Effectiveness of Aqueous Chlorine Dioxide in Minimizing Food Safety Risk Associated with Salmonella, E. coli O157:H7, and Listeria monocytogenes on Sweet Potatoes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Produce Material
2.2. Bacterial Strains
2.3. Preparation of Inoculum
2.4. Inoculation of Sweet Potatoes
2.5. Preparation of Aqueous ClO2
2.6. Aqueous ClO2 Treatment
2.7. Recovery of Pathogens and Microbiological Analyses
2.8. Statistical Analysis
3. Results
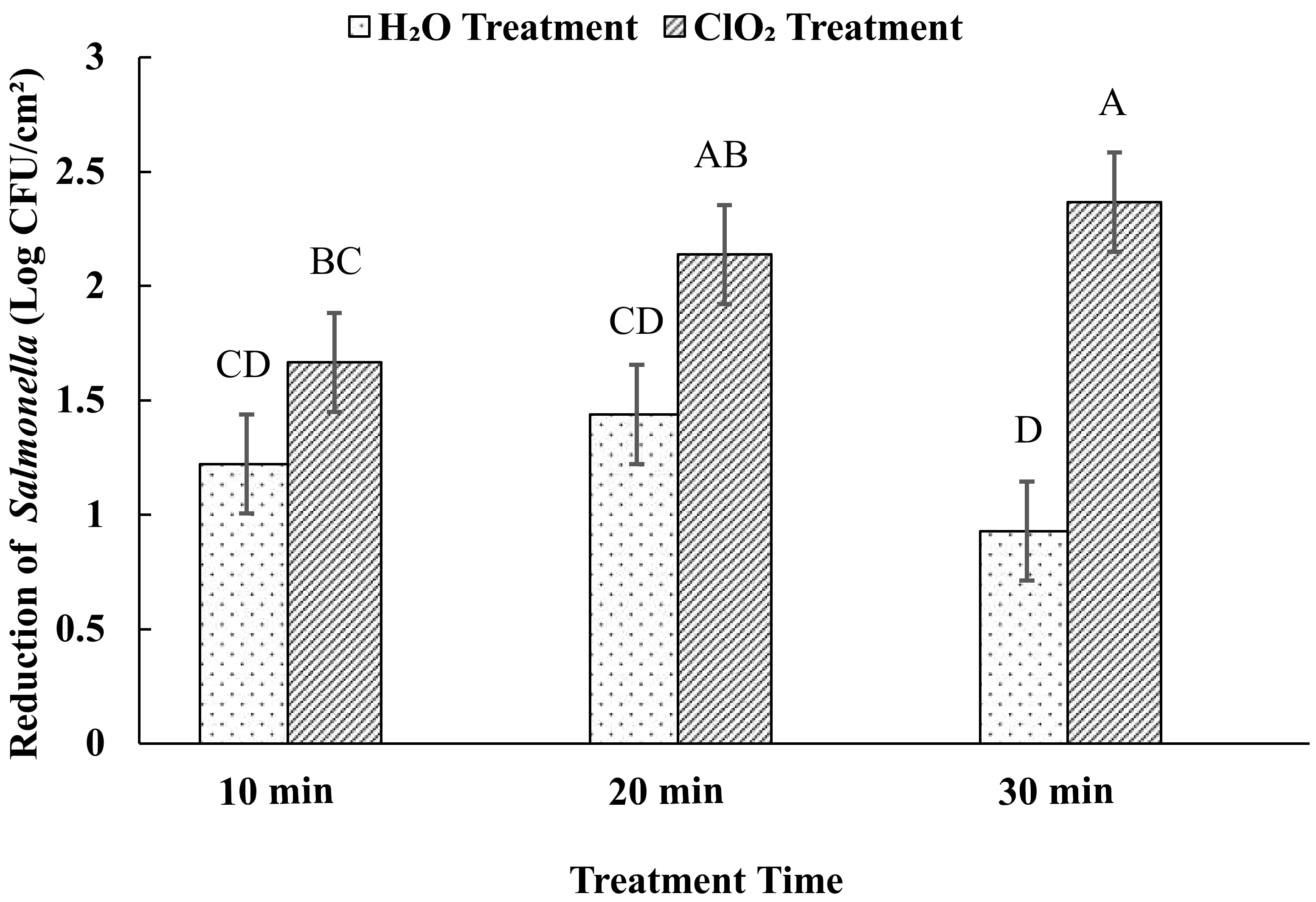
3.1. Effects of Aqueous ClO2 Treatment on Salmonella Enterica
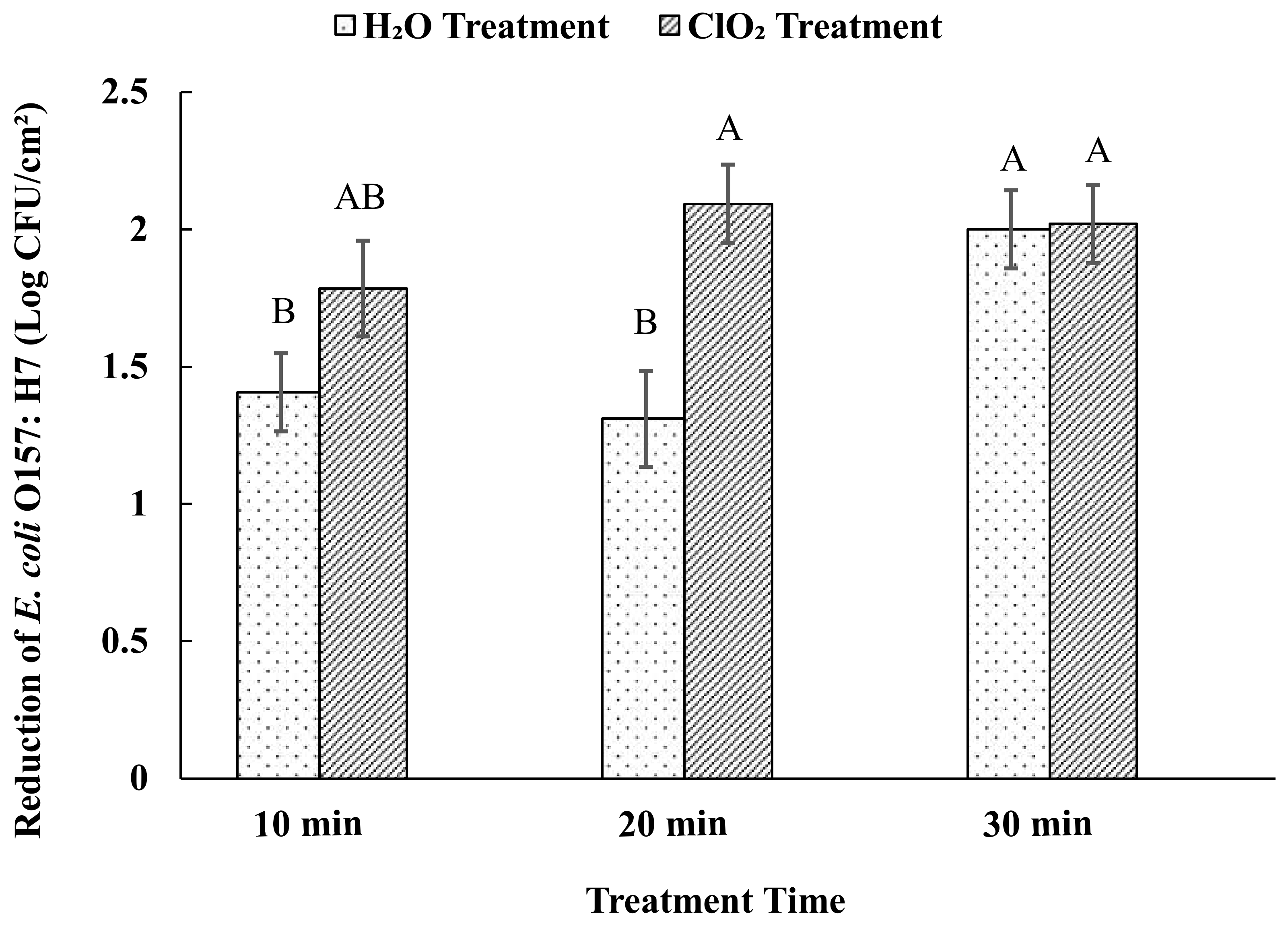
3.2. Effects of Aqueous ClO2 Treatment on E. coli O157:H7
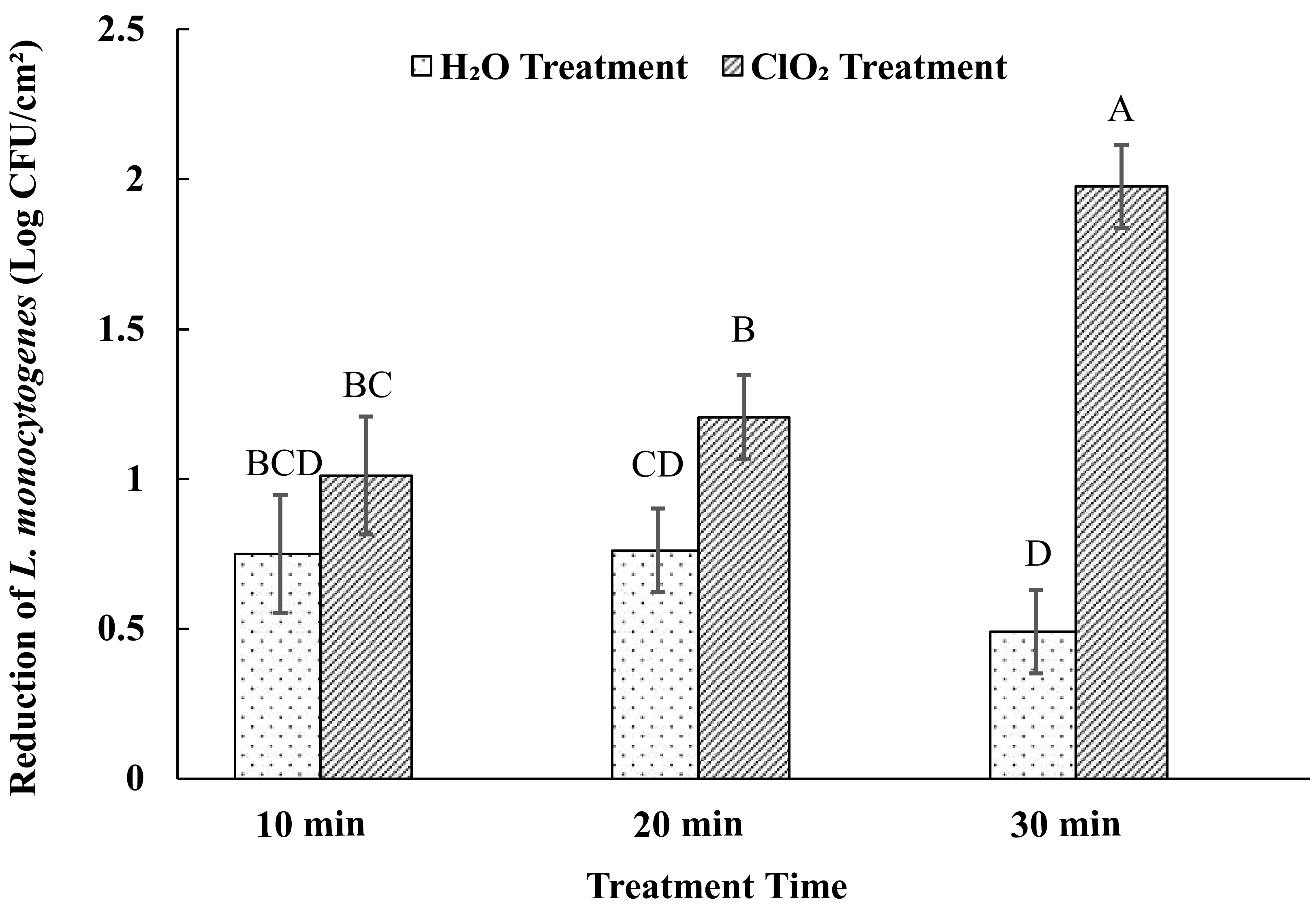
3.3. Effects of Aqueous ClO2 Treatment on Listeria Monocytogenes
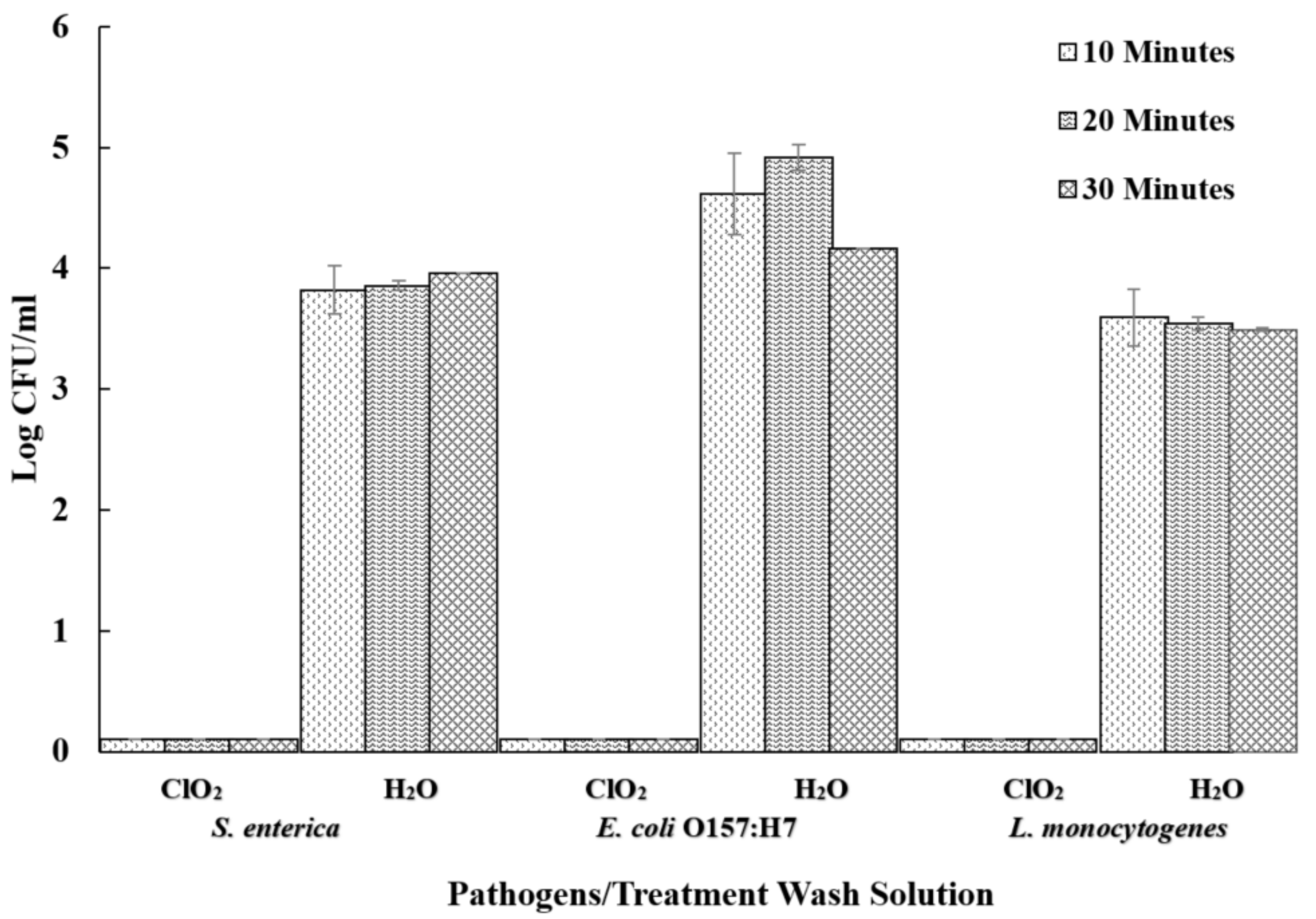
3.4. Influences of Aqueous ClO2 on Cross Contamination of Noninoculated Sweet Potatoes
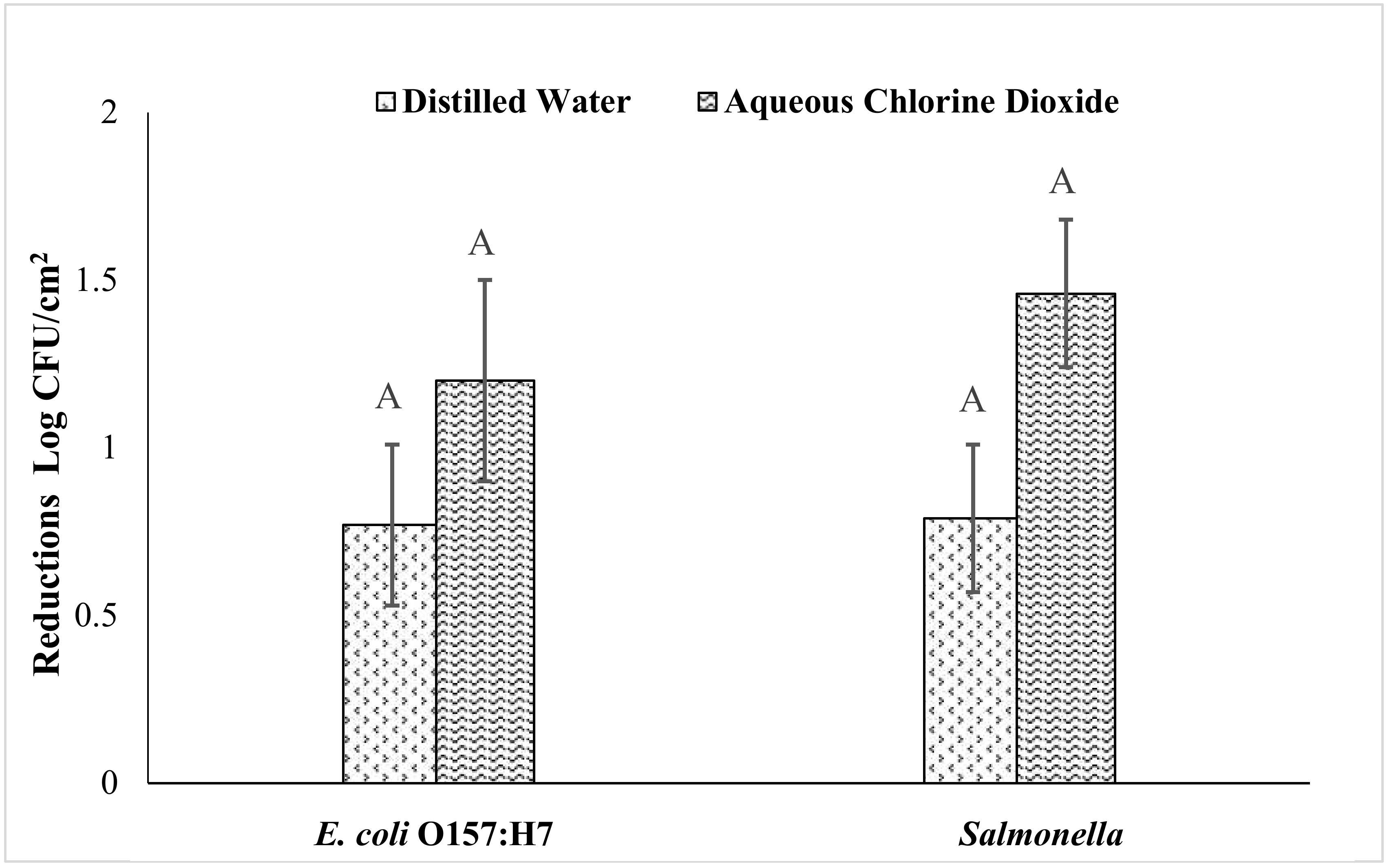
3.5. Effects of Aqueous ClO2 on E. coli O157: H7 and Salmonella on Freshly Harvested Sweet Potatoes
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Prakash, P.; Kishore, P.; Jaganathan, D.; Immanual, S.; Sivakumar, P. The Status, Performance and Impact of sweet potato cultivation on farming communities of Odisha, India. In Proceedings of the 2018 Conference, Vancouver, BC, Canada, 28 July–2 August 2018. [Google Scholar] [CrossRef]
- Prakash, C. Sweet potato biotechnology: Progress and potential. Biotechnol. Dev. Monit. 1994, 18, 1819–1822. [Google Scholar]
