Anti-Inflammatory and Cytotoxicity Effects of Cudrania tricuspidata Fruits Vinegar in a Co-Culture System with RAW264.7 Macrophages and 3T3-L1 Adipocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Antibodies
2.2. Wine and Vinegar Preparation
2.3. HPLC Analysis
2.4. Cell Culture and Co-Culture of Macrophages and Adipocytes
2.5. Cell Viability Assay
2.6. Measurement of IL-6, TNF-α, and MCP-1
2.7. NO Assay
2.8. Immunoblotting
2.9. Statistical Analysis
3. Results
3.1. Physicochemical Properties and Polyphenolic Compositions of CTFV
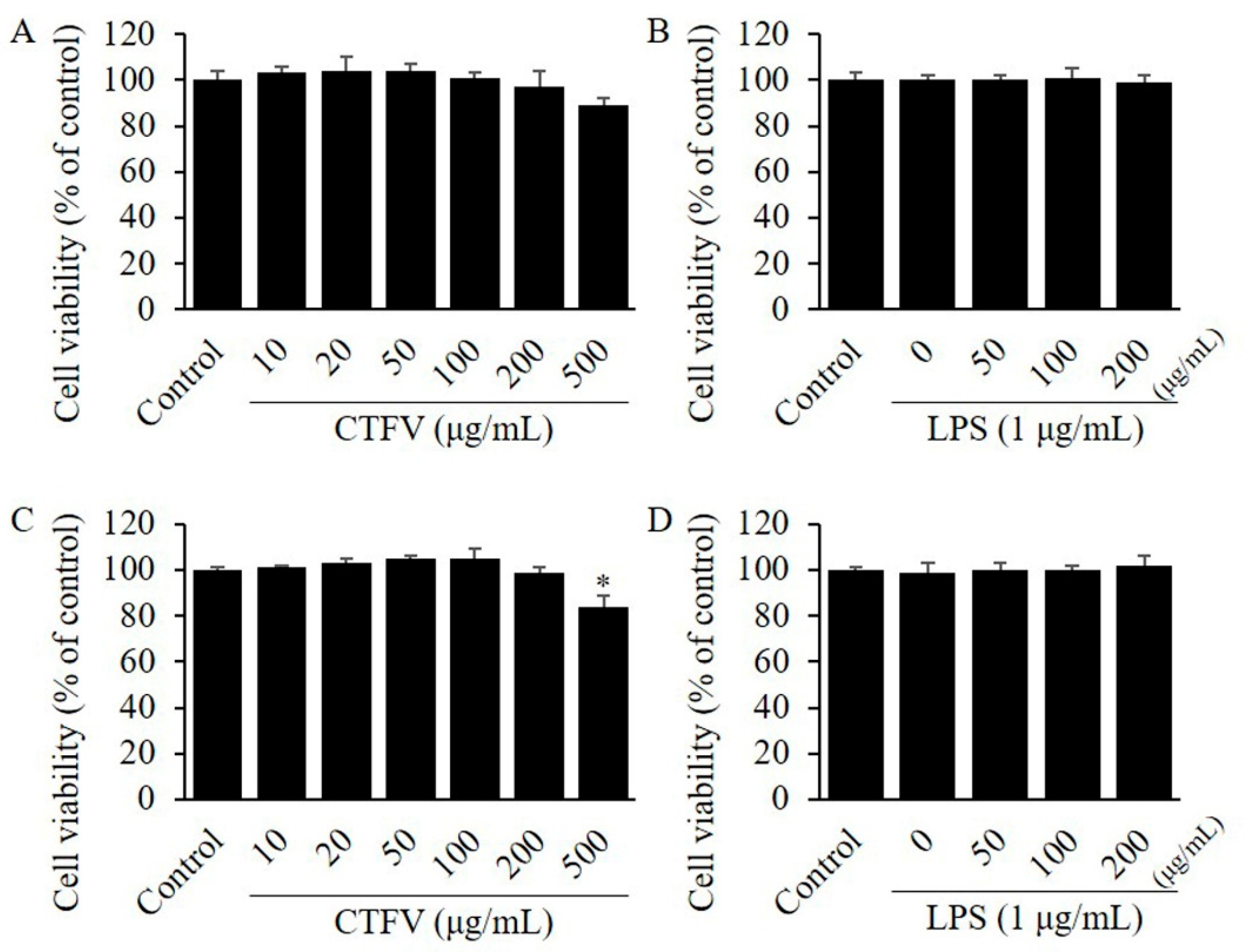
3.2. Effect of CTFV on Cell Viability in 3T3-L1, and Raw264.7
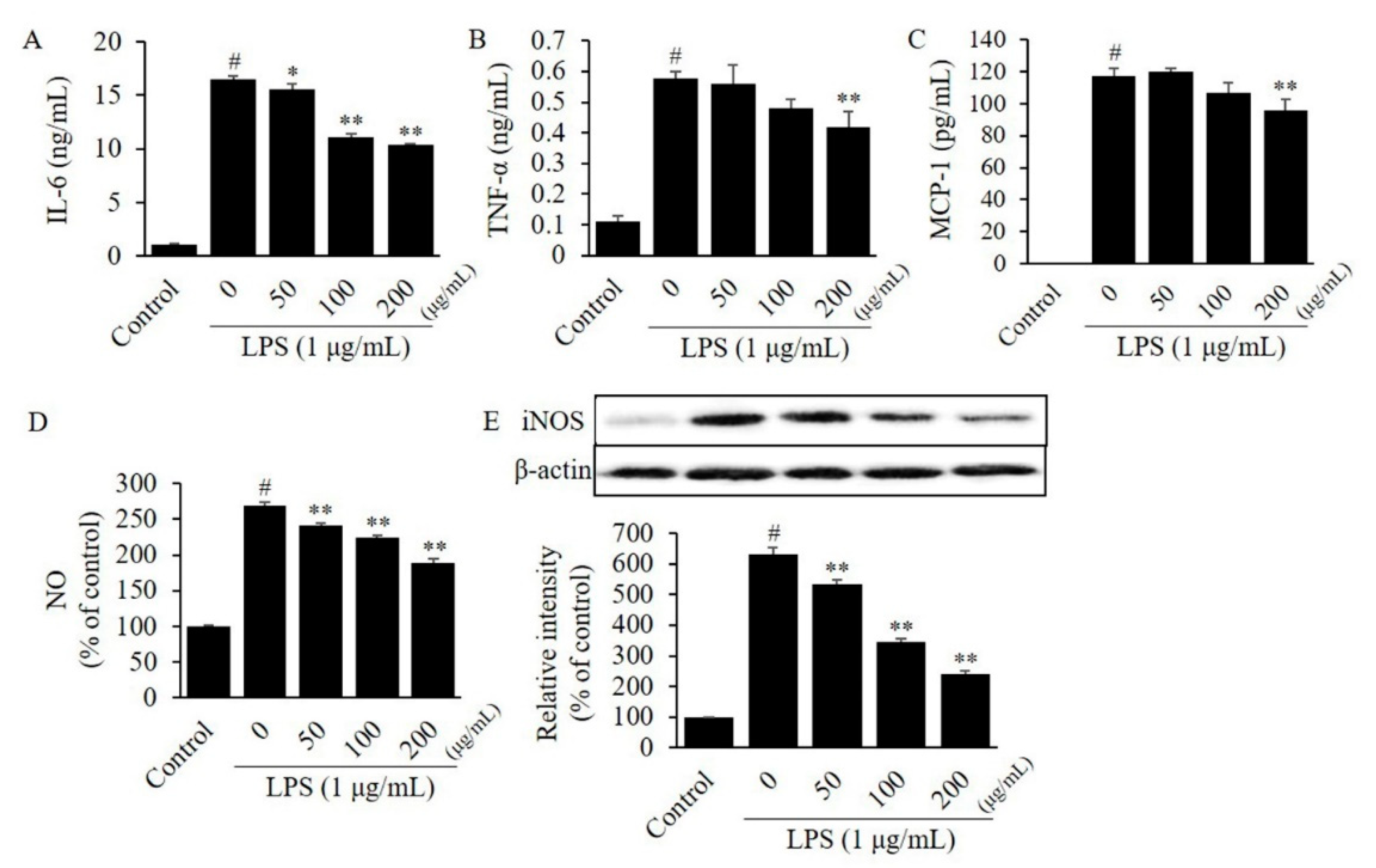
3.3. Effects of CTFV on Cytokines Levels in Macrophages or Adipocytes
3.4. Effects of CTFV on Nitrite, and iNOS Levels in Macrophages or Adipocytes
3.5. Effects of CTFV on Cytokines Levels in Co-Culture Medium
3.6. Effects of CTFV on Nitrite, and iNOS Levels in Co-Culture Medium
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, E.S.; Jo, S.W.; Yim, E.J.; Kim, Y.S.; Park, H.S.; Kim, M.K.; Cho, S.H. Fermentation characteristics of mulberry (Cudrania tricuspidata) fruits produced using microbes isolated from traditional fermented food, and development of fermented soybean food. Korean J. Food Preserv. 2014, 21, 866–877. [Google Scholar] [CrossRef]
- Lee, S.B.; Shin, J.S.; Han, H.S.; Lee, H.H.; Park, J.C.; Lee, K.T. Kaempferol 7-O-β-D-glucoside isolated from the leaves of Cudrania tricuspidata inhibits LPS-induced expression of pro-inflammatory mediators through inactivation of NF-κB, AP-1, and JAK-STAT in RAW 264.7 macrophages. Chem. Biol. Interact. 2018, 284, 101–111. [Google Scholar] [CrossRef]
- Kim, D.C.; Yoon, C.S.; Quang, T.H.; Ko, W.; Kim, J.S.; Oh, H.; Kim, Y.C. Prenylated flavonoids from Cudrania tricuspidata suppress lipopolysaccharide-induced neuroinflammatory activities in BV2 microglial cells. Int. J. Mol. Sci. 2016, 17, 255. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.H.; Kim, S.B.; Liu, Q.; Hwang, B.Y.; Lee, M.K. Prenylated xanthones from the roots of Cudrania tricuspidata as inhibitors of lipopolysaccharide-stimulated nitric oxide production. Arch. Pharm. 2017, 350, e1600263. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Quang, T.H.; Ko, W.; Kim, D.C.; Yoon, C.S.; Oh, H.; Kim, Y.C. Anti-neuroinflammatory effects of cudraflavanone A isolated from the chloroform fraction of Cudrania tricuspidata root bark. Pharm. Biol. 2018, 56, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Surmi, B.K.; Hasty, A.H. Macrophage infiltration into adipose tissue: Initiation, propagation and remodeling. Future Lipidol. 2008, 3, 545–556. [Google Scholar] [CrossRef]
