Effect of Different Method of Drying of Five Varieties Grapes (Vitis vinifera L.) on the Bunch Stem on Physicochemical, Microbiological, and Sensory Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Drying Methods
2.3. Determination of pH
2.4. Determination of Total Soluble Solids Content
2.5. Determination of Vitamin C
2.6. Determination of Instrumental Colour
2.7. Microbiological Analysis
2.8. Sensory Analysis
2.9. Statistical Assessment
3. Results and Discussion
3.1. pH and Total Soluble Solids Content
3.2. Vitamin C Content
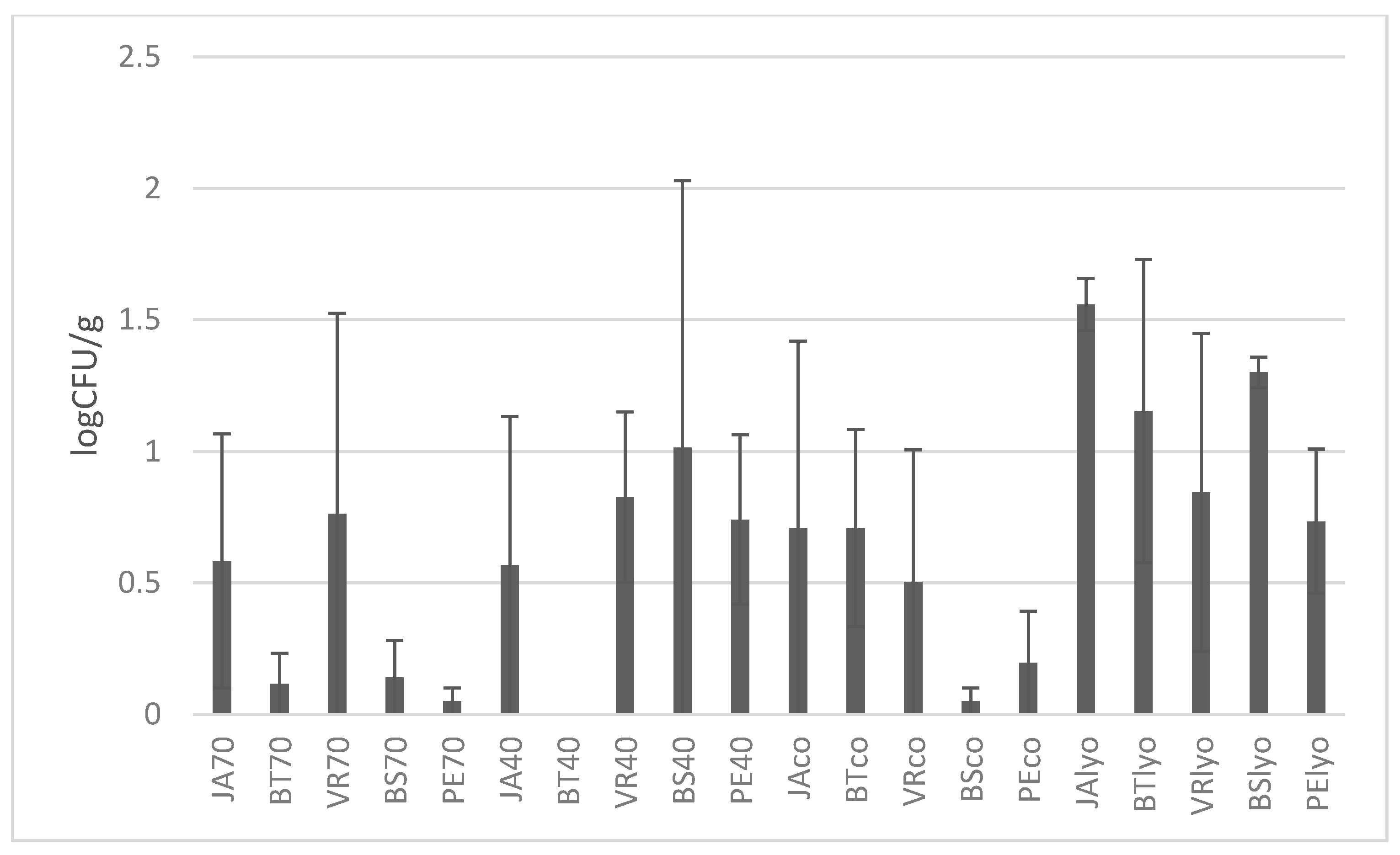
3.3. Microbiological Analysis
3.4. Instrumental Colour Measuring
3.5. Sensory Atributes
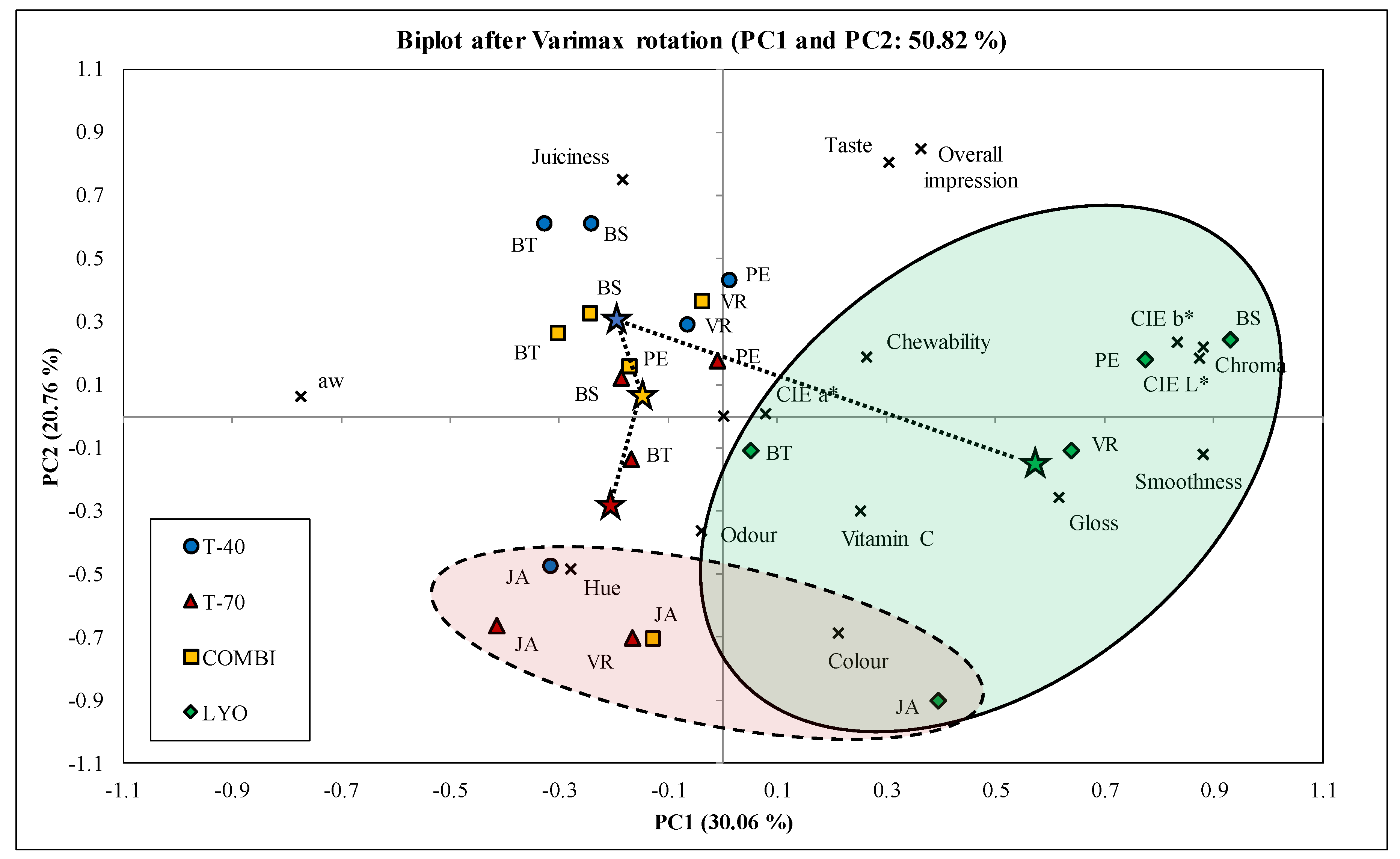
3.6. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Williamson, G.; Carughi, A. Polyphenol content and health benefits of raisins. Nutr. Res. Rev. 2010, 30, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Khiari, R.; Zemni, H.; Mihoubi, D. Raisin processing: Physicochemical, nutritional and microbiological quality characteristics as affected by drying process. Food Rev. Int. 2019, 35, 246–298. [Google Scholar] [CrossRef]
- Bhat, N.R.; Desai, B.B.; Suleiman, S.K. Grapes and Raisins. In Handbook of Fruits and Fruit Processing; Sinha, N.K., Sidhu, J.S., Barta, J., Wu, J.S.B., Cano, M.P., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 447–459. [Google Scholar]
