Non-Destructive Quality Assessment of Tomato Paste by Using Portable Mid-Infrared Spectroscopy and Multivariate Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Tomato Paste Samples
2.2. Reference Analyses
2.3. Mid-Infrared Analysis
2.4. Partial Least Squares Regression (PLSR) Analysis
3. Results and Discussion
3.1. Reference Values in Tomato Paste Samples
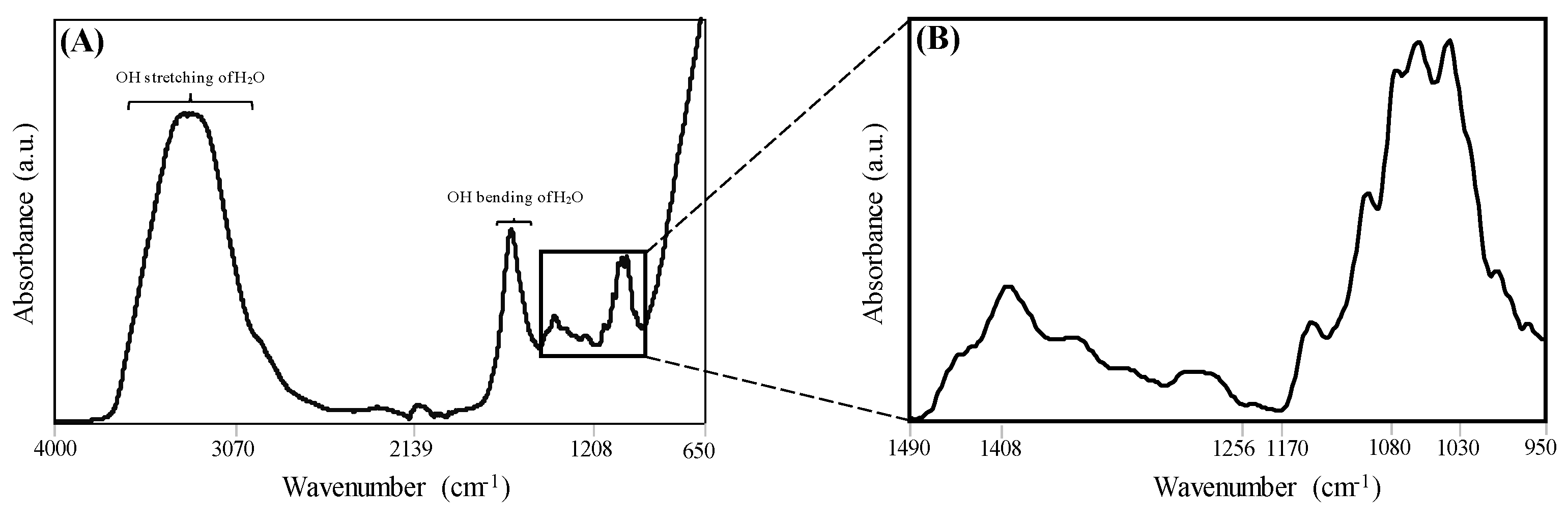
3.2. Spectral Information of Tomato Paste Samples.
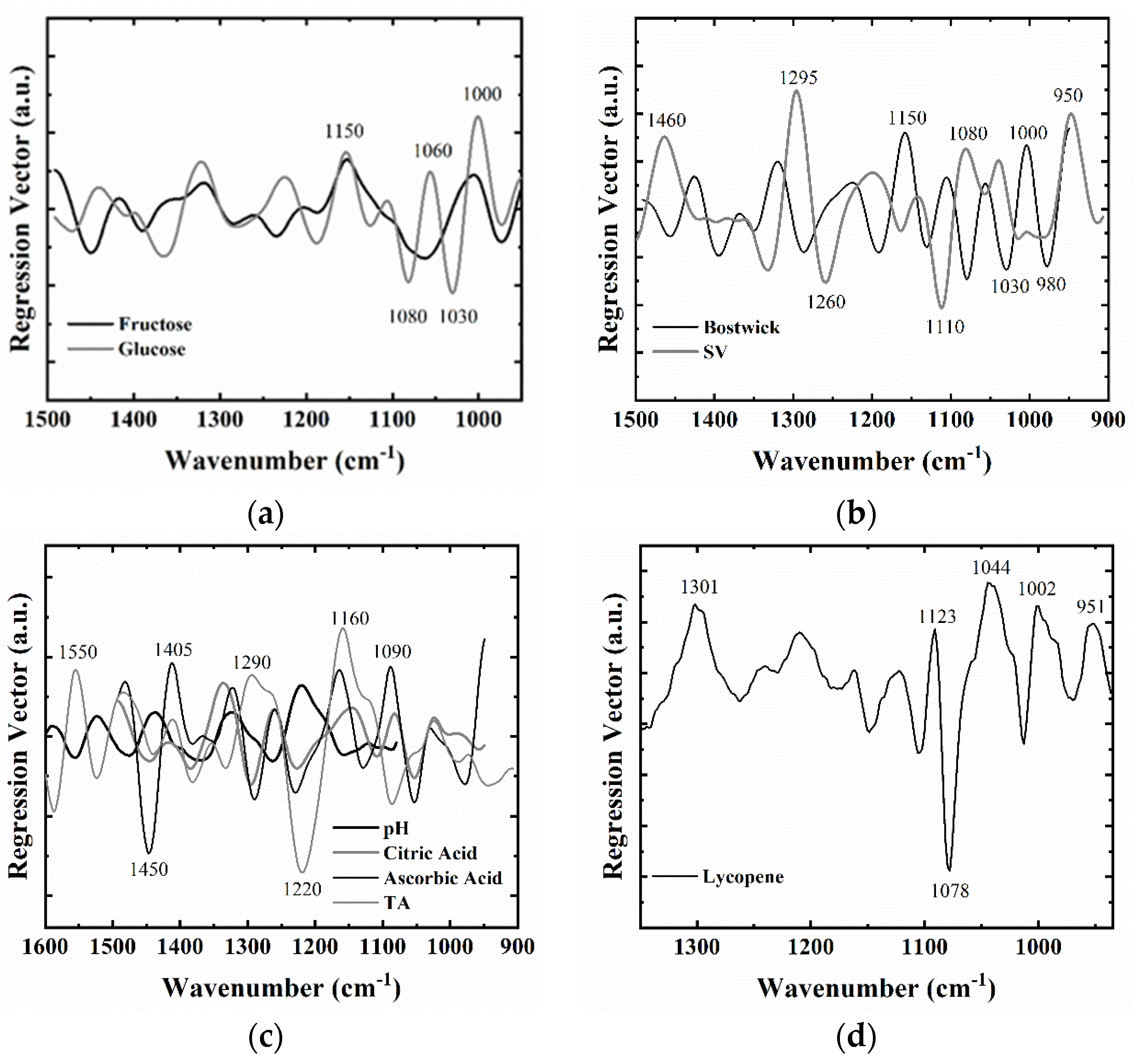
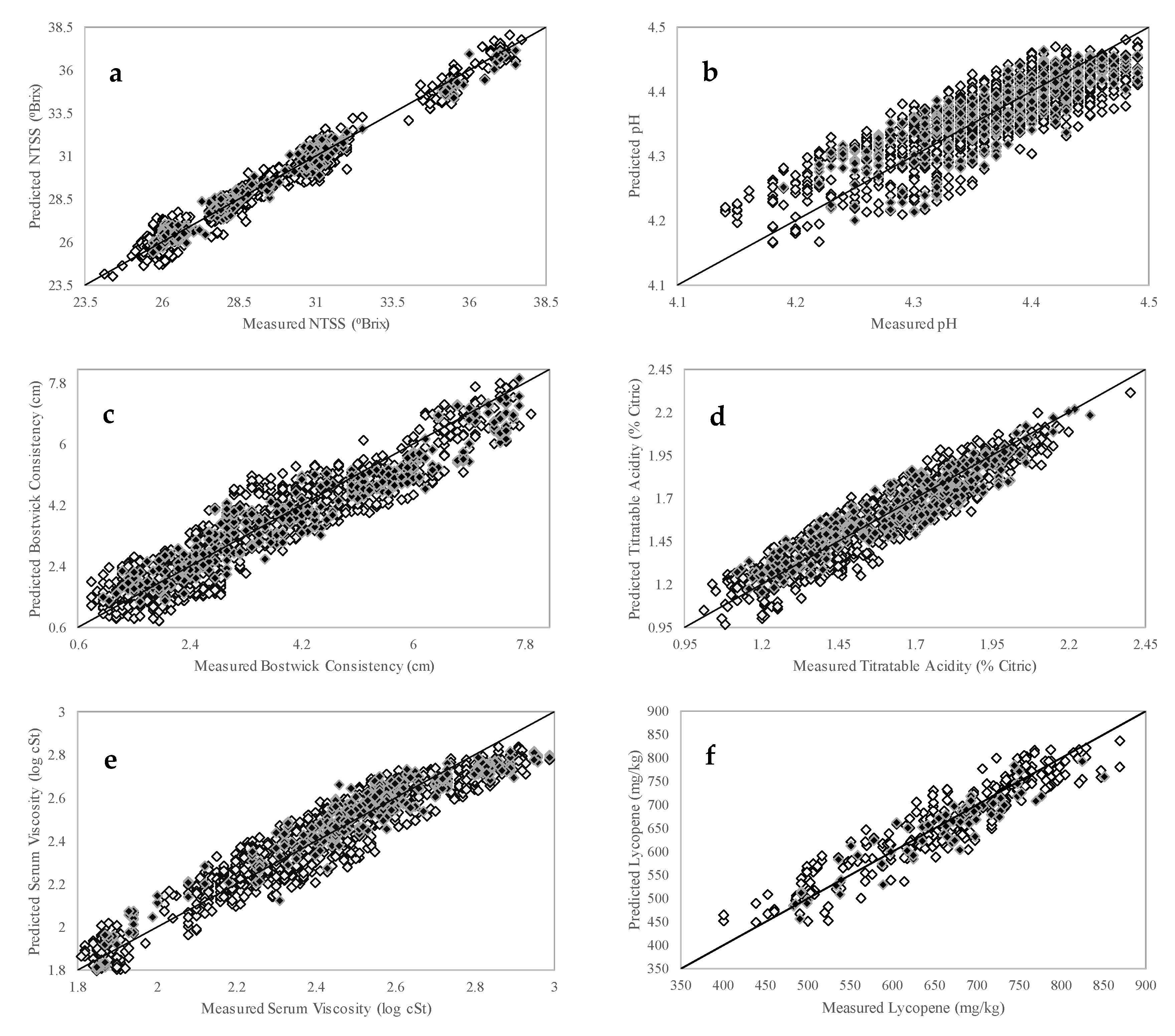
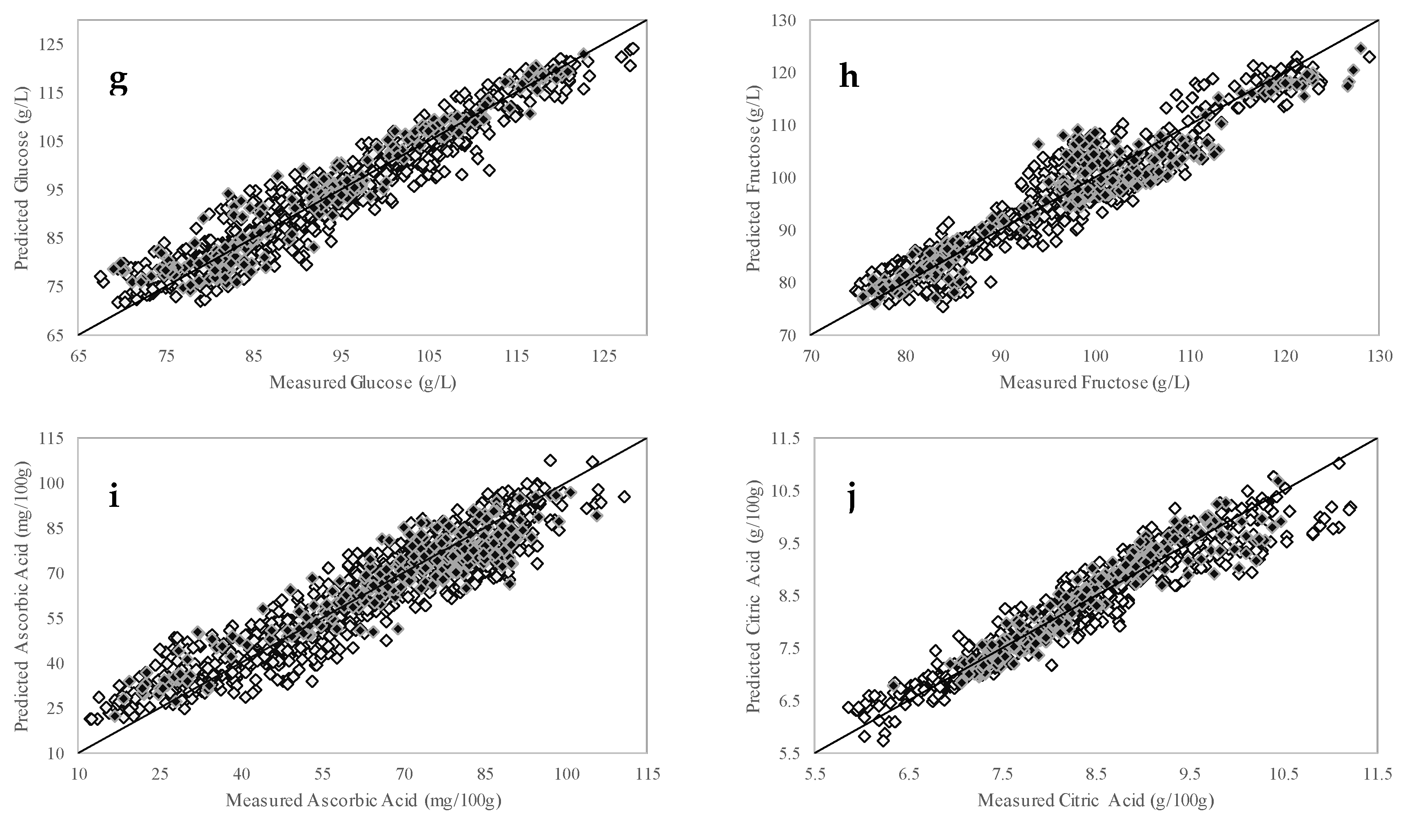
3.3. PLSR Calibration Models
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Winans, K.; Brodt, S.; Kendall, A. Life cycle assessment of California processing tomato: An evaluation of the effects of evolving practices and technologies over a 10-year (2005–2015) timeframe. Int. J. Life Cycle Assess. 2019, 25, 538–547. [Google Scholar] [CrossRef]
- Anthon, G.E.; Barrett, D.M. Pectin methylesterase activity and other factors affecting pH and titratable acidity in processing tomatoes. Food Chem. 2012, 132, 915–920. [Google Scholar] [CrossRef]
- Koh, E.; Charoenprasert, S.; Mitchell, A.E. Effects of industrial tomato paste processing on ascorbic acid, flavonoids and carotenoids and their stability over one-year storage. J. Sci. Food Agric. 2012, 92, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Anthon, G.E.; Barrett, D.M. Changes in tomato paste during storage and the effects of heating on consistency of reconstituted tomato paste. J. Texture Stud. 2010, 41, 262–278. [Google Scholar] [CrossRef]
- Zhang, L.; Schultz, M.A.; Cash, R.; Barrett, D.M.; Mccarthy, M.J. Determination of quality parameters of tomato paste using guided microwave spectroscopy. Food Control 2014, 40, 214–223. [Google Scholar] [CrossRef]
- Shobo, B.A.; Bodunde, J.G.; Makinde, E.A.; Olowe, V.I.O.; Ogunleye, M.T. Paste Quality of Tomato (Lycopersicon esculentum Mill) as Influenced by Variety and Organic Fertilizer. J. Org. Agric. Environ. 2018, 6, 10–16. [Google Scholar]