- Low, J.W.; Arimond, M.; Osman, N.; Cunguara, B.; Zano, F.; Tschirley, D. A food-based approach introducing orange-fleshed sweet potatoes increased vitamin A intake and serum retinol concentrations in young children in rural Mozambique. J. Nutr. 2007, 137, 1320–1327. [Google Scholar] [CrossRef] [PubMed]
- Peet, M. Sustainable Practices for Vegetable Production in the South; Focus Publishing, R. Pullins Co: Newburyport, MA, USA, 1996. [Google Scholar]
- Mohammad, Z.H.; Yu, H.; Neal, J.A.; Gibson, K.E.; Sirsat, S.A. Food Safety Challenges and Barriers in Southern United States Farmers Markets. Foods 2020, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, M.F.; Tauxe, R.V.; Hedberg, C.W. The growing burden of foodborne outbreaks due to contaminated fresh produce: Risks and opportunities. Epidemiols. Infect. 2009, 137, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Hanning, I.B.; Nutt, J.; Ricke, S.C. Salmonellosis outbreaks in the United States due to fresh produce: Sources and potential intervention measures. Foodborne Pathog. Dis. 2009, 6, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, V.S.; Fontenot, K.; Strahan, R.; Yemmireddy, V.K.; Parraga Estrada, K.J.; Adhikari, A. Effect of surrounding vegetation on microbial survival or die-off on watermelon surface in an agriculture setting. J. Food Saf. 2018, 38, e12520. [Google Scholar] [CrossRef]
- Islam, M.; Doyle, M.P.; Phatak, S.C.; Millner, P.; Jiang, X. Survival of Escherichia coli O157: H7 in soil and on carrots and onions grown in fields treated with contaminated manure composts or irrigation water. Food Microbiol. 2005, 22, 63–70. [Google Scholar] [CrossRef]