- Wang, J.; Mujumdar, A.S.; Mu, W.; Feng, J.; Zhang, X.; Zhang, Q.; Fang, X.; Gao, Z.; Xiao, H. Grape Drying: Current Status and Future Trends, Grape and Wine Biotechnology, Antonio Morata and Iris Loira; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- FAO-OIV. Table and Dried grapes-FAO-OIV Focus 2016. Available online: http://www.fao.org/3/a-i7042e.pdf (accessed on 6 May 2020).
- Azzouz, S.; Hermassi, I.; Toujani, M.; Belghith, A. Effect of drying temperature on the rheological characteristics of dried seedless grapes. Food Bioprod. Process. 2016, 100, 246–254. [Google Scholar] [CrossRef]
- USDA. Raisins: World Markets and Trade. Available online: https://apps.fas.usda.gov/psdonline/circulars/raisins.pdf (accessed on 7 May 2020).
- Simal, S.; Rosselló, C.; Sánchez, E.; Cañellas, J. Quality of Raisins Treated and Stored under Different Conditions. J. Agric. Food Chem. 1996, 44, 3297–3302. [Google Scholar] [CrossRef]
- Ruiz-Bejarano, M.J.; Durán-Guerrero, E.; Castro, R.; Barroso, C.G.; Rodríguez-Dodero, M.C. Use of Sensory Analysis to Investigate the Influence of Climate Chambers and Other Process Variables in the Production of Sweet Wines. Foods 2020, 9, 424. [Google Scholar] [CrossRef] [Green Version]
- Adiletta, G.; Russo, P.; Senadeera, W.; Di Matteo, M. Drying characteristics and quality of grape under physical pretreatment. J. Food Eng. 2016, 172, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Nowak, D.; Lewicki, P.P. Infrared drying of apple slices. Innov. Food Sci. Emerg. 2004, 5, 353–360. [Google Scholar] [CrossRef]
- Perera, C.O. Selected Quality Attributes of Dried Foods. Dry. Technol. 2007, 23, 717–730. [Google Scholar] [CrossRef]