- Suganami, T.; Nishida, J.; Ogawa, Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes role of free fatty acids and tumor necrosis factor a. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2062–2068. [Google Scholar] [CrossRef] [PubMed]
- Suganami, T.; Tanimoto-Koyama, K.; Nishida, J.; Itoh, M.; Yuan, X.; Mizuarai, S.; Kotani, H.; Yamaoka, S.; Miyake, K.; Aoe, S.; et al. Role of the toll-like receptor 4/NF-kB pathway in saturated fatty acid–induced inflammatory changes in the interaction between adipocytes and macrophages. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 84–91. [Google Scholar] [CrossRef]
- Ho, C.L.; Lin, C.Y.; Ka, S.M.; Chen, A.; Tasi, Y.L.; Liu, M.L.; Chiu, Y.C.; Hua, K.F. Bamboo vinegar decreases inflammatory mediator expression and NLRP3 inflammasome activation by inhibiting reactive oxygen species generation and protein kinase C-α/δ activation. PLoS ONE 2013, 8, e75738. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, J.; Yao, J.; Zhang, B.; Duan, W.; Zhao, C.; Du, P.; Song, J.; Zheng, Y.; Wang, M. Shanxi aged vinegar protects against alcohol-induced liver injury via activating Nrf2-mediated antioxidant and inhibiting TLR4-induced inflammatory response. Nutrients 2018, 10, E805. [Google Scholar] [CrossRef]
- Lee, C.S.; Yi, E.H.; Kim, H.R.; Huh, S.R.; Sung, S.H.; Chung, M.H.; Ye, S.K. Anti-dermatitis effects of oak wood vinegar on the DNCB-induced contact hypersensitivity via STAT3 suppression. J. Ethnopharmacol. 2011, 135, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Feng, J.; Wang, X.; Qi, Z.; Shi, X.; An, Y.; Zhang, Q.; Wang, C.; Liu, M.; Liu, B.; et al. Vinegar treatment prevents the development of murine experimental colitis via inhibition of inflammation and apoptosis. J. Agric. Food Chem. 2016, 64, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Golzarand, M.; Ebrahimi-Mamaghani, M.; Arefhosseini, S.R.; Ali Asgarzadeh, A. Effect of processed Berberis vulgaris in apple vinegar on blood pressure and inflammatory markers in type 2 diabetic patients. Iran. J. Diabetes Lipid Disord. 2008, 8, 15–20. [Google Scholar]