- Angulo, F.J.; Getz, J.; Taylor, J.P.; Hendricks, K.A.; Hatheway, C.L.; Barth, S.S.; Solomon, H.M.; Larson, A.E.; Johnson, E.A.; Nickey, L.N. A large outbreak of botulism: The hazardous baked potato. J. Infect. Dis. 1998, 178, 172–177. [Google Scholar] [CrossRef]
- Sobel, J.; Tucker, N.; Sulka, A.; McLaughlin, J.; Maslanka, S. Foodborne botulism in the United States, 1990–2000. Emerg. Infect. Dis. 2004, 10, 1606. [Google Scholar] [CrossRef]
- Chhetri, V.S.; Janes, M.E.; King, J.M.; Doerrler, W.; Adhikari, A. Effect of residual chlorine and organic acids on survival and attachment of Escherichia coli O157: H7 and Listeria monocytogenes on spinach leaves during storage. LWT 2019, 105, 298–305. [Google Scholar] [CrossRef]
- Han, Y.; Sherman, D.; Linton, R.; Nielsen, S.; Nelson, P. The effects of washing and chlorine dioxide gas on survival and attachment of Escherichia coli O157: H7 to green pepper surfaces. Food Microbiol. 2000, 17, 521–533. [Google Scholar] [CrossRef] [Green Version]
- Suslow, T.; Oria, M.; Beuchat, L.; Garrett, E.; Parish, M.; Harris, L.; Farber, J.; Busta, F. Production practices as risk factors in microbial food safety of fresh and fresh-cut produce. Compr. Rev. Food Sci. Food Saf. 2003, 2, 38–77. [Google Scholar] [CrossRef]
- Boyette, M.; Ritchie, D.; Carballo, S.; Blankenship, S.; Sanders, D. Chlorination and postharvest disease control. Hort Technol. 1993, 3, 395–400. [Google Scholar] [CrossRef] [Green Version]