- Hakobyan, L.; Grigoryan, K.; Trchounian, A. The dynamics of ochratoxigenic fungi contents through different stages of dried grape production. BIO Web Conf. 2017, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Alasalvar, C.; Shahidi, F. Composition, Phytochemicals, and Beneficial Health Effects of Dried Fruits: An Overview; Wiley-Blackwell and John Wiley & Sons, Inc. Publication: Hoboken, NJ, USA, 2013. [Google Scholar]
- Restani, P.; Frigerio, G.; Colombo, F.; Sousa, L.P.; Altindişli, A.; Pastor, R.F.; Lorenzo, C. Raisins in human health: A review. BIO Web Conf. 2016, 7, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Chambers, K.F.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol Effects on Cholesterol Metabolism via Bile Acid Biosynthesis, CYP7A1: A Review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molada-Tebar, A.; Riutort-Mayol, G.; Marqués-Mateu, Á.; Lerma, J.L. A Gaussian Process Model for Colour Camera Characterization: Assessment in Outdoor Levantine Rock Art Scenes. Sensors 2019, 19, 4610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 21527-2. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 2: Colony Count Technique in Products with Water Activity Less than or Equal to 0.95; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- ISO 4833-1. Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 1: Colony Count at 30 °C by the Pour Plate Technique; ISO: Geneva, Switzerland, 2019. [Google Scholar]
- XLSTAT-Pro, v. 7.5.2, Addinsoft, Paris, France; 2004. Available online: http://xlstat-pro.freedownloadscenter.com/windows/ (accessed on 7 May 2020).
