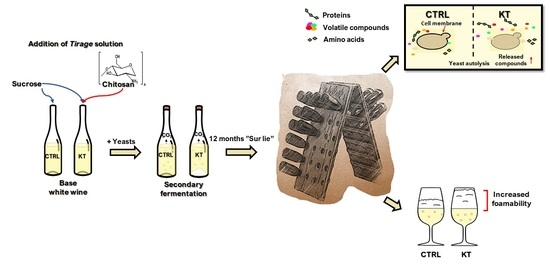
Chitosan in Sparkling Wines Produced by the Traditional Method: Influence of Its Presence during the Secondary Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples Preparation
2.3. Oenological Parameters
2.4. Organic Acids
2.5. Phenolic Acids
2.6. Total Protein Content
2.7. Amino Acids and Amines
2.7.1. Derivatization
2.7.2. HPLC Analysis
2.8. Determination of Mannose
2.9. Foamability
2.10. Sensory Analysis
2.11. Wine Volatile Compounds
2.12. Statistical Analysis
3. Results
3.1. Oenological Parameters
3.2. Organic Acids and Glycerol
3.3. Protein Content
3.4. Phenolic Acids
3.5. Amino Acids and Amines
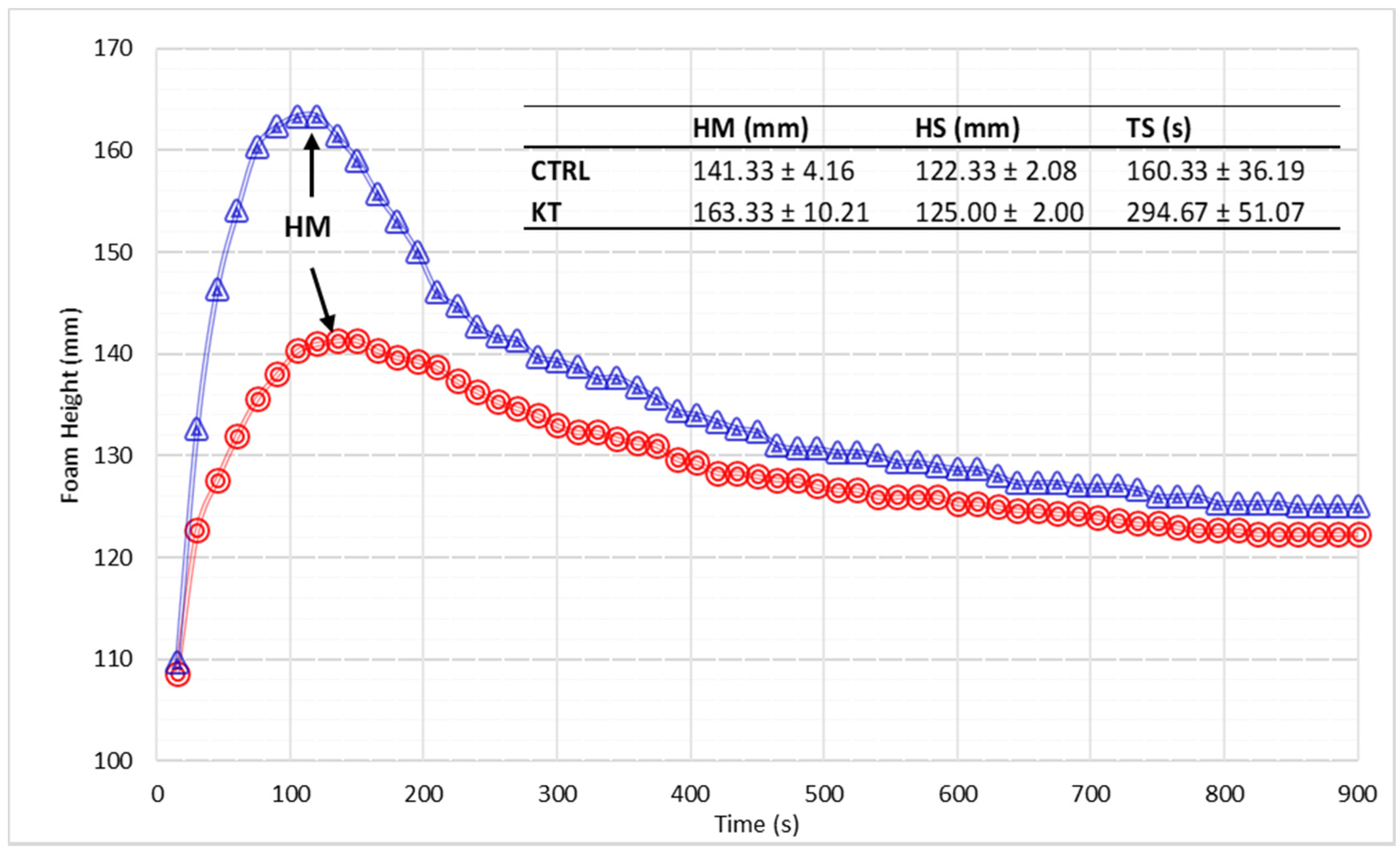
3.6. Foamability Parameters
3.7. Evolution of Volatile Compounds during Traditional Sparkling Winemaking Process
3.7.1. Fatty Acids
3.7.2. Alcohols
3.7.3. Esters
3.7.4. Other Compounds
3.8. Sensory Profile of Sparkling Wines after Secondary Fermentation and after 12 Months of Ageing Sur Lie
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berbegal, C.; Polo, L.; García-esparza, M.J.; Lizama, V.; Ferrer, S.; Pardo, I. Immobilisation of yeasts on oak chips or cellulose powder for use in bottle-fermented sparkling wine. J. Food Microbiol. 2019, 78, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Martínez-García, R.; García-Martínez, T.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J. Changes in sparkling wine aroma during the second fermentation under CO2 pressure in sealed bottle. Food Chem. 2017, 237, 1030–1040. [Google Scholar] [CrossRef] [PubMed]