- Tomás-Callejas, A.; López-Gálvez, F.; Sbodio, A.; Artés, F.; Artés-Hernández, F.; Suslow, T.V. Chlorine dioxide and chlorine effectiveness to prevent Escherichia coli O157: H7 and Salmonella cross-contamination on fresh-cut Red Chard. Food Control 2012, 23, 325–332. [Google Scholar] [CrossRef]
- Richardson, S.; Thruston, A.; Caughran, T.; Collette, T.; Patterson, K. Chemical by-products of chlorine and alternative disinfectants. Food Technol. 1998, 52, 58–61. [Google Scholar]
- Shen, C.; Norris, P.; Williams, O.; Hagan, S.; Li, K. Generation of chlorine by-products in simulated wash water. Food Chem. 2016, 190, 97–102. [Google Scholar] [CrossRef]
- Bridges, D.F.; Lacombe, A.; Wu, V.C. Integrity of the Escherichia coli O157: H7 Cell Wall and Membranes After Chlorine Dioxide Treatment. Front. Microbiol. 2020, 11, 888. [Google Scholar] [CrossRef]
- Benarde, M.A.; Israel, B.M.; Olivieri, V.P.; Granstrom, M.L. Efficiency of chlorine dioxide as a bactericide. Appl. Environ. Microbiol. 1965, 13, 776–780. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Wang, L.; Ren, N.; Ma, F. Disinfection effect of chlorine dioxide on bacteria in water. Water Res. 1997, 31, 607–613. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Rajkovic, A.; Ragaert, P.; Smigic, N.; Devlieghere, F. Chlorine dioxide for minimally processed produce preservation: A review. Trends Food Sci. Tech. 2009, 20, 17–26. [Google Scholar] [CrossRef]