- Barrett, D.M.; Garcia, E.; Wayne, J.E. Textural modification of processing tomatoes. Crit. Rev. Food Sci. Nutr. 1998, 38, 173–258. [Google Scholar] [CrossRef]
- Thakur, B.R.; Singh, R.K.; Nelson, P.E. Quality attributes of processed tomato products: A review. Food Rev. Int. 1996, 12, 375–401. [Google Scholar] [CrossRef]
- Ayvaz, H.; Sierra-Cadavid, A.; Aykas, D.P.; Mulqueeney, B.; Sullivan, S.; Rodriguez-Saona, L.E. Monitoring multicomponent quality traits in tomato juice using portable mid-infrared (MIR) spectroscopy and multivariate analysis. Food Control 2016, 66, 79–86. [Google Scholar] [CrossRef]
- Barringer, S. Canned Tomatoes: Production and Storage. In Handbook of Vegetable Preservation and Processing; Hui, Y.H., Chazala, S., Graham, D.M., Murrell, K.D., Nip, W.-K., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2004; pp. 123–134. [Google Scholar]
- Wilkerson, E.D.; Anthon, G.E.; Barrett, D.M.; Sayajon, G.F.G.; Santos, A.M.; Rodriguez-Saona, L.E. Rapid assessment of quality parameters in processing tomatoes using hand-held and benchtop infrared spectrometers and multivariate analysis. J. Agric. Food Chem. 2013, 61, 2088–2095. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Goodner, K.; Plotto, A. Interaction of volatiles, sugars, and acids on perception of tomato aroma and flavor descriptors. J. Food Sci. 2008, 73, S294–S307. [Google Scholar] [CrossRef] [PubMed]
- Ścibisz, I.; Reich, M.; Bureau, S.; Gouble, B.; Causse, M.; Bertrand, D.; Renard, C.M.G.C. Mid-infrared spectroscopy as a tool for rapid determination of internal quality parameters in tomato. Food Chem. 2011, 125, 1390–1397. [Google Scholar] [CrossRef]
- Gould, W.A. Tomato Production, Processing and Technology; CTI Publications Inc.: Maryland, USA, 1992; ISBN 0930027183. [Google Scholar]