- Kemp, B.; Alexandre, H.; Robillard, B.; Marchal, R. Effect of Production Phase on Bottle-Fermented Sparkling Wine Quality. J. Agric. Food Chem. 2015, 63, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, H.; Guilloux-Benatier, M. Yeast autolysis in sparkling wine—A review. Aust. J. Grape Wine Res. 2006, 12, 119–127. [Google Scholar] [CrossRef]
- Nunez, Y.P.; Carrascosa, A.V.; González, R.; Polo, M.C.; Martínez-Rodríguez, A.J. Effect of accelerated autolysis of yeast on the composition and foaming properties of sparkling wines elaborated by a champenoise method. J. Agric. Food Chem. 2005, 53, 7232–7237. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.; Juneja, V.K. Review of antimicrobial and antioxidative activities of chitosans in food. J. Food Prot. 2010, 73, 1737–1761. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Q. Recent Advances of Chitosan and Its Derivatives for Novel Applications in Food Science. J. Food Process. Beverages 2013, 1, 13. [Google Scholar]
- No, H.K.; Meyers, S.P.; Prinyawiwatkul, W.; Xu, Z. Applications of chitosan for improvement of quality and shelf life of foods: A review. J. Food Sci. 2007, 72, R87–R100. [Google Scholar] [CrossRef]
- Nunes, C.; Maricato, É.; Cunha, Â.; Rocha, M.A.M.; Santos, S.; Ferreira, P.; Silva, M.; Rodrigues, A.; Amado, O.; Coimbra, J.; et al. Chitosan–genipin film, a sustainable methodology for wine preservation. Green Chem. 2016, 18, 5331–5341. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Rocha, M.A.M.; Coimbra, M.A.; Nunes, C. Applications of chitosan and their derivatives in beverages: A critical review. Curr. Opin. Food Sci. 2017, 15, 61–69. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 53/2011 of 21 January 2011. Off. J. Eur. Union 2011, L19, 1–6.
