Influence of Thermomechanical Treatment and Ratio of β-Lactoglobulin and α-Lactalbumin on the Denaturation and Aggregation of Highly Concentrated Whey Protein Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Thermomechanical Treatment and Inline Rheological Analyses
2.4. Degree of Denaturation
2.5. Analysis of Protein–Protein Interactions Leading to Aggregation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Influence of Thermomechanical Treatment on the Reaction Behavior and Onset Temperatures of Model Samples
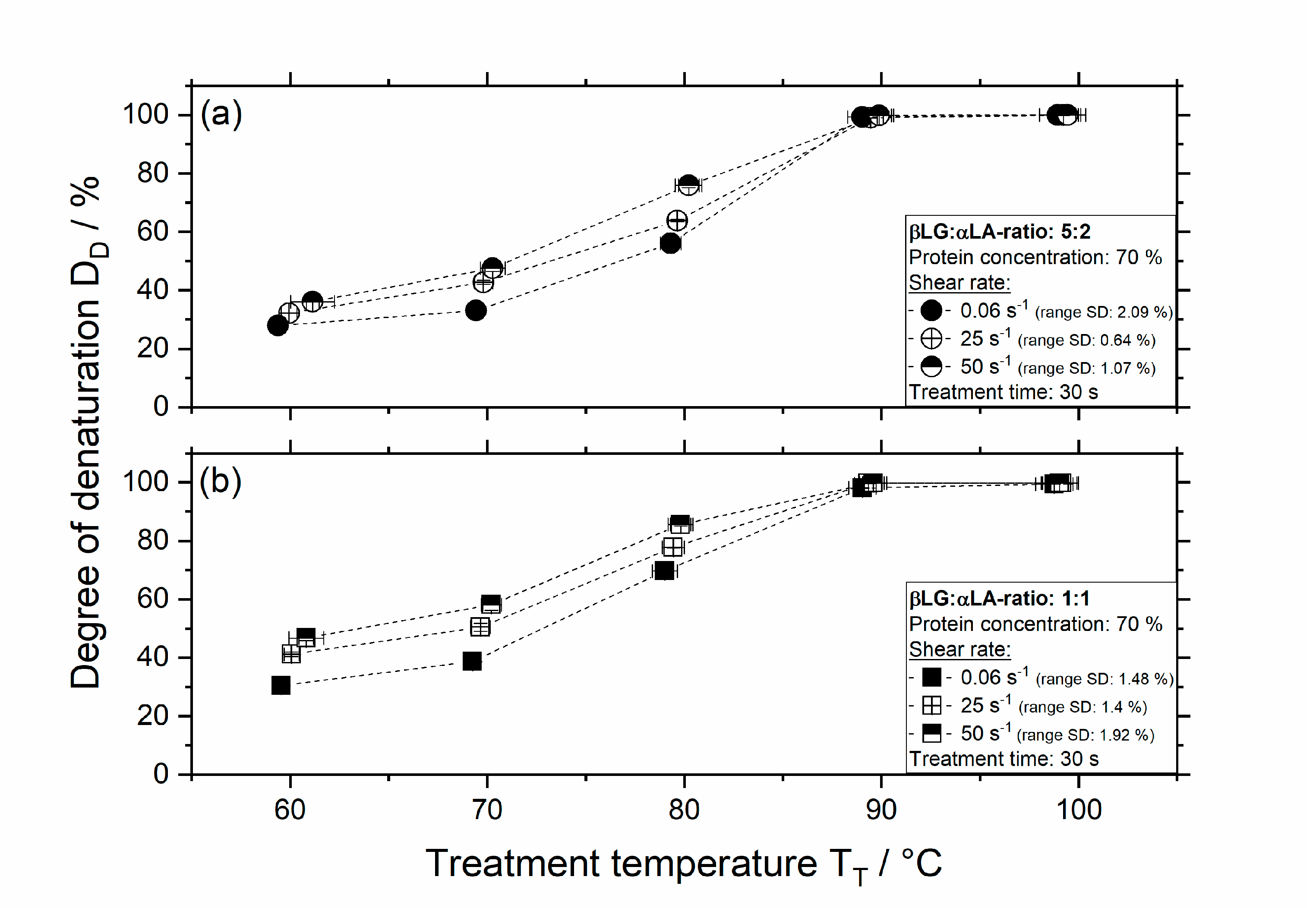
3.2. Influence of Thermomechanical Treatment on the Degree of Denaturation
3.3. Influence of Thermomechanical Treatment on the Protein–Protein Interactions Leading to Aggregation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koch, L.; Hummel, L.; Schuchmann, H.P.; Emin, M.A. Improving the emulsifying properties of whey protein isolate-citrus pectin blends by a novel reactive extrusion approach. J. Food Eng. 2018, 223, 175–188. [Google Scholar] [CrossRef]
- Manoi, K.; Rizvi, S.S.H. Rheological characterizations of texturized whey protein concentrate-based powders produced by reactive supercritical fluid extrusion. Food Res. Int. 2008, 41, 786–796. [Google Scholar] [CrossRef]