- Praeger, U.; Herppich, W.B.; Hassenberg, K. Aqueous chlorine dioxide treatment of horticultural produce: Effects on microbial safety and produce quality—A review. Crit Rev. Food Sci. Nutr. 2018, 58, 318–333. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sohn, M. Reactivity of chlorine dioxide with amino acids, peptides, and proteins. Environ. Chem. Lett. 2012, 10, 255–264. [Google Scholar] [CrossRef]
- Pao, S.; Kelsey, D.F.; LONG III, W. Spray washing of tomatoes with chlorine dioxide to minimize Salmonella on inoculated fruit surfaces and cross-contamination from revolving brushes. J. Food Prot. 2009, 72, 2448–2452. [Google Scholar] [CrossRef]
- Wu, V.C.; Kim, B. Effect of a simple chlorine dioxide method for controlling five foodborne pathogens, yeasts and molds on blueberries. Food Microbiol. 2007, 24, 794–800. [Google Scholar] [CrossRef]
- Adhikari, A.; Bary, A.; Cogger, C.; James, C.; Ünlü, G.; Killinger, K. Thermal and starvation stress response of Escherichia coli O157: H7 isolates selected from agricultural environments. J. Food Prot. 2016, 79, 1673–1679. [Google Scholar] [CrossRef]
- Adhikari, A.; Chhetri, V.S.; Bhattacharya, D.; Cason, C.; Luu, P.; Suazo, A. Effectiveness of daily rinsing of alfalfa sprouts with aqueous chlorine dioxide and ozonated water on the growth of Listeria monocytogenes during sprouting. Lett. Appl. Microbiol. 2019, 69, 252–257. [Google Scholar] [CrossRef]