- Colangelo, D.; Torchio, F.; De Faveri, D.M.; Lambri, M. The use of chitosan as alternative to bentonite for wine fining: Effects on heat-stability, proteins, organic acids, colour, and volatile compounds in an aromatic white wine. Food Chem. 2018, 264, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Castro-Marin, A.; Gabriela Buglia, A.; Riponi, C.; Chinnici, F. Volatile and fixed composition of sulphite-free white wines obtained after fermentation in the presence of chitosan. LWT—Food Sci. Technol. 2018, 93, 174–180. [Google Scholar] [CrossRef]
- Castro, A.; Culcasi, M.; Cassien, M.; Stocker, P.; Thétiot-laurent, S.; Robillard, B.; Chinnici, F.; Pietri, S. Chitosan as an antioxidant alternative to sulphites in oenology: EPR investigation of inhibitory mechanisms. Food Chem. 2019, 285, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Chinnici, F.; Natali, N.; Riponi, C. Efficacy of Chitosan in Inhibiting the Oxidation of (+)-Catechin in White Wine Model Solutions. J. Agric. Food Chem. 2014, 62, 9868–9875. [Google Scholar] [CrossRef] [PubMed]
- International Organisation of Vine and Wine (OIV). Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine (OIV): Paris, France, 2015. [Google Scholar]
- Chinnici, F.; Spinabelli, U.; Riponi, C.; Amati, A. Optimization of the determination of organic acids and sugars in fruit juices by ion-exclusion liquid chromatography. J. Food Compos. Anal. 2005, 18, 121–130. [Google Scholar] [CrossRef]
- Raposo, R.; Chinnici, F.; Ruiz-Moreno, M.J.; Puertas, B.; Cuevas, F.J.; Carbú, M.; Guerrero, R.F.; Ortíz-Somovilla, V.; Moreno-Rojas, J.M.; Cantos-Villar, E. Sulfur free red wines through the use of grapevine shoots: Impact on the wine quality. Food Chem. 2018, 243, 453–460. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Sonni, F.; Chinnici, F.; Versari, A.; Perez-Coello, M.S.; Riponi, C. Fermentation of sulphite-free white musts with added lysozyme and oenological tannins: Nitrogen consumption and biogenic amines composition of final wines. LWT—Food Sci. Technol. 2010, 43, 1501–1507. [Google Scholar] [CrossRef]
- Segarra, I.; Lao, C.; López-Tamames, E.; De La Torre-Boronat, M.C. Spectrophotometric Methods for the Analysis of Polysaccharide Levels in Winemaking Products. Am. J. Enol. Vitic. 1995, 46, 564–570. [Google Scholar]
- Albersheim, P.; Nevins, D.J.; English, P.D.; Karr, A. A method for the analysis of sugars in plant cell-wall polysaccharides by gas-liquid chromatography. Carbohydr. Res. 1967, 5, 340–345. [Google Scholar] [CrossRef]
- Maujean, A.; Poinsaut, P.; Dantan, H.; Brissonnet, F.; Cossiez, E. Étude de la tenue et de la qualité de mousse des vins effervescents II—Mise au point d’une technique de mesure de la moussabilité, de la tenue et de la stabilité de la mousse des vins effervescents. Bull. OIV 1990, 63, 405–427. [Google Scholar]