- Queguiner, C.; Dumay, E.; Salou-Cavalier, C.; Cheftel, J.C. Microcoagulation of a whey protein isolate by extrusion cooking at acid pH. J. Food Sci. 1992, 57, 610–616. [Google Scholar] [CrossRef]
- Wolz, M.; Kastenhuber, S.; Kulozik, U. High moisture extrusion for microparticulation of whey proteins—Influence of process parameters. J. Food Eng. 2016, 185, 56–61. [Google Scholar] [CrossRef]
- Dissanayake, M.; Liyanaarachchi, S.; Vasiljevic, T. Functional properties of whey proteins microparticulated at low pH. J. Dairy Sci. 2012, 95, 1667–1679. [Google Scholar] [CrossRef]
- McKenzie, H.A.; Sawyer, W.H. Effect of pH on beta-lactoglobulins. Nature 1967, 214, 1101–1104. [Google Scholar] [CrossRef]
- Verheul, M.; Roefs, S.P.F.M.; Kruif, K.G.d. Kinetics of heat-induced aggregation of β-lactoglobulin. J. Agric. Food Chem. 1998, 46, 896–903. [Google Scholar] [CrossRef]
- Georges, C.; Guinand, S.; Tonnelat, J. Etude thermodynamique de la dissociation réversible de la β-lactoglobuline B pour des pH supérieurs à 5,5. Biochim. Et Biophys. Acta 1962, 59, 737–739. [Google Scholar] [CrossRef]
- Loveday, S.M. β-Lactoglobulin heat denaturation: A critical assessment of kinetic modelling. Int. Dairy J. 2016, 52, 92–100. [Google Scholar] [CrossRef]