- Anthon, G.E.; Lestrange, M.; Barrett, D.M. Changes in pH, acids, sugars and other quality parameters during extended vine holding of ripe processing tomatoes. J. Sci. Food Agric. 2011, 91, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.; Barrett, D.M. Evaluation of processing tomatoes from two consecutive growing seasons: Quality attributes, peelability and yield. J. Food Process. Preserv. 2006, 30, 20–36. [Google Scholar] [CrossRef]
- Monti, L.M. The Breeding of Tomatoes for Peeling. Acta Hortic. 1980, 100, 341–354. [Google Scholar] [CrossRef]
- Abushita, A.A.; Daood, H.G.; Biacs, P.A. Change in carotenoids and antioxidant vitamins in tomato as a function of varietal and technological factors. J. Agric. Food Chem. 2000, 48, 2075–2081. [Google Scholar] [CrossRef]
- Capanoglu, E.; Beekwilder, J.; Boyacioglu, D.; Hall, R.; De Vos, R. Changes in antioxidant and metabolite profiles during production of tomato paste. J. Agric. Food Chem. 2008, 56, 964–973. [Google Scholar] [CrossRef]
- Baranska, M.; Schütze, W.; Schulz, H. Determination of lycopene and β-carotene content in tomato fruits and related products: Comparison of FT-raman, ATR-IR, and NIR spectroscopy. Anal. Chem. 2006, 78, 8456–8461. [Google Scholar] [CrossRef]
- De Oliveira, G.A.; Bureau, S.; Renard, C.M.G.C.; Pereira-Netto, A.B.; De Castilhos, F. Comparison of NIRS approach for prediction of internal quality traits in three fruit species. Food Chem. 2014, 143, 223–230. [Google Scholar] [CrossRef]
- Torres, I.; Pérez-Marín, D.; De la Haba, M.J.; Sánchez, M.T. Fast and accurate quality assessment of Raf tomatoes using NIRS technology. Postharvest Biol. Technol. 2015, 107, 9–15. [Google Scholar] [CrossRef]
- Ibáñez, G.; Cebolla-Cornejo, J.; Martí, R.; Roselló, S.; Valcárcel, M. Non-destructive determination of taste-related compounds in tomato using NIR spectra. J. Food Eng. 2019, 263, 237–242. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, M.; Adhikari, B.; Guo, Z. Nondestructive Detection of Postharvest Quality of Cherry Tomatoes Using a Portable NIR Spectrometer and Chemometric Algorithms. Food Anal. Methods 2019, 12, 914–925. [Google Scholar] [CrossRef]