- López, R.; Aznar, M.; Cacho, J.; Ferreira, V. Determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 2002, 966, 167–177. [Google Scholar] [CrossRef]
- Pozo-Bayón, M.A.; Hernández, M.T.; Martín-Álvarez, P.J.; Polo, M.C. Study of Low Molecular Weight Phenolic Compounds during the Aging of Sparkling Wines Manufactured with Red and White Grape Varieties. J. Agric. Food Chem. 2003, 51, 2089–2095. [Google Scholar] [CrossRef]
- Dizy, M.; Polo, M.C. Changes in concentration of nitrogenous compounds during fermentation of white grape musts at pilot plant scale/Cambios en la concentración de compuestos nitrogenados durante la fermentación de mosto a escala piloto. Food Sci. Technol. Int. 2007, 2, 87–93. [Google Scholar] [CrossRef]
- Kelly-Treadwell, P.H. Protease activity in yeast: Its relationship to autolysis and Champagne character. Aust. Grapegrow. Winemak. 1988, 292, 58–66. [Google Scholar]
- Lopez-Moya, F.; Lopez-Llorca, L. Omics for Investigating Chitosan as an Antifungal and Gene Modulator. J. Fungi 2016, 2, 11. [Google Scholar] [CrossRef]
- Spagna, G.; Pifferi, P.G.; Rangoni, C.; Mattivi, F.; Nicolini, G.; Palmonari, R. The stabilization of white wines by adsorption of phenolic compounds on chitin and chitosan. Food Res. Int. 1996, 29, 241–248. [Google Scholar] [CrossRef]
- Sartor, S.; Toaldo, I.M.; Panceri, C.P.; Caliari, V.; Luna, A.S.; de Gois, J.S.; Bordignon-Luiz, M.T. Changes in organic acids, polyphenolic and elemental composition of rosé sparkling wines treated with mannoproteins during over-lees aging. Food Res. Int. 2019, 124, 34–42. [Google Scholar] [CrossRef]
- Del Barrio-Galán, R.; Úbeda, C.; Gil, M.; Sieczkowski, N.; Peña-Neira, Á. Different application dosages of a specific inactivated dry yeast (SIDY): Effect on the polysaccharides, phenolic and volatile contents and color of Sauvignon blanc wines. OENO One 2018, 52, 333–346. [Google Scholar]
- Mazauric, J.P.; Salmon, J.M. Interactions between yeast lees and wine polyphenols during simulation of wine aging: II. Analysis of desorbed polyphenol compounds from yeast lees. J. Agric. Food Chem. 2006, 54, 3876–3881. [Google Scholar] [CrossRef] [PubMed]
- Fornairon-Bonnefond, C.; Caramasa, C.; Moutounet, M.; Salmon, J.M. New trends on yeast autolysis and wine ageing on lees: A bibliographic review. J. Int. Sci. Vigne Vin 2002, 36, 49–69. [Google Scholar] [CrossRef] [Green Version]
- Leroy, M.J.; Charpentier, M.; Duteurtre, B.; Feuillat, M.; Charpentier, C. Yeast Autolysis during Champagne Aging. Am. J. Enol. Vitic. 1990, 41, 21–28. [Google Scholar]
- Martínez-Rodriguez, A.J.; Polo, M.C. Effect of the addition of bentonite to the tirage solution on the nitrogen composition and sensory quality of sparkling wines. Food Chem. 2003, 81, 383–388. [Google Scholar] [CrossRef]
- Vanrell, G.; Canals, R.; Esteruelas, M.; Fort, F.; Canals, J.M.; Zamora, F. Influence of the use of bentonite as a riddling agent on foam quality and protein fraction of sparkling wines (Cava). Food Chem. 2007, 104, 148–155. [Google Scholar] [CrossRef]