- Hoffmann, M.A.M.; Mil, P.J.J.M.V. Heat-induced aggregation of β-lactoglobulin: Role of the free thiol group and disulfide bonds. J. Agric. Food Chem. 1997, 45, 2942–2948. [Google Scholar] [CrossRef]
- Sienkiewicz, T. Nomenklatur und einige eigenschaften der molkenproteine. 2. Mitt. α-Lactalbumin, immunoglobuline, proteose-peptone, minorproteine und enzyme. Nahrung 1981, 25, 335–343. [Google Scholar] [CrossRef]
- Apenten, R.K.O. Thermodynamic parameters for 3-state thermal denaturation of human and bovine α-lactalbumin. Thermochim. Acta 1995, 262, 1–12. [Google Scholar] [CrossRef]
- Chaplin, L.C.; Lyster, R.L.J. Irreversible heat denaturation of bovine α-lactalbumin. J. Dairy Res. 1986, 53, 249–258. [Google Scholar] [CrossRef]
- Schnack, U.; Klostermeyer, H. Thermal decomposition of alpha-lactalbumin: I. Destruction of cystine residues. Milchwissenschaft 1980, 35, 206–208. [Google Scholar]
- Schokker, E.P.; Singh, H.; Creamer, L.K. Heat-induced aggregation of β-lactoglobulin A and B with α-lactalbumin. Int. Dairy J. 2000, 10, 843–853. [Google Scholar] [CrossRef]
- Quevedo, M.; Karbstein, H.P.; Emin, M.A. Denaturation behavior and kinetics of single- and multi-component protein systems at extrusion-like conditions. Foods 2020, submitted. [Google Scholar]