- Çağlarirmak, N.; Ochratoxin, A. Hydroxymethylfurfural and Vitamin C Levels of Sun-Dried Grapes and Sultanas. J. Food Process. Preserv. 2006, 30, 549–562. [Google Scholar] [CrossRef]
- Carranza-Concha, J.; Benlloch, M.; Camacho, M.M.; Martínez-Navarrete, N. Effects of drying and pretreatment on the nutritional and functional quality of raisins. Food Bioprod. Process. 2012, 90, 243–248. [Google Scholar] [CrossRef]
- Xiao, H.W.; Pang, C.L.; Wang, L.H.; Bai, J.W.; Yang, W.X.; Gao, Z.J. Drying kinetics and quality of Monukka seedless grapes dried in an air-impingement jet dryer. Biosyst. Eng. 2010, 105, 233–240. [Google Scholar] [CrossRef]
- Clary, C.D.; Mejia-Meza, E.; Wang, S.; Petrucci, V.E. Improving Grape Quality Using Microwave Vacuum Drying Associated with Temperature Control. J. Food Sci. 2007, 72, E23–E28. [Google Scholar] [CrossRef]
- Thakur, A.K.; Saharan, V.K.; Gupta, R.K. Drying of ‘Perlette’ grape under different physical treatment for raisin making. J. Food Sci. Technol. 2010, 47, 626–631. [Google Scholar] [CrossRef] [Green Version]
- Zemni, H.; Sghaier, A.; Khiari, R.; Chebil, S.; Ben Ismail, H.; Nefzaoui, R.; Hamdi, Z.; Lasram, S. Physicochemical, Phytochemical and Mycological Characteristics of Italia Muscat Raisins Obtained Using Different Pre-Treatment and Drying Techniques. Food Bioprocess. Technol. 2017, 10, 479–490. [Google Scholar] [CrossRef]
- McCoy, S.; Chang, J.W.; McNamara, K.T.; Oliver, H.F.; Deering, A.J. Quality and Safety Attributes of Afghan Raisins before and after Processing. Food Sci. Nutr. 2015, 3, 56–64. [Google Scholar] [CrossRef]
- Doerflinger, F.; Pagay, V. Objective assessment of dried sultana grape quality using digital image analysis. Aust. J. Grape Wine Res. 2018, 24, 234–240. [Google Scholar] [CrossRef]
- Serratosa, M.P.; Lopez-Toledano, A.; Merida, J.; Medina, M. Changes in Colour and Phenolic Compounds during the Raisining of Grape Cv. Pedro Ximenez. J. Agric. Food Chem. 2008, 56, 2810–2816. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mujumdar, A.S.; Wang, H.; Fang, X.M.; Xiao, H.W.; Raghavan, V. Effect of drying method and cultivar on sensory attributes, textural profiles, and volatile characteristics of grape raisins. Dry. Technol. 2019. [Google Scholar] [CrossRef]
- Doets, E.L.; Kremer, S. The silver sensory experience—A review of senior consumers’ food perception, liking and intake. Food Qual. Prefer. 2016, 48, 316–332. [Google Scholar] [CrossRef]