- Martínez-Lapuente, L.; Ayestarán, B.; Guadalupe, Z. Influence of Wine Chemical Compounds on the Foaming Properties of Sparkling Wines. In Grapes and Wines; Jordão, A.M., Cosme, F., Eds.; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar]
- Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B.; Pérez-Magariño, S. Role of major wine constituents in the foam properties of white and rosé sparkling wines. Food Chem. 2015, 174, 330–338. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/srd (accessed on 22 January 2020).
- Flavornet. Available online: http://www.flavornet.org/flavornet.html (accessed on 22 January 2020).
- Ferreira, V.; Felipe, J.; Juan, F. Flavor of Wine. In Food Flavors; Henryk, J., Ed.; CRC Press: Boca Raton, FL, USA, 2011; pp. 269–300. [Google Scholar]
- Hadwiger, L.A.; Kendra, D.F.; Fristensky, B.W.; Wagoner, W. Chitosan Both Activates Genes in Plants and Inhibits RNA Synthesis in Fungi. In Chitin in Nature and Technology; Springer: Boston, MA, USA, 1986; pp. 209–214. [Google Scholar]
- Wakil, S.J.; Stoops, J.K.; Joshi, V.C. Fatty Acid Synthesis and its Regulation. Ann. Rev. Biochem. 1983, 52, 537–579. [Google Scholar] [CrossRef]
- Rapp, A.; Versini, G. Influence of nitrogen compounds in grapes on aroma compounds of wines. In Developments in Food Science; Elsevier: Amsterdam, The Netherlands, 1995; Volume 37, pp. 1659–1694. [Google Scholar]
- Ribereau-Gayon, P.; Glories, Y.; Maujean, A.; Dubordieu, D. Handbook of Enology, Volume 2: The Chemistry of Wine Stabilization and Treatments, 2nd ed.; John Wiley Sons, Ltd.: West Sussex, UK, 2001; Volume 2. [Google Scholar]
- Hazelwood, L.A.; Daran, J.M.; Van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [Green Version]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef] [Green Version]


| Base Wine | 2nd AF | 12 Months “Sur lie” | |||
|---|---|---|---|---|---|
| CTRL | KT | CTRL | KT | ||
| Titratable acidity (g/L) | 5.75 ± 0.07 ab | 5.90 ± 0.14 a | 5.85 ± 0.07 a | 5.59 ± 0.06 ab | 5.51 ± 0.01 b |
| pH | 3.10 ± 0.01 a | 3.10 ± 0.01 a | 3.10 ± 0.02 a | 3.11 ± 0.01 a | 3.12 ± 0.01 a |
| Volatile acidity (g/L) | 0.29 ± 0.02 a | 0.28 ± 0.01 a | 0.30 ± 0.01 a | 0.32 ± 0.01 a | 0.28 ± 0.01 a |
| Alcohol (%v/v) | 10.32 ± 0.71 b | 11.42 ± 0.09 a | 11.36 ± 0.19 a | 11.40 ± 0.06 a | 11.30 ± 0.03 a |
| Optical Density 420 nm | 0.092 ± 0.001 a | 0.093 ± 0.01 a | 0.089 ± 0.012 a | 0.101 ± 0.001 a | 0.104 ± 0.001 a |
| TPI (GAE) | 172.3 ± 0.02 ab | 162.8 ± 0.07 b | 180.4 ± 0.28 a | 177.1 ± 0.03 ab | 176.9 ± 0.03 ab |
| Total proteins (mg/L) | 22.43 ± 0.45 d | 30.53 ± 1.28 b | 32.99 ± 1.37 b | 25.55 ± 0.85 c | 38.25 ± 1.01 a |
| Base Wine | 2nd AF | 12 Months “Sur lie” | |||
|---|---|---|---|---|---|
| CTRL | KT | CTRL | KT | ||
| Tartaric acid | 3.57 ± 0.01 a | 3.58 ± 0.14 a | 3.66 ± 0.04 a | 2.53 ± 0.02 b | 2.61 ± 0.14 b |