- Seo, H.B.; Jeon, B.D.; Ryu, S.P. Persimmon vinegar ripening with the mountain-cultivated ginseng ingestion reduces blood lipids and lowers inflammatory cytokines in obese adolescents. J. Exerc. Nutr. Biochem. 2015, 19, 1–10. [Google Scholar] [CrossRef]
- Ali, Z.; Ma, H.; Ayim, I.; Wali, A. Efficacy of new beverage made of dates vinegar and garlic juice in improving serum lipid profile parameters and inflammatory biomarkers of mildly hyperlipidemic adults: A double blinded, randomized, placebo controlled study. J. Food Biochem. 2018, 42, e12545. [Google Scholar] [CrossRef]
- Ali, Z.; Ma, H.; Wali, A.; Ayim, I.; Sharif, M.N. Daily date vinegar consumption improves hyperlipidemia, β-carotenoid and inflammatory biomarkers in mildly hypercholesterolemic adults. J. Herbal Med. 2019, 17-18, 100265. [Google Scholar] [CrossRef]
- Yagnik, D.; Serafin, V.J.; Shah, A. Antimicrobial activity of apple cider vinegar against Escherichia coli, Staphylococcus aureus and Candida albicans; downregulating cytokine and microbial protein expression. Sci. Rep. 2018, 8, 1732. [Google Scholar] [CrossRef]
- Mohamad, N.E.; Yeap, S.K.; Abu, N.; Lim, K.L.; Zamberi, N.R.; Nordin, N.; Sharifuddin, S.A.; Long, K.; Alitheen, N.B. In vitro and in vivo antitumour effects of coconut water vinegar on 4T1 breast cancer cells. Food Nutr. Res. 2019, 63, 1616. [Google Scholar] [CrossRef]
- Ali, Z.; Ma, H.; Rashid, M.T.; Wali, A.; Younas, S. Preliminary study to evaluate the phytochemicals and physiochemical properties in red and black date’s vinegar. Food Sci. Nutr. 2019, 7, 1976–1985. [Google Scholar] [CrossRef]
- Choi, C.Y.; Park, E.H.; Ryu, S.J.; Shin, W.C.; Kim, M.D. Metabolome analysis and aroma characteristics of fermented fruit vinegar. Microbiol. Biotechnol. Lett. 2018, 46, 416–424. [Google Scholar] [CrossRef]
- Park, S.Y.; Chae, K.S.; Son, R.H.; Jung, J.H.; Im, Y.R.; Kwon, J.W. Quality characteristics and antioxidant activity of bokbunja (black raspberry) vinegars. Food Eng. Prog. 2012, 16, 340–346. [Google Scholar]
- Oh, H.; Jang, S.; Jun, H.I.; Jeong, D.Y.; Kim, Y.S.; Song, G.S. Production of concentrated blueberry vinegar using blueberry juice and its antioxidant and antimicrobial activities. J. Korean Soc. Food Sci. Nutr. 2017, 46, 695–702. [Google Scholar]