- Camps, C.; Gilli, C. Prediction of local and global tomato texture and quality by FT-NIR spectroscopy and chemometric. Eur. J. Hortic. Sci. 2017, 82, 126–133. [Google Scholar] [CrossRef]
- Sheng, R.; Cheng, W.; Li, H.; Ali, S.; Akomeah Agyekum, A.; Chen, Q. Model development for soluble solids and lycopene contents of cherry tomato at different temperatures using near-infrared spectroscopy. Postharvest Biol. Technol. 2019, 156, 110952. [Google Scholar] [CrossRef]
- Ding, X.; Guo, Y.; Ni, Y.; Kokot, S. A novel NIR spectroscopic method for rapid analyses of lycopene, total acid, sugar, phenols and antioxidant activity in dehydrated tomato samples. Vib. Spectrosc. 2016, 82, 1–9. [Google Scholar] [CrossRef]
- Akpolat, H.; Barineau, M.; Jackson, K.A.; Aykas, D.P.; Rodriguez-Saona, L.E. Portable infrared sensing technology for phenotyping chemical traits in fresh market tomatoes. Lwt 2020, 124, 109164. [Google Scholar] [CrossRef]
- Bureau, S.; Reling, P.; Bogé, M.; Garcia, C.; Renard, C.M.G.C.; Giovinazzo, R.; Page, D. Rapid characterization of industry-type tomato using mid-infrared spectroscopy. Acta Hortic. 2017, 1159, 183–188. [Google Scholar] [CrossRef]
- Beullens, K.; Kirsanov, D.; Irudayaraj, J.; Rudnitskaya, A.; Legin, A.; Nicolaï, B.M.; Lammertyn, J. The electronic tongue and ATR-FTIR for rapid detection of sugars and acids in tomatoes. Sens. Actuators B Chem. 2006, 116, 107–115. [Google Scholar] [CrossRef]
- Nicolai, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Anthon, G.; Barrett, D.M. Standardization of a rapid spectrophotometric method for lycopene analysis. Acta Hortic. 2007, 758, 111–128. [Google Scholar] [CrossRef]
- Shi, J.; Le Maguer, M.; Bryan, M.; Kakuda, Y. Kinetics of lycopene degradation in tomato puree by heat and light irradiation. J. Food Process Eng. 2003, 25, 485–498. [Google Scholar] [CrossRef]