| Pyruvic acid | 26.1 ± 0.23 b | 24.3 ± 2.19 b | 22.4 ± 1.46 b | 36.8 ± 0.42 a | 40.6 ± 1.54 a |
| Malic acid | 0.13 ± 0.01 a | 0.16 ± 0.04 a | 0.14 ± 0.01 a | 0.19 ± 0.01 a | 0.19 ± 0.01 a |
| Shikimic acid | 60.7 ± 0.35 a | 54.4 ± 2.15 a | 56.1 ± 0.75 a | 56.3 ± 1.22 a | 55.9 ± 1.22 a |
| Lactic acid | 2.37 ± 0.03 a | 2.30 ± 0.08 a | 2.35 ± 0.07 a | 2.35 ± 0.08 a | 2.36 ± 0.08 a |
| Acetic acid | 0.18 ± 0.01 ab | 0.16 ± 0.01 bc | 0.19 ± 0.02 a | 0.13 ± 0.01 d | 0.14 ± 0.01 cd |
| Succinic acid | 0.55 ± 0.01 a | 0.52 ± 0.04 a | 0.60 ± 0.03 a | 0.69 ± 0.06 a | 0.69 ± 0.06 a |
| Glycerol | 3.21 ± 0.05 a | 3.39 ± 0.12 a | 3.40 ± 0.11 a | 3.40 ± 0.12 a | 3.35 ± 0.10 a |
| Sum | 6.80 ± 0.03 a | 6.70 ± 0.22 a | 6.93 ± 0.08 a | 5.88 ± 0.16 b | 5.98 ± 0.16 b |
| Base Wine | 2nd AF | 12 Months “Sur lie” | |||
|---|---|---|---|---|---|
| CTRL | KT | CTRL | KT | ||
| Hydroxybenzoic acids and flavanols | |||||
| Gallic | 21.79 ± 0.26 a | 21.21 ± 0.50 a | 21.05 ± 1.04 a | 23.17 ± 0.16 a | 22.69 ± 0.07 a |
| Syringic | 0.74 ± 0.05 a | 0.85 ± 0.07 a | 1.04 ± 0.03 a | 1.18 ± 0.03 a | 0.94 ± 0.49 a |
| p-Hydroxybenzoic | 1.15 ± 0.01 a | 0.15 ± 0.02 c | 0.09 ± 0.09 c | 0.77 ± 0.22 b | 0.62 ± 0.04 b |
| (+)-Catechin | 3.58 ± 0.07 a | 3.53 ± 0.12 a | 3.54 ± 0.21 a | 3.16 ± 0.03 a | 2.60 ± 0.04 b |
| Hydroxycinnamic acids | |||||
| t-Caftaric acid | 5.39 ± 0.08 a | 4.14 ± 0.05 c | 4.12 ± 0.08 c | 4.64 ± 0.01 b | 4.49 ± 0.03 bc |
| GRP | 5.81 ± 0.09 a | 3.47 ± 0.10 b | 3.17 ± 0.05 c | 5.87 ± 0.03 a | 5.75 ± 0.06 a |
| t-Coutaric acid | 1.92 ± 0.06 a | 1.87 ± 0.02 a | 1.87 ± 0.01 a | 1.89 ± 0.08 a | 1.83 ± 0.01 a |
| c-Coutaric acid | 2.46 ± 0.01 a | 1.37 ± 0.01 bc | 1.36 ± 0.01 c | 1.69 ± 0.05 b | 1.63 ± 0.02 b |
| Fertaric acid | 4.13 ± 0.07 a | 3.11 ± 0.02 c | 3.07 ± 0.06 c | 3.60 ± 0.01 b | 3.65 ± 0.07 b |
| Caffeic acid | 1.72 ± 0.01 a | 0.81 ± 0.11 bc | 0.78 ± 0.16 c | 1.16 ± 0.07 b | 1.11 ± 0.03 b |
| p-Coumaric acid | 1.57 ± 0.05 a | 0.56 ± 0.10 c | 0.51 ± 0.04 c | 0.89 ± 0.01 b | 0.92 ± 0.04 b |
| Ferulic acid | 1.74 ± 0.02 a | 0.77 ± 0.08 c | 0.76 ± 0.05 c | 0.98 ± 0.05 b | 0.97 ± 0.04 b |
| Flavonols | |||||
| Quercetin | 0.11 ± 0.01 a | 0.12 ± 0.01 a | 0.09 ± 0.01 a | 0.11 ± 0.01 a | 0.10 ± 0.01 a |
| Other | |||||
| Tyrosol | 3.20 ± 0.2 b | 3.81 ± 0.04 a | 3.72 ± 0.13 a | 3.94 ± 0.05 a | 3.81 ± 0.05 a |
| Base Wine | 2nd AF | 12 Months Storage | |||
|---|---|---|---|---|---|
| CTRL | KT | CTRL | KT | ||
| Aspartic acid | 7.45 ± 0.02 a | 1.34 ± 0.03 e | 1.67 ± 0.04 d | 3.29 ± 0.05 c | 3.65 ± 0.03 b |
| Glutamic acid | 10.52 ± 0.88 a | 4.24 ± 0.20 d | 5.82 ± 0.00 cd | 6.40 ± 0.05 bc | 8.01 ± 0.09 b |
| Serine | 6.41 ± 0.56 a | 0.97 ± 0.03 b | 1.02 ± 0.03 b | 1.95 ± 0.01 b | 1.94 ± 0.04 b |
| Asparagine | 4.70 ± 0.23 a | 5.27 ± 0.00 a | 5.11 ± 0.17 a | 5.56 ± 0.52 a | 5.71 ± 0.14 a |
| Glutamine | 25.65 ± 1.72 b | 34.38 ± 0.44 a | 34.27 ± 0.75 a | 36.34 ± 0.79 a | 34.31 ± 1.76 a |