- Bang, J.; Kim, H.; Kim, H.; Beuchat, L.; Kim, Y.; Ryu, J.H. Reduction of Escherichia coli O157: H7 on radish seeds by sequential application of aqueous chlorine dioxide and dry-heat treatment. Lett. Appl. Microbiol. 2011, 53, 424–429. [Google Scholar] [CrossRef]
- Pao, S.; Kelsey, D.; Khalid, M.; Ettinger, M. Using aqueous chlorine dioxide to prevent contamination of tomatoes with Salmonella enterica and Erwinia carotovora during fruit washing. J. Food Prot. 2007, 70, 629–634. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, S.H.; Park, J.; Park, J.; Chung, M.; Kwon, K.; Chung, K.; Won, M.; Song, K.B. Inactivation of Escherichia coli O157: H7, Salmonella typhimurium, and Listeria monocytogenes on stored iceberg lettuce by aqueous chlorine dioxide treatment. J. Food Sci. 2008, 73, M418–M422. [Google Scholar] [CrossRef]
- Huang, T.S.; Xu, C.; Walker, K.; West, P.; Zhang, S.; Weese, J. Decontamination efficacy of combined chlorine dioxide with ultrasonication on apples and lettuce. J. Food Sci. 2006, 71, M134–M139. [Google Scholar] [CrossRef]
- Song, H.-J.; Choi, D.-W.; Song, K.B. Effect of aqueous chlorine dioxide and UV-C treatment on the microbial reduction and color of cherry tomatoes. Hortic. Environ. Biotechnol. 2011, 52, 488. [Google Scholar] [CrossRef]
- Jin, H.H.; Lee, S.Y. Combined effect of aqueous chlorine dioxide and modified atmosphere packaging on inhibiting Salmonella typhimurium and Listeria monocytogenes in mungbean sprouts. J. Food Sci. 2007, 72, M441–M445. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Singh, R.; Bhunia, A.; Stroshine, R. Effect of inoculation and washing methods on the efficacy of different sanitizers against Escherichia coli O157: H7 on lettuce. Food Microbiol. 2002, 19, 183–193. [Google Scholar] [CrossRef]