- Berk, Z. Food Process Engineering and Technology, 2nd ed.; Food Science and Technology International Series; Elsevier/Academic Press: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Angulo, O.; Fidelibus, M.W.; Heymann, H. Grape cultivar and drying method affect sensory characteristics and consumer preference of raisins. J. Sci. Food Agric. 2007, 87, 865–870. [Google Scholar] [CrossRef]
- Chervin, C.; Aked, J.; Crisosto, C.H. Grapes. In Crop Post-Harvest: Science and Technology; Rees, D., Farrell, G., Orchard, J., Eds.; Wiley-Blackwell: Oxford, UK, 2012; pp. 187–211. [Google Scholar]
- Gee, M. Some Flavor and Colour Changes during Low Temperature Dehydration of Grapes. J. Food Sci. 1980, 45, 146–147. [Google Scholar] [CrossRef]
- Schwarz, M.; Rodríguez-Dodero, M.C.; Jurado, M.S.; Puertas, B.; Barroso, C.G.; Guillén, D.A. Analytical Characterization and Sensory Analysis of Distillates of Different Varieties of Grapes Aged by an Accelerated Method. Foods 2020, 9, 277. [Google Scholar] [CrossRef] [Green Version]



| Variety | Designation | Grapes Colour | Grapes Description | Origin/Variety Number VIVC |
|---|---|---|---|---|
| Jakubské | JA | blue | medium large, cylindrical shape, seeds | France/9280 |
| Beauty seedless | BT | blue | large, egg-shaped, seedless | USA/1065 |
| Vrboska | VR | pink | medium large, conical shape, seeds | Czech Republic/17515 |
| Bezsemenné | BS | white | large, round shape, seedless | Czech Republic/new cultivar AMPELOS |
| Perlette | PE | white | large, cylindrical shape, seedless | USA/9168 |
| Variety | pH | Total Soluble Solids (°Brix) |
|---|---|---|
| JA | 3.21 ± 0.03 b | 13.50 ± 0.29 ab |
| BT | 3.36 ± 0.01 a | 14.33 ± 0.66 ab |
| VR | 3.37 ± 0.01 a | 15.67 ± 0.66 b |
| BS | 3.14 ± 0.01 b | 15.00 ± 0.60 ab |
| PE | 3.42 ± 0.01 a | 13.33 ± 1.45 a |
| Variety | Parameter | Fresh | 40 | 70 | co | Lyo |
|---|---|---|---|---|---|---|
| PE | L*(D65) | 48.71 ± 3.85 a | 27.40 ± 2.46 b | 27.47 ± 1.83 b | 26.42 ± 1.31 b | 67.37 ± 3.09 c |
| a*(D65) | −2.92 ± 1.12 a | 5.88 ± 0.88 b | 3.74 ± 0.62 c | 5.59 ± 1.56 b | −2.64 ± 1.35 a | |
| b*(D65) | 20.23 ± 3.20 a | 7.14 ± 1.53 b | 4.22 ± 0.59 c | 5.60 ± 1.83 bc | 42.62 ± 3.10 d | |
| ΔE*ab | 0.00 | 26.51 *** | 27.42 *** | 27.99 *** | 29.15 *** | |
| BS | L*(D65) | 39.70 ± 1.31 a | 28.34 ± 0.86 b | 19.96 ± 1.32 c | 26.56 ± 0.62 b | 71.88 ± 3.87 d |
| a*(D65) | −3.39 ± 0.64 a | 3.09 ± 0.57 b | 2.65 ± 0.54 b | 3.17 ± 0.42 b | −5.04 ± 3.64 a | |