- Quevedo, M.; Kulozik, U.; Karbstein, H.P.; Emin, M.A. Kinetics of denaturation and aggregation of highly concentrated β-Lactoglobulin under defined thermomechanical treatment. J. Food Eng. 2020, 274, 109825. [Google Scholar] [CrossRef]
- Quevedo, M.; Karbstein, H.P.; Emin, M.A. Influence of thermomechanical treatment and pH on the denaturation kinetics of highly concentrated whey protein isolate. J. Food Eng. 2020, 292, 110294. [Google Scholar] [CrossRef]
- Quevedo, M.; Jandt, U.; Kulozik, U.; Karbstein, H.P.; Emin, M.A. Investigation on the influence of high protein concentrations on the thermal reaction behaviour of β-lactoglobulin by experimental and numerical analyses. Int. Dairy J. 2019, 97, 99–110. [Google Scholar] [CrossRef]
- Quevedo, M.; Kulozik, U.; Karbstein, H.P.; Emin, M.A. Effect of thermomechanical treatment on the aggregation behaviour and colloidal functionality of β-Lactoglobulin at high concentrations. Int. Dairy J. 2020, 104, 104654. [Google Scholar] [CrossRef]
- Toro-Sierra, J.; Tolkach, A.; Kulozik, U. Fractionation of α-Lactalbumin and β-Lactoglobulin from whey protein isolate using selective thermal aggregation, an optimized membrane separation procedure and resolubilization techniques at pilot plant scale. Food Bioprocess Technol. 2013, 6, 1032–1043. [Google Scholar] [CrossRef]
- Haller, N.; Kulozik, U. Separation of whey protein aggregates by means of continuous centrifugation. Food Bioprocess Technol. 2019, 12, 1052–1067. [Google Scholar] [CrossRef]
- Koch, L.; Emin, M.A.; Schuchmann, H.P. Reaction behaviour of highly concentrated whey protein isolate under defined heat treatments. Int. Dairy J. 2017, 71, 114–121. [Google Scholar] [CrossRef]
- Calvo, M.M.; Leaver, J.; Banks, J.M. Influence of other whey proteins on the heat-induced aggregation of α-lactalbumin. Int. Dairy J. 1993, 3, 719–727. [Google Scholar] [CrossRef]
- Walkenström, P.; Hermansson, A.-M. Effects of shear on pure and mixed gels of gelatin and particulate whey protein. Food Hydrocoll. 1998, 12, 77–87. [Google Scholar] [CrossRef]
- Tolstoguzov, V.B. Thermoplastic extrusion-the mechanism of the formation of extrudate structure and properties. J. Am. Oil Chem. Soc. 1993, 70, 417–424. [Google Scholar] [CrossRef]
- Clark, A.H. Biopolymer gels. Curr. Opin. Colloid Interface Sci. 1996, 1, 712–717. [Google Scholar] [CrossRef]