- Singh, N.; Singh, R.; Bhunia, A.; Stroshine, R. Efficacy of chlorine dioxide, ozone, and thyme essential oil or a sequential washing in killing Escherichia coli O157: H7 on lettuce and baby carrots. LWT 2002, 35, 720–729. [Google Scholar] [CrossRef]
- Rodgers, S.L.; Cash, J.N.; Siddiq, M.; Ryser, E.T. A comparison of different chemical sanitizers for inactivating Escherichia coli O157: H7 and Listeria monocytogenes in solution and on apples, lettuce, strawberries, and cantaloupe. J. Food Prot. 2004, 67, 721–731. [Google Scholar] [CrossRef]
- Han, Y.; Linton, R.; Nielsen, S.; Nelson, P. Reduction of Listeria monocytogenes on green peppers (Capsicum annuum L.) by gaseous and aqueous chlorine dioxide and water washing and its growth at 7 C. J. Food Prot. 2001, 64, 1730–1738. [Google Scholar] [CrossRef]
- Zhang, S.; Farber, J. The effects of various disinfectants against Listeria monocytogenes on fresh-cut vegetables. Food Microbiol. 1996, 13, 311–321. [Google Scholar] [CrossRef]
- Park, S.-H.; Kang, D.-H. Influence of surface properties of produce and food contact surfaces on the efficacy of chlorine dioxide gas for the inactivation of foodborne pathogens. Food Control. 2017, 81, 88–95. [Google Scholar] [CrossRef]
- FDA. Code of Federal Regulations. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=173 (accessed on 1 September 2020).
