1 | 1 | 1 | 1 |
| Cervical, anterior horn | 1 | 1, 2 | 1, 2 | 1 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calderón-Garcidueñas, L.; Stommel, E.W.; Lachmann, I.; Waniek, K.; Chao, C.-K.; González-Maciel, A.; García-Rojas, E.; Torres-Jardón, R.; Delgado-Chávez, R.; Mukherjee, P.S. TDP-43 CSF Concentrations Increase Exponentially with Age in Metropolitan Mexico City Young Urbanites Highly Exposed to PM2.5 and Ultrafine Particles and Historically Showing Alzheimer and Parkinson’s Hallmarks. Brain TDP-43 Pathology in MMC Residents Is Associated with High Cisternal CSF TDP-43 Concentrations. Toxics 2022, 10, 559. https://doi.org/10.3390/toxics10100559
Calderón-Garcidueñas L, Stommel EW, Lachmann I, Waniek K, Chao C-K, González-Maciel A, García-Rojas E, Torres-Jardón R, Delgado-Chávez R, Mukherjee PS. TDP-43 CSF Concentrations Increase Exponentially with Age in Metropolitan Mexico City Young Urbanites Highly Exposed to PM2.5 and Ultrafine Particles and Historically Showing Alzheimer and Parkinson’s Hallmarks. Brain TDP-43 Pathology in MMC Residents Is Associated with High Cisternal CSF TDP-43 Concentrations. Toxics. 2022; 10(10):559. https://doi.org/10.3390/toxics10100559
Chicago/Turabian StyleCalderón-Garcidueñas, Lilian, Elijah W. Stommel, Ingolf Lachmann, Katharina Waniek, Chih-Kai Chao, Angélica González-Maciel, Edgar García-Rojas, Ricardo Torres-Jardón, Ricardo Delgado-Chávez, and Partha S. Mukherjee. 2022. "TDP-43 CSF Concentrations Increase Exponentially with Age in Metropolitan Mexico City Young Urbanites Highly Exposed to PM2.5 and Ultrafine Particles and Historically Showing Alzheimer and Parkinson’s Hallmarks. Brain TDP-43 Pathology in MMC Residents Is Associated with High Cisternal CSF TDP-43 Concentrations" Toxics 10, no. 10: 559. https://doi.org/10.3390/toxics10100559
APA StyleCalderón-Garcidueñas, L., Stommel, E. W., Lachmann, I., Waniek, K., Chao, C.-K., González-Maciel, A., García-Rojas, E., Torres-Jardón, R., Delgado-Chávez, R., & Mukherjee, P. S. (2022). TDP-43 CSF Concentrations Increase Exponentially with Age in Metropolitan Mexico City Young Urbanites Highly Exposed to PM2.5 and Ultrafine Particles and Historically Showing Alzheimer and Parkinson’s Hallmarks. Brain TDP-43 Pathology in MMC Residents Is Associated with High Cisternal CSF TDP-43 Concentrations. Toxics, 10(10), 559. https://doi.org/10.3390/toxics10100559