| b*(D65) | 12.16 ± 2.17a | 2.58 ± 0.74 b | 2.58 ± 0.46 b | 4.61 ± 0.66 c | 42.34 ± 3.65 d | |
| ΔE*ab | 0.00 | 16.21 *** | 22.76 *** | 16.51 *** | 44.15 *** | |
| JA | L*(D65) | 27.96 ± 1.50 a | 24.81 ± 2.79 b | 22.05 ± 1.30 c | 25.24 ± 1.27 b | 29.80 ± 2.44 a |
| a*(D65) | 0.40 ± 0.31 a | 0.64 ± 0.44 a | 2.10 ± 0.58 b | 0.97 ± 0.48 a | 1.50 ± 2.53 ab | |
| b*(D65) | −1.47 ± 0.69 a | −1.94 ± 0.84 a | −0.35 ± 0.25 b | −0.57 ± 0.30 b | −0.86 ± 0.91 ab | |
| ΔE*ab | 0 | 3.19 * | 6.25 ** | 2.91 | 2.24 | |
| VR | L*(D65) | 28.85 ± 0.81 a | 25.30 ± 1.64 b | 25.02 ± 1.65 b | 29.14 ± 1.92 a | 41.17 ± 6.22 c |
| a*(D65) | 8.59 ± 1.30 a | 4.36 ± 0.80 b | 3.31 ± 0.61 b | 4.82 ± 0.44 b | 23.39 ± 4.74 c | |
| b*(D65) | 3.62 ± 0.96 a | 2.98 ± 1.37 a | 2.51 ± 1.04 a | 5.61 ± 0.91 b | 13.04 ± 3.40 c | |
| ΔE*ab | 0 | 5.56 * | 6.61 ** | 4.28 * | 21.43 *** | |
| BT | L*(D65) | 28.67 ± 2.10 a | 27.32 ± 0.89 ac | 23.42 ± 1.46 b | 26.10 ± 1.31 c | 22.90 ± 1.95 b |
| a*(D65) | 0.65 ± 0.40 a | −0.02 ± 0.19 b | 0.67 ± 0.19 a | 1.38 ± 0.63 c | −0.01 ± 0.37 b | |
| b*(D65) | −1.99 ± 0.93 a | −3.07 ± 0.51 b | −0.49 ± 0.11 c | −0.50 ± 0.24 c | −2.22 ± 0.76 a | |
| ΔE*ab | 0 | 1.84 | 5.45 * | 3.05 * | 5.81 * |
| Variety | Smoothness | Gloss | Colour | Odour | Chewability | Juiciness | Taste | Overall Impression | |
|---|---|---|---|---|---|---|---|---|---|
| JA | 70 | 11.3 ± 3.3 a | 10.0 ± 2.0 a | 65.6 ± 8.8 | 74.3 ± 6.9 | 57.4 ± 9.0 a | 43.0 ± 7.3 | 52.7 ± 9.8 | 51.1 ± 7.9 |
| 40 | 22.0 ± 4.0 a | 12.9 ± 2.6 a | 76.3 ± 5.4 | 71.9 ± 6.2 | 66.6 ± 8.0 ab | 59.0 ± 7.2 | 64.6 ± 8.5 | 55.8 ± 7.9 | |
| co | 17.8 ± 5.1 a | 26.5 ± 7.7 b | 78.4 ± 4.9 | 71.9 ± 7.3 | 68.3 ± 9.4 ab | 44.0 ± 8.3 | 64.3 ± 9.6 | 53.5 ± 9.5 | |
| lyo | 72.9 ± 5.8 b | 58.3 ± 6.8 c | 78.3 ± 8.2 | 79.9 ± 4.8 | 78.2 ± 7.2 b | 42.6 ± 10.1 | 67.9 ± 8.4 | 63.4 ± 8.6 | |
| PE | 70 | 27.2 ± 3.6 a | 44.7 ± 8.2 | 54.5 ± 10.6 | 62.6 ± 8.4 | 61.5 ± 6.8 ab | 57.1 ± 4.4 | 73.0 ± 5.2 | 71.5 ± 5.4 |
| 40 | 25.5 ± 5.6 a | 34.0 ± 7.1 | 49.9 ± 10.0 | 69.8 ± 4.8 | 71.0 ± 5.9 b | 67.2 ± 6.7 | 74.9 ± 6.9 | 73.3 ± 6.4 | |
| co | 21.8 ± 5.6 a | 22.6 ± 6.3 | 49.5 ± 9.9 | 67.4 ± 4.5 | 58.6 ± 5.5 a | 54.3 ± 5.4 | 65.7 ± 6.2 | 64.2 ± 5.8 | |
| lyo | 46.1 ± 4.1 b | 43.1 ± 7.6 | 55.8 ± 10.1 | 61.1 ± 7.0 | 60.5 ± 7.0 ab | 45.0 ± 7.1 | 74.3 ± 6.3 | 73.0 ± 6.6 | |
| BS | 70 | 22.1 ± 5.8 a | 15.7 ± 6.3 | 58.2 ± 8.4 | 72.0 ± 7.0 | 40.7 ± 6.9 a | 47.8 ± 7.9 | 79.0 ± 4.5 | 66.8 ± 7.5 |