- Wechtersbach, L.; Cigić, B. Reduction of dehydroascorbic acid at low pH. J. Biochem. Biophys. Methods 2007, 70, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Melado-Herreros, A.; Fernandez-Valle, M.E.; Barreiro, P. Application of NMR to Resolve Food Structure, Composition and Quality. In Applications of NMR Spectroscopy, Applications in Food Sciences; Atta-ur-Rahman, Chaudhary, M.I., Eds.; Bentham Science Publishers: Sharjah, UAE, 2016; Volume 4, p. 44. ISBN 978-1-68108-143-4. [Google Scholar]
- Kawamura, K.; Tsujimoto, Y.; Rabenarivo, M.; Asai, H.; Andriamananjara, A.; Rakotoson, T. Vis-NIR spectroscopy and PLS regression with waveband selection for estimating the total C and N of paddy soils in Madagascar. Remote Sens. 2017, 9, 1081. [Google Scholar] [CrossRef]
- Abdi, H. Partial least squares regression and projection on latent structure regression (PLS Regression). Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 97–106. [Google Scholar] [CrossRef]
- Carmen Sánchez, M.; Valencia, C.; Gallegos, C.; Ciruelos, A.; Latorre, A. Influence of processing on the rheological properties of tomato paste. J. Sci. Food Agric. 2002, 82, 990–997. [Google Scholar] [CrossRef]
- Deda, M.S.; Bloukas, J.G.; Fista, G.A. Effect of tomato paste and nitrite level on processing and quality characteristics of frankfurters. Meat Sci. 2007, 76, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Kadakal, Ç.; Artik, N. Degradation kinetics of ergosterol in tomato paste serum. Eur. Food Res. Technol. 2008, 227, 683–688. [Google Scholar] [CrossRef]
- Khachik, F.; Beecher, G.R.; Holden, J.; Tenorio, M.D.; Barrera, M.R.; Goli, M.B.; Lusby, W.R. Effect of Food Preparation on Qualitative and Quantitative Distribution of Major Carotenoid Constituents of Tomatoes and Several Green Vegetables. J. Agric. Food Chem. 1992, 40, 390–398. [Google Scholar] [CrossRef]
- Shatta, A.A.B. Impact of Processing Steps on Physicochemical and Rheological Properties of Tomato Paste (Cold-Break). MOJ Food Process. Technol. 2017, 5, 263–271. [Google Scholar] [CrossRef]
- USDA Tomato Products, Canned, Paste, without Salt Added. Available online: https://fdc.nal.usda.gov/fdc-app.html#/food-details/170459/nutrients (accessed on 10 July 2020).