- Tolkach, A.; Kulozik, U. Effect of pH and T on the reaction kinetic parameters of the thermal denaturation of β-lg. Milchwissenschaft 2005, 60, 249–252. [Google Scholar]
- Spiegel, T.; Huss, M. Whey protein aggregation under shear conditions - effects of pH-value and removal of calcium. Int. J. Food Sci. Technol. 2002, 37, 559–568. [Google Scholar] [CrossRef]
- Oldfield, D.J.; Singh, H.; Taylor, M.W.; Pearce, K.N. Kinetics of Denaturation and aggregation of whey proteins in skim milk heated in an Ultra-High Temperature (UHT) pilot plant. Int. Dairy J. 1998, 8, 311–318. [Google Scholar] [CrossRef]
- Zúñiga, R.N.; Tolkach, A.; Kulozik, U.; Aguilera, J.M. Kinetics of formation and physicochemical characterization of thermally-induced beta-lactoglobulin aggregates. J. Food Sci. 2010, 75, E261–E268. [Google Scholar] [CrossRef] [PubMed]
- Uhrínová, S.; Smith, M.H.; Jameson, G.B.; Uhrín, D.; Sawyer, L.; Barlow, P.N. Structural changes accompanying pH-induced dissociation of the beta-lactoglobulin dimer. Biochemistry 2000, 39, 3565–3574. [Google Scholar] [CrossRef] [PubMed]
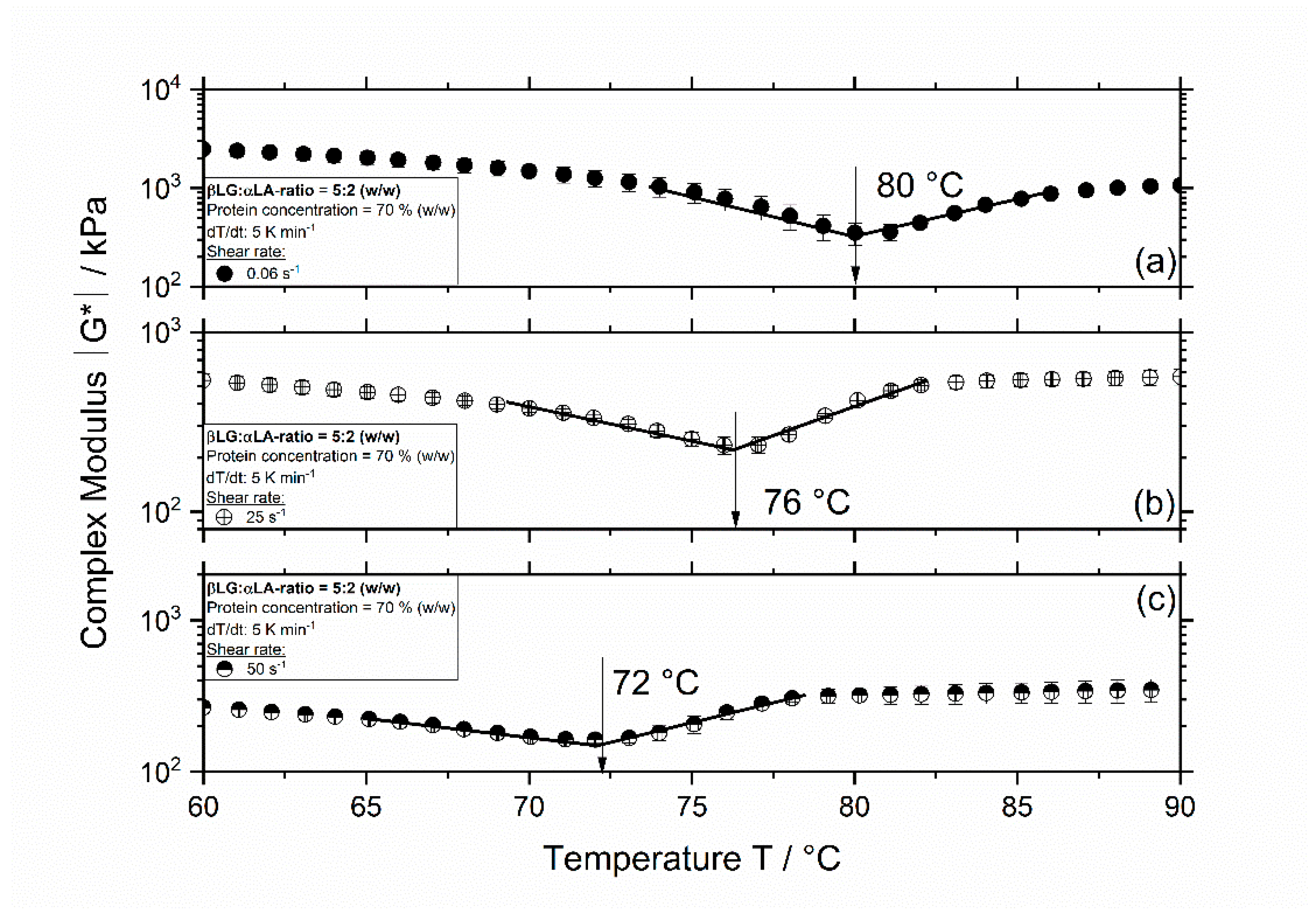
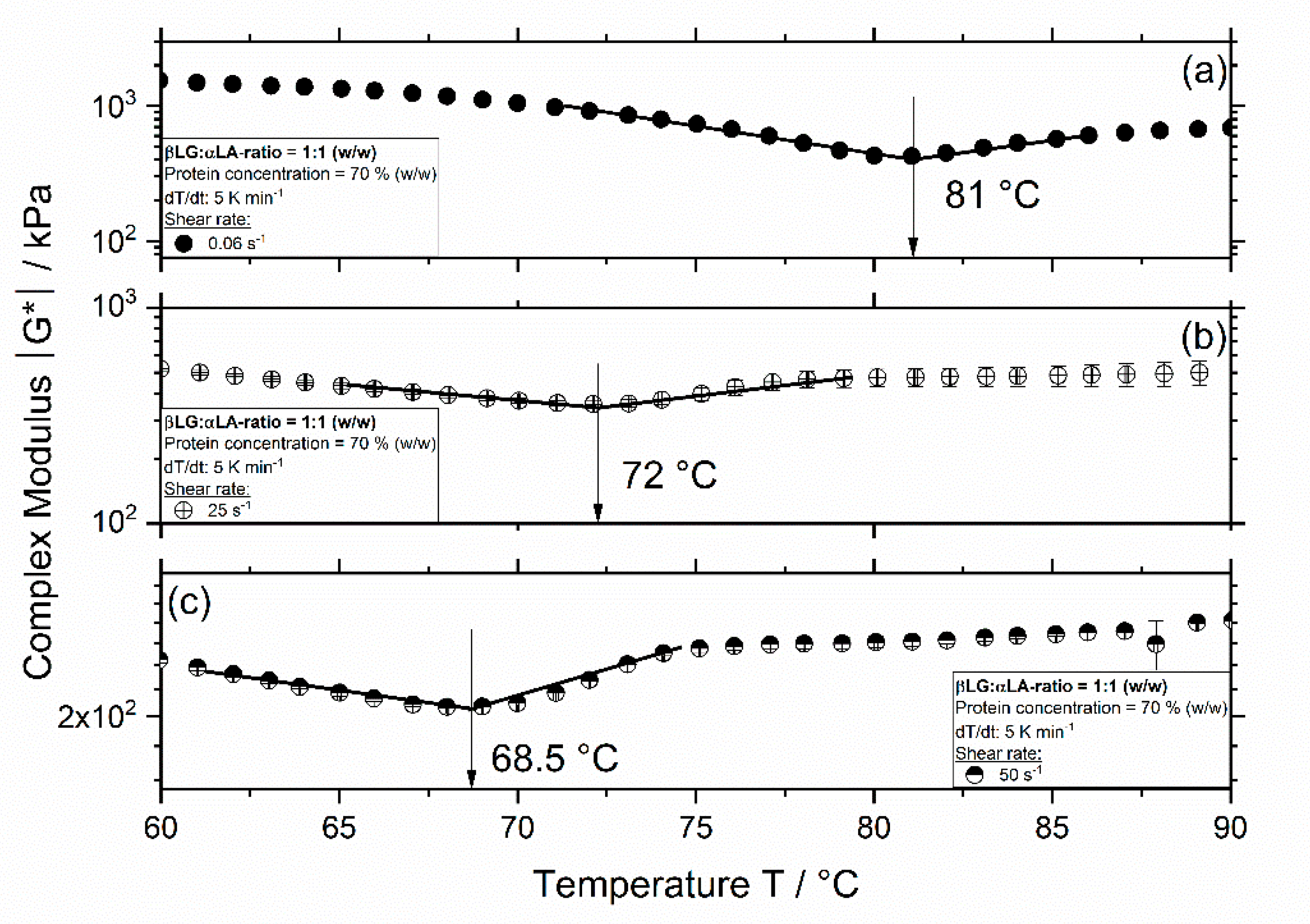
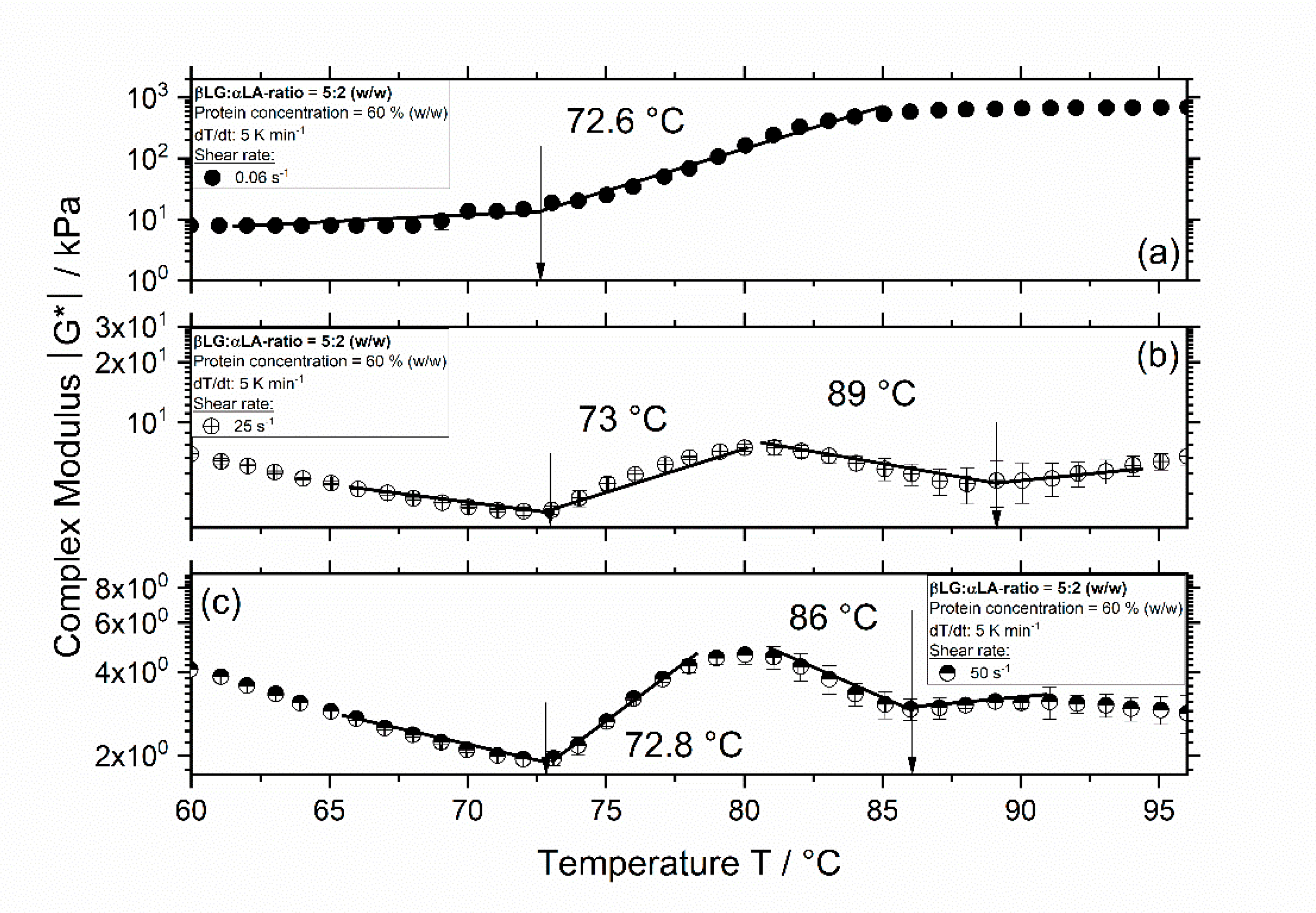
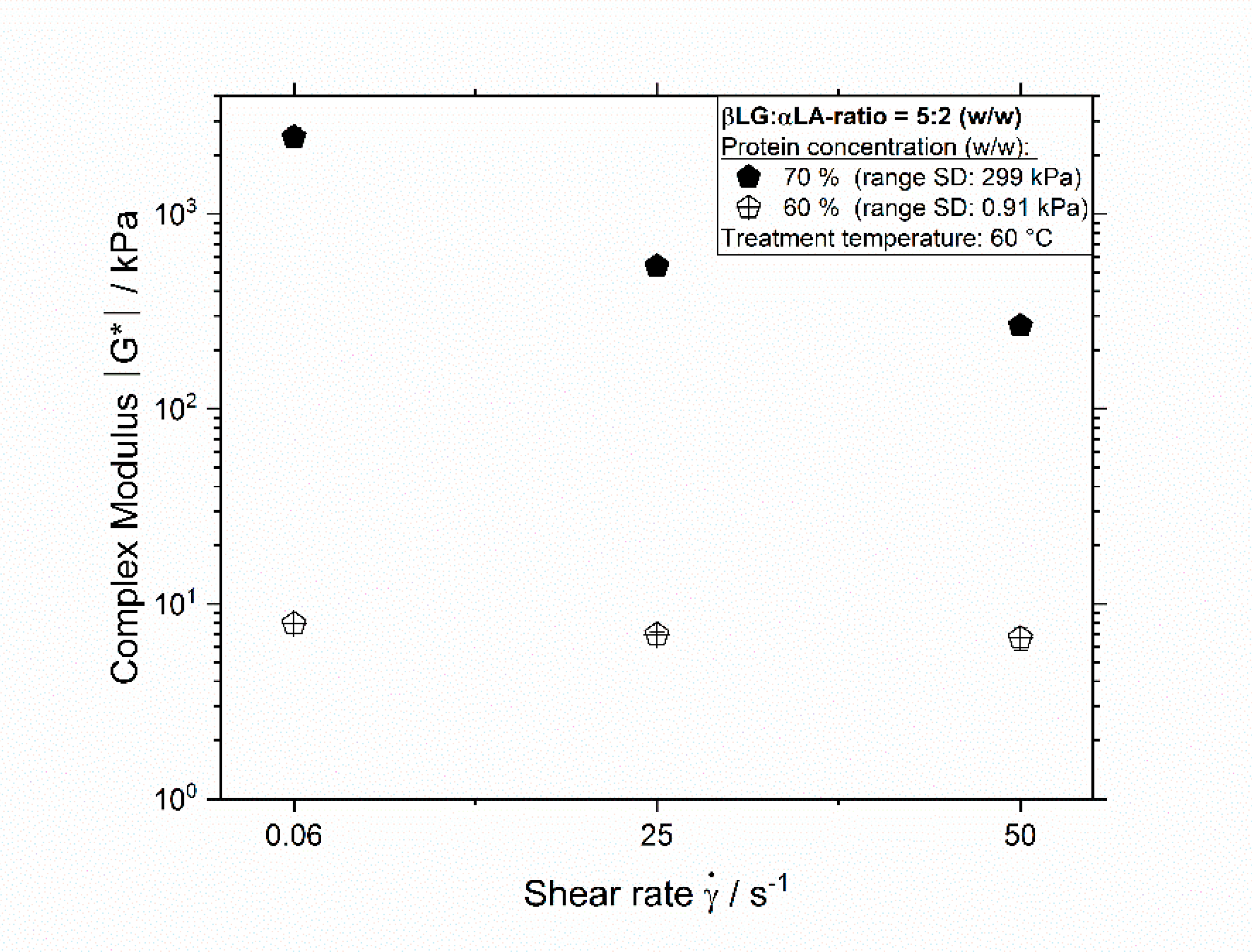