| 40 | 24.7 ± 3.7 a | 14.0 ± 4.8 | 52.9 ± 9.1 | 75.8 ± 5.8 | 66.0 ± 9.0 ab | 65.5 ± 7.8 | 86.0 ± 2.9 | 75.8 ± 5.1 | |
| co | 13.4 ± 3.3 a | 15.4 ± 4.3 | 55.5 ± 8.3 | 63.6 ± 5.8 | 56.4 ± 7.4 ab | 50.3 ± 8.9 | 76.6 ± 5.6 | 69.0 ± 6.1 | |
| lyo | 70.3 ± 4.4 b | 26.9 ± 9.4 | 69.5 ± 9.9 | 73.8 ± 8.7 | 70.7 ± 8.9 b | 56.1 ± 8.8 | 82.2 ± 6.6 | 78.6 ± 6.3 | |
| VR | 70 | 20.8 ± 4.4 a | 24.5 ± 6.3 | 62.7 ± 7.3 | 72.4 ± 5.8 | 33.0 ± 7.0 a | 28.3 ± 5.0 a | 58.4 ± 8.4 | 51.4 ± 4.3 |
| 40 | 35.4 ± 4.9 a | 20.9 ± 5.2 | 62.9 ± 7.9 | 72.7 ± 5.1 | 65.0 ± 6.9 b | 66.1 ± 6.7 c | 80.8 ± 3.7 | 72.7 ± 4.3 | |
| co | 28.7 ± 5.2 a | 21.1 ± 5.8 | 59.4 ± 9.2 | 67.4 ± 4.2 | 66.0 ± 6.3 b | 61.2 ± 5.1 c | 80.9 ± 4.4 | 71.9 ± 5.2 | |
| lyo | 59.0 ± 4.4b | 48.2 ± 8.6 | 73.8 ± 8.2 | 62.8 ± 5.6 | 63.3 ± 7.2 b | 45.1 ± 8.3 b | 79.5 ± 6.6 | 70.3 ± 7.1 | |
| BT | 70 | 16.3 ± 6.1 a | 46.8 ± 9.2 a | 67.2 ± 6.2 | 61.8 ± 5.9 | 48.9 ± 8.0 a | 44.8 ± 8.7 | 73.0 ± 5.9 | 68.4 ± 6.8 |
| 40 | 19.1 ± 5.8 a | 20.9 ± 5.2 b | 62.0 ± 6.1 | 68.0 ± 5.7 | 74.2 ± 5.1 b | 76.4 ± 4.9 | 80.6 ± 6.2 | 81.4 ± 5.8 | |
| co | 19.4 ± 5.5 a | 19.1 ± 5.1 b | 63.4 ± 5.6 | 65.8 ± 3.8 | 66.3 ± 6.4 b | 58.6 ± 5.3 | 75.6 ± 6.1 | 72.5 ± 6.3 | |
| lyo | 46.0 ± 5.9 b | 37.4 ± 5.2 a | 73.7 ± 5.4 | 69.9 ± 0.6 | 66.1 ± 7.4 b | 53.8 ± 7.4 | 75.6 ± 6.2 | 73.7 ± 7.9 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langová, R.; Jůzl, M.; Cwiková, O.; Kos, I. Effect of Different Method of Drying of Five Varieties Grapes (Vitis vinifera L.) on the Bunch Stem on Physicochemical, Microbiological, and Sensory Quality. Foods 2020, 9, 1183. https://doi.org/10.3390/foods9091183
Langová R, Jůzl M, Cwiková O, Kos I. Effect of Different Method of Drying of Five Varieties Grapes (Vitis vinifera L.) on the Bunch Stem on Physicochemical, Microbiological, and Sensory Quality. Foods. 2020; 9(9):1183. https://doi.org/10.3390/foods9091183
Chicago/Turabian StyleLangová, Radka, Miroslav Jůzl, Olga Cwiková, and Ivica Kos. 2020. "Effect of Different Method of Drying of Five Varieties Grapes (Vitis vinifera L.) on the Bunch Stem on Physicochemical, Microbiological, and Sensory Quality" Foods 9, no. 9: 1183. https://doi.org/10.3390/foods9091183
APA StyleLangová, R., Jůzl, M., Cwiková, O., & Kos, I. (2020). Effect of Different Method of Drying of Five Varieties Grapes (Vitis vinifera L.) on the Bunch Stem on Physicochemical, Microbiological, and Sensory Quality. Foods, 9(9), 1183. https://doi.org/10.3390/foods9091183