- Porretta, S.; Sandei, L.; Crucitti, P.M.; Poli, G.; Attolini, M.G. Comparison of the main analytical methods used in quality control of tomato paste. Int. J. Food Sci. Technol. 1992, 27, 145–152. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S.; Kadiroğlu, P.; Kola, O.; Kesen, S.; Uçar, B.; Çetiner, B. Bioactive compounds and antioxidant potential in tomato pastes as affected by hot and cold break process. Food Chem. 2017, 220, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Underwood, J.C. Factors Influencing Quality of Tomato Paste. I. Chemical Composition of California Commercial Tomato Paste. J. Food Sci. 1950, 15, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Sinelli, N.; Spinardi, A.; Di Egidio, V.; Mignani, I.; Casiraghi, E. Evaluation of quality and nutraceutical content of blueberries (Vaccinium corymbosum L.) by near and mid-infrared spectroscopy. Postharvest Biol. Technol. 2008, 50, 31–36. [Google Scholar] [CrossRef]
- Duarte, I.F.; Barros, A.; Delgadillo, I.; Almeida, C.; Gil, A.M. Application of FTIR spectroscopy for the quantification of sugars in mango juice as a function of ripening. J. Agric. Food Chem. 2002, 50, 3104–3111. [Google Scholar] [CrossRef]
- Grube, M.; Bekers, M.; Upite, D.; Kaminska, E. Infrared spectra of some fructans. Spectrosc. Int. J. 2002, 16, 289–296. [Google Scholar] [CrossRef]
- Sivakesava, S.; Irudayaraj, J. Determination of sugars in aqueous mixtures using mid-infrared spectroscopy. Appl. Eng. Agric. 2000, 16, 543–550. [Google Scholar] [CrossRef]
- Kačuráková, M.; Mathlouthi, M. FTIR and laser-Raman spectra of oligosaccharides in water: Characterization of the glycosidic bond. Carbohydr. Res. 1996, 284, 145–157. [Google Scholar] [CrossRef]
- Kanou, M.; Kameoka, T.; Suehara, K.I.; Hashimoto, A. Mid-infrared spectroscopic analysis of saccharides in aqueous solutions with sodium chloride. Biosci. Biotechnol. Biochem. 2017, 81, 735–742. [Google Scholar] [CrossRef]
- Kędzierska-Matysek, M.; Matwijczuk, A.; Florek, M.; Barłowska, J.; Wolanciuk, A.; Matwijczuk, A.; Chruściel, E.; Walkowiak, R.; Karcz, D.; Gładyszewska, B. Application of FTIR spectroscopy for analysis of the quality of honey. BIO Web Conf. 2018, 10, 02008. [Google Scholar] [CrossRef]
- Cael, J.J.; Koening, J.L.; Blackwell, J. Infrared and raman spectroscopy of carbohydrates part 4. Identification of configuration- and conformation-sensitive modes for d-glucose by normal coordinate analysis. Carbohydr. Res. 1974, 32, 79–91. [Google Scholar] [CrossRef]
- Hineno, M. Infrared spectra and normal vibration of β-d-glucopyranose. Carbohydr. Res. 1977, 56, 219–227. [Google Scholar] [CrossRef]
- Irudayaraj, J.; Tewari, J. Simultaneous monitoring of organic acids and sugars in fresh and processed apple juice by Fourier transform infrared-attenuated total reflection spectroscopy. Appl. Spectrosc. 2003, 57, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Birarda, G.; Holman, E.A.; Fu, S.; Weikel, K.; Hu, P.; Blankenberg, F.G.; Holman, H.Y.; Taylor, A. Synchrotron infrared imaging of advanced glycation endproducts (AGEs) in cardiac tissue from mice fed high glycemic diets. Biomed. Spectrosc. Imaging 2013, 2, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Halim, Y.; Schwartz, S.; Francis, D.; Baldauf, N.A.; Rodriguez-Saona, L.E. Direct Determination of Lycopene Content in Tomatoes (Lycopersicon esculentum) by Attenuated Total Reflectance Infrared Spectroscopy and Multivariate Analysis. J. AOAC 2006, 89, 1257–1262. [Google Scholar] [CrossRef]