| βLG:αLA Mixing Ratio | Protein Concentration/% | Total Mass Sample/g | βLG/g | αLA/g | Deionized Water/g |
|---|---|---|---|---|---|
| 1:1 | 70 | 100 | 35.02 | 35.02 | 29.96 |
| 5:2 | 50.08 | 19.96 | 29.96 | ||
| 1:1 | 60 | 100 | 30.02 | 30.02 | 39.96 |
| 5:2 | 42.93 | 17.11 | 39.96 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quevedo, M.; Kulozik, U.; Karbstein, H.P.; Emin, M.A. Influence of Thermomechanical Treatment and Ratio of β-Lactoglobulin and α-Lactalbumin on the Denaturation and Aggregation of Highly Concentrated Whey Protein Systems. Foods 2020, 9, 1196. https://doi.org/10.3390/foods9091196
Quevedo M, Kulozik U, Karbstein HP, Emin MA. Influence of Thermomechanical Treatment and Ratio of β-Lactoglobulin and α-Lactalbumin on the Denaturation and Aggregation of Highly Concentrated Whey Protein Systems. Foods. 2020; 9(9):1196. https://doi.org/10.3390/foods9091196
Chicago/Turabian StyleQuevedo, Maria, Ulrich Kulozik, Heike P. Karbstein, and M. Azad Emin. 2020. "Influence of Thermomechanical Treatment and Ratio of β-Lactoglobulin and α-Lactalbumin on the Denaturation and Aggregation of Highly Concentrated Whey Protein Systems" Foods 9, no. 9: 1196. https://doi.org/10.3390/foods9091196