| Glycine | 55.04 ± 2.17 a | 43.37 ± 0.05 c | 49.08 ± 1.04 b | 51.89 ± 0.25 ab | 56.97 ± 1.77 a |
| Histidine | 17.57 ± 0.19 a | 7.55 ± 0.10 c | 7.95 ± 0.19 c | 10.10 ± 0.12 b | 10.58 ± 0.23 b |
| Threonine | 2.20 ± 0.76 a | 1.00 ± 0.06 a | 0.91 ± 0.05 a | 1.47 ± 0.06 a | 1.55 ± 0.08 a |
| Arginine | 17.94 ± 0.04 b | 14.43 ± 0.09 d | 16.47 ± 0.22 c | 16.45 ± 0.08 c | 19.23 ± 0.28 a |
| Alanine | 6.52 ± 0.09 a | 2.23 ± 0.07 c | 3.03 ± 0.11 bc | 3.23 ± 0.30 b | 3.42 ± 0.30 b |
| Tyrosine | 4.84 ± 0.03 b | 3.71 ± 0.01 d | 4.18 ± 0.07 c | 4.80 ± 0.00 b | 5.21 ± 0.01 a |
| Ammonium | 28.43 ± 0.43 c | 45.98 ± 0.51 b | 45.61 ± 0.67 b | 47.34 ± 0.59 ab | 48.56 ± 0.20 a |
| Ethanolamine | 15.36 ± 0.12 c | 15.43 ± 0.20 c | 15.80 ± 0.07 bc | 16.4 ± 0.23 ab | 16.45 ± 0.08 a |
| Valine | 10.43 ± 0.89 a | 3.32 ± 0.04 c | 4.62 ± 0.06 bc | 4.79 ± 0.03 bc | 6.01 ± 0.06 b |
| Methionine | 5.59 ± 0.07 a | 1.85 ± 0.15 b | 1.93 ± 0.20 b | 1.90 ± 0.18 b | 1.98 ± 0.26 b |
| Isoleucine | 9.61 ± 0.69 a | 0.94 ± 0.00 c | 1.30 ± 0.16 c | 2.14 ± 0.17 bc | 2.65 ± 0.02 b |
| Leucine | 18.64 ± 0.32 a | 3.94 ± 0.03 e | 4.92 ± 0.12 d | 5.87 ± 0.02 c | 6.83 ± 0.05 b |
| Phenylalanine | 7.10 ± 0.08 a | 2.57 ± 0.07 d | 3.45 ± 0.07 c | 3.34 ± 0.02 c | 4.38 ± 0.15 b |
| Ornithine | 2.46 ± 0.14 d | 4.05 ± 0.06 bc | 3.90 ± 0.06 c | 4.52 ± 0.04 a | 4.36 ± 0.13 ab |
| Lysine | 35.61 ± 0.27 a | 11.38 ± 0.60 d | 14.29 ± 1.14 c | 15.63 ± 0.05 c | 18.6 ± 0.48 b |
| Putrescine | 18.93 ± 0.42 a | 14.48 ± 0.84 b | 15.07 ± 0.44 b | 15.38 ± 0.44 b | 16.38 ± 0.58 b |
| SUM amino acids | 248.3 ± 3.46 a | 146.5 ± 0.32 e | 163.9 ± 3.04 d | 179.7 ± 1.94 c | 195.4 ± 4.31 b |
| SUM amines | 36.75 ± 0.15 a | 34.96 ± 1.43 a | 35.77 ± 1.58 a | 36.3 ± 0.39 a | 37.19 ± 0.10 a |
| Compound | tR (min) | LRI | Identification a |
|---|---|---|---|
| Isobutyl alcohol | 5.70 | 1106 | Std, MS, LRI |
| Isoamyl acetate | 6.74 | 1133 | Std, MS, LRI |
| n-butanol | 7.19 | 1145 | Std, MS, LRI |
| 3-penten-2-ol | 7.80 | 1149 | Std, MS, LRI |
| 3-methyl-1-butanol | 8.92 | 1190 | Std, MS, LRI |
| Ethyl n-caproate | 9.86 | 1218 | Std, MS, LRI |
| Ethyl pyruvate | 11.33 | 1267 | Std, MS, LRI |
| 2-hexanol | 12.47 | 1304 | MS, LRI |
| 3-methyl-1-pentanol | 13.51 | 1331 | Std, MS, LRI |
| Ethyl lactate | 13.86 | 1340 | Std, MS, LRI |
| n-hexanol | 14.19 | 1349 | Std, MS, LRI |
| 2-hydroxy-3-pentanone | 14.63 | 1360 | Std, MS, LRI |
| 3-ethoxy-1-propanol | 15.10 | 1372 | Std, MS, LRI |
| 3-hexen-1-ol | 15.37 | 1379 | Std, MS, LRI |
| Ethyl octanoate | 17.40 | 1432 | Std, MS, LRI |
| Linalool oxide | 18.60 | 1463 | SMS, LRI |
| Furfural | 18.78 | 1467 | Std, MS, LRI |
| c-5-hydroxy-2-methyl-1,3-dioxane | 20.19 | 1503 | MS, LRI |
| Ethyl-3-hydroxybutyrate | 21.05 | 1524 | Std, MS, LRI |
| 2-methyl-3-thiolannone | 21.36 | 1531 | MS, LRI |
| 2,3-butanediol | 23.08 | 1572 | Std, MS, LRI |
| Ethyl 3-hydroxypropionate | 23.93 | 1584 | MS, LRI |