- Wills, R.; McGlasson, B.; Graham, D.; Joyce, D.; Rushing, J.W. Postharvest: An introduction to the physiology and handling of fruit, vegetables and ornamentals. J. Veg. Crop Prod. 1999, 4, 83–84. [Google Scholar]
- Chhetri, V.S.; Fontenot, K.; Strahan, R.; Yemmireddy, V.K.; Cason, C.; Kharel, K.; Adhikari, A. Attachment strength and on-farm die-off rate of Escherichia coli on watermelon surfaces. PLoS ONE 2019, 14, e0210115. [Google Scholar] [CrossRef]
- Gautam, D.; Dobhal, S.; Payton, M.E.; Fletcher, J.; Ma, L.M. Surface survival and internalization of Salmonella through natural cracks on developing cantaloupe fruits, alone or in the presence of the melon wilt pathogen Erwinia tracheiphila. PLoS ONE 2014, 9, e105248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Kim, M.; Song, K. Efficacy of aqueous chlorine dioxide and fumaric acid for inactivating pre-existing microorganisms and Escherichia coli O157: H7, Salmonella typhimurium, and Listeria monocytogenes on broccoli sprouts. Food Control. 2009, 20, 1002–1005. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, C. Combined effects of aqueous chlorine dioxide and ultrasonic treatments on postharvest storage quality of plum fruit (Prunus salicina L.). Postharvest Biol. Technol. 2011, 61, 117–123. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, H.J.; Lim, G.O.; Jang, S.A.; Song, K.B. The effects of aqueous chlorine dioxide or fumaric acid treatment combined with UV-C on postharvest quality of ‘Maehyang’strawberries. Postharvest Biol. Technol. 2010, 56, 254–256. [Google Scholar] [CrossRef]
- Yang, H.; Zheng, J.; Huang, C.; Zhao, X.; Chen, H.; Sun, Z. Effects of combined aqueous chlorine dioxide and chitosan coatings on microbial growth and quality maintenance of fresh-cut bamboo shoots (Phyllostachys praecox f. prevernalis.) during storage. Food Bioprocess Technol. 2015, 8, 1011–1019. [Google Scholar] [CrossRef]
| Pathogen | Treatment Time | Recovery Average of Pathogens (log CFU/cm2) | |
|---|---|---|---|
| Aqueous ClO2 | Water Treatment | ||
| Salmonella | 10 | ND | 3.40 ± 0.0 |
| 20 | ND | 2.84 ± 0.2 | |
| 30 | ND | 2.84 ± 0.1 | |
| E. coli O157:H7 | 10 | ND | 2.97 ± 0.7 |
| 20 | ND | 2.95 ± 0.5 | |
| 30 | ND | 2.38 ± 0.3 | |
| L. monocytogenes | 10 | ND | 3.00 ± 0.6 |
| 20 | ND | 3.18 ± 0.5 | |
| 30 | ND | 3.47 ± 1.1 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luu, P.; Chhetri, V.S.; Janes, M.E.; King, J.M.; Adhikari, A. Effectiveness of Aqueous Chlorine Dioxide in Minimizing Food Safety Risk Associated with Salmonella, E. coli O157:H7, and Listeria monocytogenes on Sweet Potatoes. Foods 2020, 9, 1259. https://doi.org/10.3390/foods9091259
Luu P, Chhetri VS, Janes ME, King JM, Adhikari A. Effectiveness of Aqueous Chlorine Dioxide in Minimizing Food Safety Risk Associated with Salmonella, E. coli O157:H7, and Listeria monocytogenes on Sweet Potatoes. Foods. 2020; 9(9):1259. https://doi.org/10.3390/foods9091259
Chicago/Turabian StyleLuu, Phillip, Vijay Singh Chhetri, Marlene E. Janes, Joan M. King, and Achyut Adhikari. 2020. "Effectiveness of Aqueous Chlorine Dioxide in Minimizing Food Safety Risk Associated with Salmonella, E. coli O157:H7, and Listeria monocytogenes on Sweet Potatoes" Foods 9, no. 9: 1259. https://doi.org/10.3390/foods9091259
APA StyleLuu, P., Chhetri, V. S., Janes, M. E., King, J. M., & Adhikari, A. (2020). Effectiveness of Aqueous Chlorine Dioxide in Minimizing Food Safety Risk Associated with Salmonella, E. coli O157:H7, and Listeria monocytogenes on Sweet Potatoes. Foods, 9(9), 1259. https://doi.org/10.3390/foods9091259