- Baek, S.Y.; Lee, C.H.; Park, Y.K.; Choi, H.S.; Mun, J.Y.; Yeo, S.H. Quality characteristics of fermented vinegar prepared with the detoxified Rhus verniciflua extract. J. Korean Soc. Food Preserv. 2015, 22, 674–682. [Google Scholar] [CrossRef]
- Kim, D.W.; Lee, W.J.; Asmelash Gebru, Y.; Choi, H.S.; Yeo, S.H.; Jeong, Y.J.; Kim, S.; Kim, Y.H.; Kim, M.K. Comparison of bioactive compounds and antioxidant activities of Maclura tricuspidata fruit extracts at different maturity stages. Molecules 2019, 24, E567. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, S. Mechanisms of attenuation of clot formation and acute thromboembolism by syringic acid in mice. J. Funct. Foods 2018, 43, 112–122. [Google Scholar] [CrossRef]
- Kim, Y.; Park, Y.; Namkoong, S.; Lee, J. Esculetin inhibits the inflammatory response by inducing heme oxygenase-1 in cocultured macrophages and adipocytes. Food Funct. 2014, 5, 2371–2377. [Google Scholar] [CrossRef]
- Ohira, H.; Fujioka, Y.; Katagiri, C.; Mamoto, R.; Aoyama-Ishikawa, M.; Amako, K.; Izumi, Y.; Nishiumi, S.; Yoshida, M.; Usami, M.; et al. Butyrate attenuates inflammation and lipolysis generated by the interaction of adipocytes and macrophages. J. Atheroscler. Thromb. 2013, 20, 425–442. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, N.H.; Kim, S.J.; Lee, H.J.; Kim, S. Fucoxanthin inhibits the inflammation response in paw edema model through suppressing MAPKs, Akt, and NFκB. J. Biochem. Mol. Toxicol. 2016, 30, 111–119. [Google Scholar] [CrossRef]
- Kadowaki, T.; Wamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- Cox, A.R.; Chernis, N.; Bader, D.A.; Saha, P.; Masschelin, P.M.; Felix, J.; Lian, Z.; Putluri, V.; Rajapakshe, K.; Kim, K.H.; et al. STAT1 dissociates adipose tissue inflammation from insulin sensitivity in obesity. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zeyda, M.; Stulnig, T. Obesity, inflammation, and insulin resistance-a mini-review. Gerontology 2009, 55, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Cancello, R.; Clement, K. Is obesity an inflammatory illness? Role of low-grade inflammation and macropohage infiltration in human white adipose tissue. RCOG 2006, 113, 1141–1147. [Google Scholar]
- Fuggetta, M.P.; Zonfrillo, M.; Villivà, C.; Bonmassar, E.; Ravagnan, G. Inflammatory microenvironment and adipogenic differentiation in obesity: The inhibitory effect of theobromine in a model of human obesity in vitro. Mediators Inflamm. 2019, 2019, 1515621. [Google Scholar] [CrossRef] [PubMed]