| t-4-hydroxymethyl-2-methyl-1,3 dioxolane | 24.35 | 1606 | MS, LRI |
| 2-furancarboxylic acid, ethyl ester | 24.55 | 1616 | MS, LRI |
| n-butyric acid | 24.71 | 1624 | Std, MS, LRI |
| Decanoic acid, ethyl ester | 25.38 | 1659 | Std, MS, LRI |
| Pentanoic acid | 25.87 | 1689 | MS, LRI |
| Furfuryl alcohol | 26.02 | 1695 | Std, MS, LRI |
| Diethyl succinate | 26.44 | 1710 | Std, MS, LRI |
| 3-methylthio-1-propanol | 27.48 | 1746 | Std, MS, LRI |
| 1,3-propanediol diacetate | 28.03 | 1766 | MS, LRI |
| Ethyl 4-hydroxybutanoate | 29.79 | 1840 | Std, MS, LRI |
| 2-phenylethyl-acetate | 30.01 | 1851 | Std, MS, LRI |
| t-5-hydroxy-2-methyl-1,3-dioxane | 30.11 | 1856 | MS, LRI |
| Hexanoic acid | 30.39 | 1870 | Std, MS, LRI |
| Benzyl alcohol | 31.16 | 1905 | Std, MS, LRI |
| 2-phenylethanol | 31.92 | 1931 | Std, MS, LRI |
| Benzothiazole | 32.96 | 1966 | MS, LRI |
| 2,3-dihydroxypyrazine | 33.99 | 2001 | Std, MS, LRI |
| Diethyl Malate | 34.70 | 2038 | MS, LRI |
| Octanoic acid | 34.96 | 2052 | Std, MS, LRI |
| Diethyl-2-hydroxypentanedioate | 37.32 | 2197 | Std, MS, LRI |
| 4-vinyl-2-methoxyphenol | 37.78 | 2220 | Std, MS, LRI |
| Ethyl 5-oxotetrahydrofuran-2-furancarboxylate | 38.82 | 2270 | MS, LRI |
| decanoic acid | 39.31 | 2293 | Std, MS, LRI |
| Ethyl 2-hydroxy-3-phenylpropanoate | 39.39 | 2297 | MS, LRI |
| Glycerol | 40.20 | 2328 | Std, MS, LRI |
| Diethyl tartrate | 40.33 | 2182 | MS, LRI |
| Ethyl hydrogen succinate | 40.94 | 2356 | MS, LRI |
| 4-vinyl phenol | 41.26 | 2368 | Std, MS, LRI |
| Benzoic acid | 41.85 | 2390 | Std, MS, LRI |
| 3-furoic acid | 42.08 | 2399 | MS, LRI |
| Dodecanoic acid | 42.78 | 2444 | Std, MS, LRI |
| HMF | 43.12 | 2467 | Std, MS, LRI |
| Acetovanillone | 47.96 | 2662 | MS, LRI |
| n-hexadecanoic acid | 49.08 | 2803 | Std, MS, LRI |
| 4-hydroxy-benzenethanol | 51.20 | 2917 | Std, MS, LRI |
| Octadecanoic acid | 53.25 | 2998 | Std, MS, LRI |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro Marín, A.; Riponi, C.; Chinnici, F. Chitosan in Sparkling Wines Produced by the Traditional Method: Influence of Its Presence during the Secondary Fermentation. Foods 2020, 9, 1174. https://doi.org/10.3390/foods9091174
Castro Marín A, Riponi C, Chinnici F. Chitosan in Sparkling Wines Produced by the Traditional Method: Influence of Its Presence during the Secondary Fermentation. Foods. 2020; 9(9):1174. https://doi.org/10.3390/foods9091174
Chicago/Turabian StyleCastro Marín, Antonio, Claudio Riponi, and Fabio Chinnici. 2020. "Chitosan in Sparkling Wines Produced by the Traditional Method: Influence of Its Presence during the Secondary Fermentation" Foods 9, no. 9: 1174. https://doi.org/10.3390/foods9091174
APA StyleCastro Marín, A., Riponi, C., & Chinnici, F. (2020). Chitosan in Sparkling Wines Produced by the Traditional Method: Influence of Its Presence during the Secondary Fermentation. Foods, 9(9), 1174. https://doi.org/10.3390/foods9091174