- Windham, W.R.; Mertens, D.R.; Barton, F.E., II. Protocol for NIRS Calibration: Sample Selection and Equation Development and Validation. In Agriculture Handbook No. 643 Near Infrared Reflectance Spectroscopy (NIRS): Analysis of Forage Quality; Marten, G.C., Shenk, J.S., Barton, F.E., II, Eds.; United States Departmentt of Agriculture: Washington, DC, USA, 1989; Volume 643, pp. 96–103. [Google Scholar]
| Company | Year | Number of Samples | NTSS | pH | Bostwick | TA | Serum Viscosity c | Lycopene | Glucose | Fructose | Ascorbic Acid | Citric Acid | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 2015 | 120 | range | 25.6–36.0 | 4.3–4.5 | 2.5–10.8 | 1.0–1.9 | 2.3–2.6 | |||||
| Avg a ± std b | 30.7 ± 2.8 | 4.4 ± 0.0 | 5.1 ± 2.0 | 1.5 ± 0.2 | 2.4 ± 0.1 | ||||||||
| 2016 | 150 | range | 25.8–37.1 | 4.2–4.4 | 1.6–9.1 | 1.1–2.0 | 2.1–2.9 | 70.3–116.6 | 75.9–117.6 | 56.8–97.0 | 6.3–10.6 | ||
| avg ± std | 30.1 ± 2.8 | 4.3 ± 0.1 | 4.1 ± 1.9 | 1.5 ± 0.2 | 2.6 ± 0.2 | 93.3 ± 10.3 | 96.4 ± 9.6 | 77.6 ± 8.9 | 8.4 ± 0.9 | ||||
| 2017 | 196 | range | 25.4–37.0 | 4.2–4.5 | 0.8–11.9 | 1.0–1.8 | 2.2–2.8 | 74.1–128.2 | 75.2–130.5 | 49.0–110.7 | 6.4–11.7 | ||
| avg ± std | 28.6 ± 2.4 | 4.4 ± 0.0 | 3.5 ± 2.2 | 1.3 ± 0.2 | 2.5 ± 0.1 | 92.0 ± 11.5 | 92.3 ± 11.0 | 79.4 ± 10.9 | 8.4 ± 0.9 | ||||
| 2019 | 87 | range | 25.7–38.0 | 4.2–4.5 | 1.5–9.2 | 1.1–2.1 | 2.3–2.8 | 400.6–869.1 | |||||
| avg ± std | 31.1 ± 2.8 | 4.4 ± 0.1 | 5.4 ± 2.1 | 1.4 ± 0.2 | 2.5 ± 0.2 | 644.5 ± 112.1 | |||||||
| General | 553 | range | 25.4–38.0 | 4.2–4.5 | 0.8–11.9 | 1.0–2.1 | 2.1–2.9 | 70.3–128.2 | 75.2–130.5 | 49.0–110.7 | 6.3–11.7 | ||
| avg ± std | 29.8 ± 2.9 | 4.4 ± 0.1 | 4.3 ± 2.2 | 1.4 ± 0.2 | 2.5 ± 0.1 | 92.6 ± 11.0 | 94.1 ± 10.6 | 78.7 ± 10.1 | 8.4 ± 0.9 | ||||
| B | 2016 | 79 | range | 28.5–37.5 | 4.1–4.5 | 4.0–7.6 | 1.3 -2.2 | 1.9–2.4 | 88.1–120.1 | 92.1–121.4 | 59.7–104.8 | 6.8–10.4 | |
| avg ± std | 32.2 ± 2.9 | 4.3 ± 0.1 | 5.6–1.2 | 1.6 ± 0.3 | 2.1 ± 0.2 | 104.0 ± 9.0 | 104.9 ± 7.8 | 75.6 ± 8.9 | 8.4 ± 0.9 | ||||
| 2017 | 116 | range | 28.0–36.2 | 4.1–4.5 | 1.1–6.4 | 1.3 -1.9 | 1.8–2.4 | 87.0–128.0 | 94.0–128.8 | 55.8–98.4 | 5.9–10.0 | ||
| avg ± std | 30.6 ± 1.2 | 4.4 ± 0.1 | 4.8 ± 1.1 | 1.6 ± 0.1 | 2.1 ± 0.2 | 102.4 ± 6.0 | 101 ± 5.2 | 78.8 ± 11.1 | 8.3 ± 0.7 | ||||
| 2019 | 103 | range | 24.1–38.1 | 4.2–4.5 | 1.2–8.5 | 1.0–1.9 | 1.8–2.6 | ||||||
| avg ± std | 31.6 ± 4.3 | 4.4 ± 0.1 | 4.3 ± 2.1 | 1.5 ± 0.2 | 2.2 ± 0.2 | ||||||||
| General | 298 | range | 24.1–38.1 | 4.1–4.5 | 1.1–8.5 | 1.0–2.2 | 1.8–2.6 | 87.0–128.0 | 92.1–128.8 | 55.8–104.8 | 5.9–10.4 | ||
| avg ± std | 31.5 ± 3.0 | 4.3 ± 0.1 | 4.8 ± 1.6 | 1.6 ± 0.2 | 2.1 ± 0.2 | 102.0 ± 7.4 | 102.6 ± 6.6 | 77.5 ± 10.4 | 8.3 ± 0.8 | ||||
| C | 2016 | 222 | range | 27.8–37.5 | 4.1–4.5 | 2.3–7.9 | 1.5–2.2 | 1.9–2.5 | 81.2–122.6 | 89.2–128.0 | 32.6–100.7 | 7.8–9.6 | |
| avg ± std | 31.0 ± 1.9 | 4.3 ± 0.1 | 4.4 ± 1.3 | 1.8 ± 0.2 | 2.2 ± 0.2 | 95.9 ± 8.8 | 101.0 ± 7.9 | 65.5 ± 12.8 | 8.6 ± 0.4 | ||||
| 2017 | 290 | range | 26.0–36.5 | 4.1–4.5 | 0.8–7.1 | 1.3–2.4 | 1.9–2.7 | 71.0–122.9 | 77.8–123.8 | 13.5–109.8 | 7.1–11.2 | ||
| avg ± std | 29.1 ± 2.8 | 4.4 ± 0.1 | 3.1 ± 1.6 | 1.7 ± 0.2 | 2.4 ± 0.2 | 90.6 ± 12.6 | 94.4 ± 12.1 | 55.0 ± 19.9 | 8.5 ± 1.1 | ||||
| 2019 | 110 | range | 26.1–31.6 | 4.2–4.5 | 1.0–4.9 | 1.3–2.3 | 1.9–2.7 | 614.3–829.3 | |||||
| avg ± std | 29.2 ± 2.1 | 4.4 ± 0.1 | 3.0 ± 1.2 | 1.7 ± 0.2 | 2.3 ± 0.2 | 690.9 ± 43.6 | |||||||
| General | 622 | range | 26.0–37.5 | 4.1–4.5 | 0.8–7.9 | 1.3–2.4 | 1.9–2.7 | 71.0–127.9 | 77.8–128.0 | 13.5–109.8 | 7.1–11.2 | ||