- Cinti, S.; Mitchell, G.; Barbatelli, G.; Murano, I.; Ceresi, E.; Faloia, E.; Wang, S.; Fortier, M.; Greenberg, A.S.; Obin, M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005, 46, 2347–2355. [Google Scholar] [CrossRef] [PubMed]
- Zeyda, M.; Farmer, D.; Todoric, J.; Aszmann, O.; Speiser, M.; Gyori, G.; Zlabinger, G.J.; Stulnig, T.M. Human adipose tissue macrophages are of an anti-inflammatory phenotype but capable of excessive pro-inflammatory mediator production. Int. J. Obes. 2007, 31, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef]
- Christiansen, T.; Richelsen, B.; Bruun, J. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int. J. Obesity 2005, 29, 146. [Google Scholar] [CrossRef]
- Hassimotto, N.M.; Genovese, M.I.; Lajolo, F.M. Absorption and metabolism of cyanidin-3- glucoside and cyanidin-3-rutinoside extracted from wild mulberry (Morus nigra L.) in rats. Nutr. Res. 2008, 28, 198–207. [Google Scholar] [CrossRef]
- Maury, E.; Brichard, S.M. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol. Cell. Endocrinol. 2010, 314, 1–16. [Google Scholar] [CrossRef]
- Koppula, S.; Kumar, H.; Kim, I.S.; Choi, D.K. Reactive oxygen species and inhibitors of inflammatory enzymes, NADPH oxidase, and iNOS in experimental models of Parkinson’s disease. Mediat. Inflamm. 2012, 2012, 823902. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Cheng, G.; Conner, D.A.; Huang, Y.; Kucherlapati, R.S.; Munn, L.L.; Ruddle, N.H.; Jain, R.K.; Fukumura, D.; Padera, T.P. Impaired lymphatic contraction associated with immunosuppression. Proc. Natl. Acad. Sci. USA 2011, 108, 18784–18789. [Google Scholar] [CrossRef] [PubMed]
- Scallan, J.P.; Davis, M.J. Genetic removal of basal nitric oxide enhances contractile activity in isolated murine collecting lymphatic vessels. J. Physiol. 2013, 591, 2139–2156. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.L.; Tesfay, W.; Garcia-Barilla, M.C.; Cases, J.A.; Tronco, A.M. Evaluation of the aroma profile of sherry wine vinegar during an experimental aging lagging in wood. J. Agric. Food Chem. 2002, 50, 3173–3178. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.S.; Kim, C.M.; Buller, A.J. Vinegar improves insulin sensitivity to a high carbohydrate meal in subjects with insulin resistance or type-2 diabetes mellitus. Diabetes Care 2004, 27, 281–282. [Google Scholar] [CrossRef]