| avg ± std | 29.8 ± 2.6 | 4.4 ± 0.1 | 3.5 ± 1.6 | 1.8 ± 0.2 | 2.3 ± 0.2 | 92.9 ± 11.4 | 97.3 ± 10.9 | 59.6 ± 17.9 | 8.6 ± 0.8 | ||||
| D | 2015 | 47 | range | 25.0–26.6 | 4.2–4.4 | 1.1–2.4 | 1.3–1.5 | 2.6–2.7 | |||||
| avg ± std | 25.9 ± 0.3 | 4.3 ± 0.0 | 1.8 ± 0.4 | 1.4 ± 0.0 | 2.7 ± 0.0 | ||||||||
| 2016 | 48 | range | 25.3–26.5 | 4.4–4.5 | 2.3–2.9 | 1.3–1.4 | 2.9–3.0 | 76.9–84.6 | 78.1–89.0 | 43.9–63.4 | 5.9–7.1 | ||
| avg ± std | 26 ± 0.3 | 4.4 ±0.0 | 2.6 ± 0.2 | 1.4 ± 0.0 | 3.0 ± 0.0 | 80.7 ± 1.8 | 83.5 ± 2.7 | 50.8 ± 5.3 | 6.5 ± 0.3 | ||||
| 2017 | 203 | range | 25.1–28.5 | 4.3–4.5 | 1.0–2.9 | 1.2–1.6 | 2.4–2.9 | 67.5–99.3 | 74.7–100.6 | 12.1–72.2 | 6.6–8.8 | ||
| avg ± std | 26.2 ± 0.8 | 4.4 ± 0.0 | 1.9 ± 0.4 | 1.4 ± 0.1 | 2.7 ± 0.1 | 80.7 ± 6.9 | 82.4 ± 3.9 | 38.0 ± 13.0 | 7.4 ± 0.4 | ||||
| 2019 | 72 | range | 25.3–28.3 | 4.3–4.5 | 1.4–2.2 | 1.3–1.5 | 2.5–2.9 | ||||||
| avg ± std | 26.5 ± 1.0 | 4.4 ± 0.0 | 1.9 ± 0.2 | 1.4 ± 0.1 | 2.7 ± 0.1 | ||||||||
| General | 370 | range | 25.0–28.5 | 4.2–4.5 | 1.0–2.9 | 1.2–1.6 | 2.4–3.0 | 67.5–104.3 | 74.7–100.6 | 12.1–72.2 | 5.9–8.8 | ||
| avg ± std | 26.2 ± 0.8 | 4.4 ± 0.0 | 2.0 ± 0.5 | 1.4 ± 0.1 | 2.7 ± 0.1 | 80.7 ± 6.2 | 82.6 ± 3.7 | 40.4 ± 12.9 | 7.2 ± 0.5 |
| Parameter | Calibration Model | External Validation Model | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | N a | Factor | SECV b | Rcv c | Range | N d | SEP e | RPre f | RPD g | |
| NTSS (°Brix) | 24.1–38.1 | 1436 | 3 | 0.44 | 0.99 | 25.7–37.5 | 359 | 0.40 | 0.99 | 7.3 |
| pH | 4.14–4.49 | 1419 | 6 | 0.04 | 0.85 | 4.19–4.49 | 355 | 0.04 | 0.83 | 1.8 |
| Bostwick Consistency (cm) | 0.8–7.9 | 1382 | 5 | 0.55 | 0.94 | 1.0–7.7 | 345 | 0.58 | 0.96 | 2.9 |
| Titratable Acidity (% Citric) | 0.99–2.40 | 1406 | 6 | 0.08 | 0.94 | 1.12–2.27 | 352 | 0.09 | 0.93 | 2.8 |
| Serum Viscosity (log cSt) | 1.81–2.99 | 1304 | 6 | 0.08 | 0.96 | 1.85–2.99 | 326 | 0.08 | 0.96 | 3.5 |
| Lycopene (mg/kg) | 400.6–869.1 | 138 | 6 | 35.75 | 0.93 | 483.4–851.1 | 35 | 35.11 | 0.93 | 2.7 |
| Glucose (g/L) | 67.5–128.2 | 1043 | 5 | 3.16 | 0.96 | 68.9–122.6 | 261 | 3.39 | 0.97 | 3.5 |
| Fructose (g/L) | 74.7–128.8 | 1032 | 4 | 3.11 | 0.96 | 75.4–128.0 | 258 | 3.88 | 0.96 | 2.9 |
| Ascorbic Acid (mg/100 g) | 12.1–110.7 | 1040 | 6 | 6.99 | 0.94 | 16.7–105.6 | 260 | 7.32 | 0.93 | 2.7 |
| Citric Acid (g/100 g) | 5.9–11.2 | 1031 | 5 | 0.27 | 0.96 | 6.3–10.5 | 258 | 0.27 | 0.96 | 3.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aykas, D.P.; Rodrigues Borba, K.; Rodriguez-Saona, L.E. Non-Destructive Quality Assessment of Tomato Paste by Using Portable Mid-Infrared Spectroscopy and Multivariate Analysis. Foods 2020, 9, 1300. https://doi.org/10.3390/foods9091300
Aykas DP, Rodrigues Borba K, Rodriguez-Saona LE. Non-Destructive Quality Assessment of Tomato Paste by Using Portable Mid-Infrared Spectroscopy and Multivariate Analysis. Foods. 2020; 9(9):1300. https://doi.org/10.3390/foods9091300
Chicago/Turabian StyleAykas, Didem Peren, Karla Rodrigues Borba, and Luis E. Rodriguez-Saona. 2020. "Non-Destructive Quality Assessment of Tomato Paste by Using Portable Mid-Infrared Spectroscopy and Multivariate Analysis" Foods 9, no. 9: 1300. https://doi.org/10.3390/foods9091300
APA StyleAykas, D. P., Rodrigues Borba, K., & Rodriguez-Saona, L. E. (2020). Non-Destructive Quality Assessment of Tomato Paste by Using Portable Mid-Infrared Spectroscopy and Multivariate Analysis. Foods, 9(9), 1300. https://doi.org/10.3390/foods9091300