- Cocchi, M.; Durante, C.; Grandi, M.; Lambertini, P.; Manzini, D.; Marchetti, A. Simultaneous determination of sugars and organic acids in aged vinegar and chemometric data analysis. Talanta 2006, 69, 1166–1175. [Google Scholar] [CrossRef]
- Jeong, M.R.; Jang, Y.S.; Yun, S.I.; Lee, Y.E. Anti-inflammatory activity of fermented fig vinegar. FASEB J. 2008, 22, 703.2. [Google Scholar]
- Lansky, E.P.; Paavilainen, H.M.; Pawlus, A.D.; Newman, R.A. Ficus spp. (fig): Ethnobotany and potential as anticancer and anti-inflammatory agents. J. Ethnopharmacol. 2008, 119, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Bounihi, A.; Bitam, A.; Bouazza, A.; Yargui, L.; Koceir, E.A. Fruit vinegars attenuate cardiac injury via anti-inflammatory and anti-adiposity actions in high-fat diet-induced obese rats. Pharm. Biol. 2017, 55, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.J.; Jo, S.W.; Lee, E.S.; Park, H.S.; Ryu, M.S.; Uhm, T.B.; Kim, H.Y.; Cho, S.H. Fermentation characteristics of mulberry (Cudrania tricuspidata) fruit vinegar produced by acetic acid bacteria isolated from traditional fermented foods. Korean J. Food Preserv. 2015, 22, 108–118. [Google Scholar] [CrossRef]
- De Boer, A.A.; Monk, J.M.; Robinson, L.E. Docosahexaenoic acid decreases pro-inflammatory mediators in an in vitro murine adipocyte macrophage co-culture model. PLoS ONE 2014, 9, e85037. [Google Scholar] [CrossRef] [PubMed]
- Menard, S.; Cerf-Bensussan, N.; Heyman, M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal. Immunol. 2010, 3, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Rogoll, D.; Bergmann, H.; Hellenschmidt, D.; Heinze, J.; Scheppach, W.; Melcher, R.; Richling, E. Influence of apple polyphenols on the intestinal barrier in a colonic cell model. J. Appl. Bot. Food Qual. 2010, 83, 110–117. [Google Scholar]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nut. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Remesy, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Bohn, T.; McDougall, G.J.; Alegría, A.; Alminger, M.; Arrigoni, E.; Aura, A.M.; Brito, C.; Cilla, A.; El, S.N.; Karakaya, S.; et al. Mind the gap—Deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—A position paper focusing on carotenoids and polyphenols. Mol. Nutr. Food Res. 2015, 59, 1307–1323. [Google Scholar] [CrossRef]
- Sang, S.; Lee, M.J.; Hou, Z.; Ho, C.T.; Yang, C.S. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 2005, 53, 9478–9484. [Google Scholar] [CrossRef]
- Li, N.; Taylor, L.S.; Ferruzzi, M.G.; Mauer, L.J. Kinetic study of catechin stability: Effects of pH, concentration, and temperature. J. Agric. Food Chem. 2012, 60, 12531–12539. [Google Scholar] [CrossRef]
- Xiao, J.; Högger, P. Stability of dietary polyphenols under the cell culture conditions: Avoiding erroneous conclusions. J. Agric. Food Chem. 2015, 63, 1547–1557. [Google Scholar] [CrossRef]
- Zeng, L.; Ma, M.; Li, C.; Luo, L. Stability of tea polyphenols solution with different pH at different temperatures. Int. J. Food Prop. 2017, 20, 1–18. [Google Scholar] [CrossRef]



| Concentration of Polyphenolic Compounds (µg/g dw) | |||||
|---|---|---|---|---|---|
| Chlorogenic Acid | Caffeic Acid | Rutin | Total Flavonoid (mg/mL) | Total Phenol (mg/mL) | |
| CTFV | 454.1 ± 5.2 | 60.8 ± 0.4 | 40.1 ± 2.9 | 0.12 ± 0.01 | 4.3 ± 0.2 |
| Concentration of the Parishin Derivatives (µg/g dw) | |||
|---|---|---|---|
| CTFV | Gastrodin | p-Hydroxybenzyl alcohol | Parishin A |
| 273.1 ± 4.6 | 182.9 ± 0.9 | 41.7 ± 0.3 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.-H.; Park, S.-E.; Yeo, S.-H.; Kim, S. Anti-Inflammatory and Cytotoxicity Effects of Cudrania tricuspidata Fruits Vinegar in a Co-Culture System with RAW264.7 Macrophages and 3T3-L1 Adipocytes. Foods 2020, 9, 1232. https://doi.org/10.3390/foods9091232
Choi J-H, Park S-E, Yeo S-H, Kim S. Anti-Inflammatory and Cytotoxicity Effects of Cudrania tricuspidata Fruits Vinegar in a Co-Culture System with RAW264.7 Macrophages and 3T3-L1 Adipocytes. Foods. 2020; 9(9):1232. https://doi.org/10.3390/foods9091232
Chicago/Turabian StyleChoi, Jun-Hui, Se-Eun Park, Soo-Hwan Yeo, and Seung Kim. 2020. "Anti-Inflammatory and Cytotoxicity Effects of Cudrania tricuspidata Fruits Vinegar in a Co-Culture System with RAW264.7 Macrophages and 3T3-L1 Adipocytes" Foods 9, no. 9: 1232. https://doi.org/10.3390/foods9091232
APA StyleChoi, J.-H., Park, S.-E., Yeo, S.-H., & Kim, S. (2020). Anti-Inflammatory and Cytotoxicity Effects of Cudrania tricuspidata Fruits Vinegar in a Co-Culture System with RAW264.7 Macrophages and 3T3-L1 Adipocytes. Foods, 9(9), 1232. https://doi.org/10.3390/foods9091232





