Yogurts Supplemented with Juices from Grapes and Berries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Milk, Juices and Starter Culture
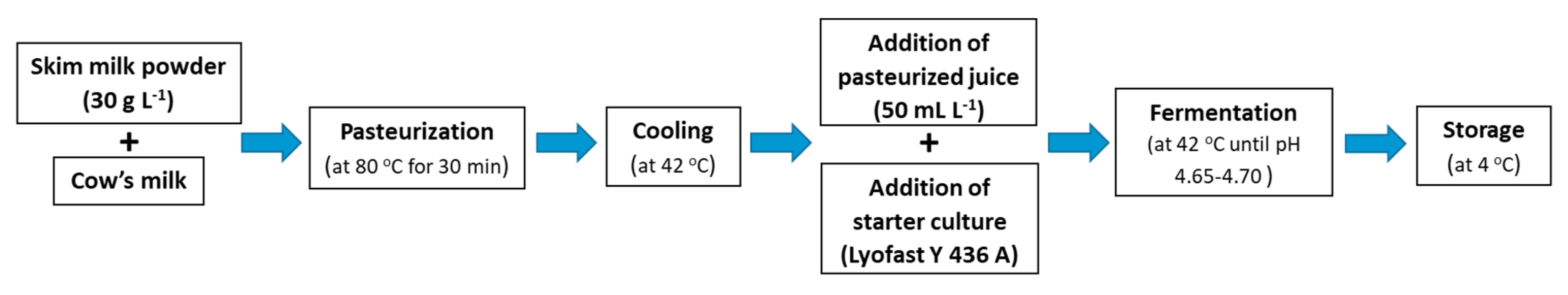
2.2. Yogurt Production
2.3. Analyses
2.3.1. Total Acidity and pH
2.3.2. Water Holding Capacity and Syneresis
2.3.3. Color
2.3.4. Preparation of Yogurt Water Extract
2.3.5. Free Radical-Scavenging Activity
2.3.6. Total Phenolic Content
2.3.7. Reducing Sugars
2.4. Microbiological Analysis
2.5. Statistical Analysis
3. Results and Discussion
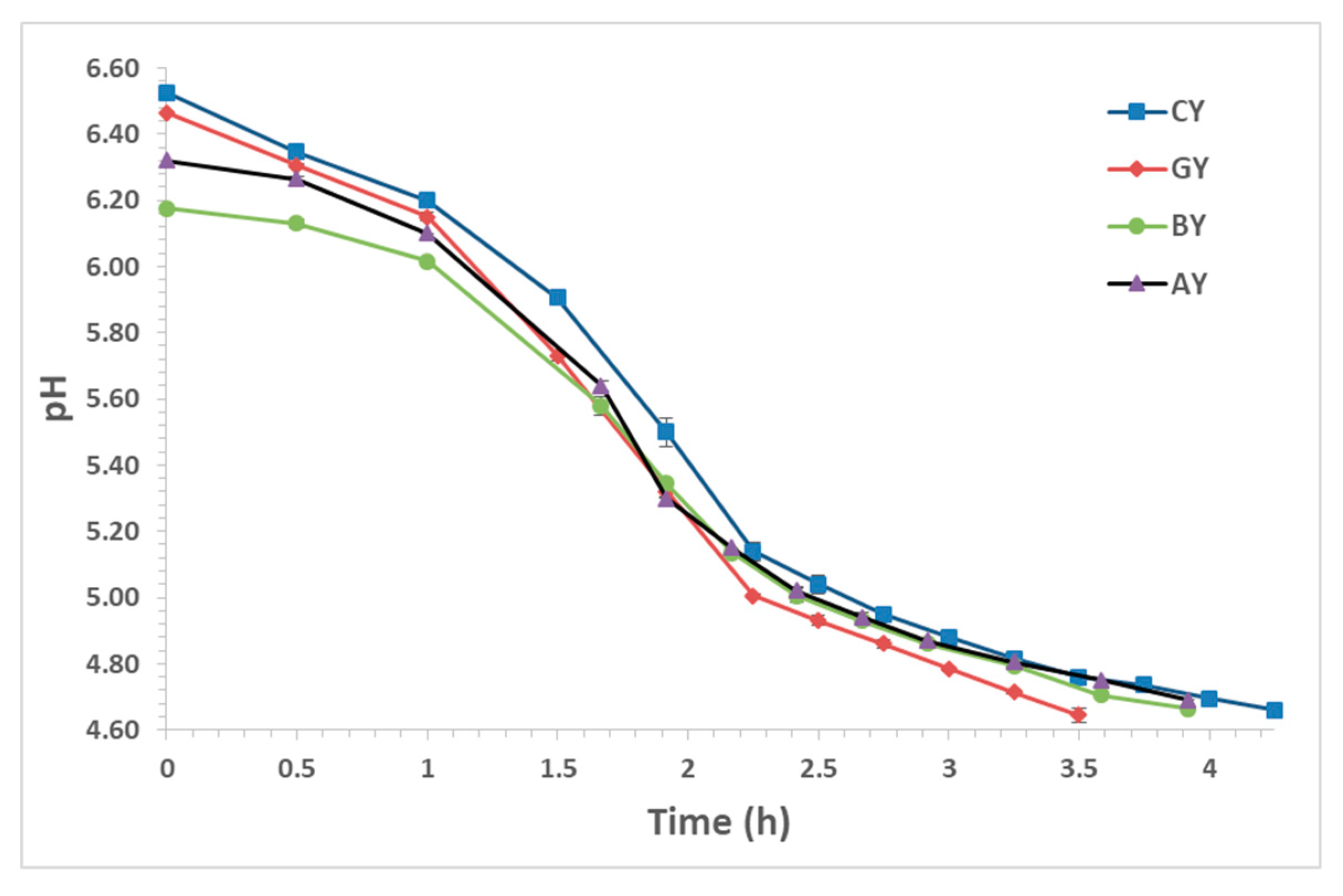
3.1. Acidification Trend
3.2. Physicochemical Characteristics and Antioxidant Activity
3.3. Color Characteristics
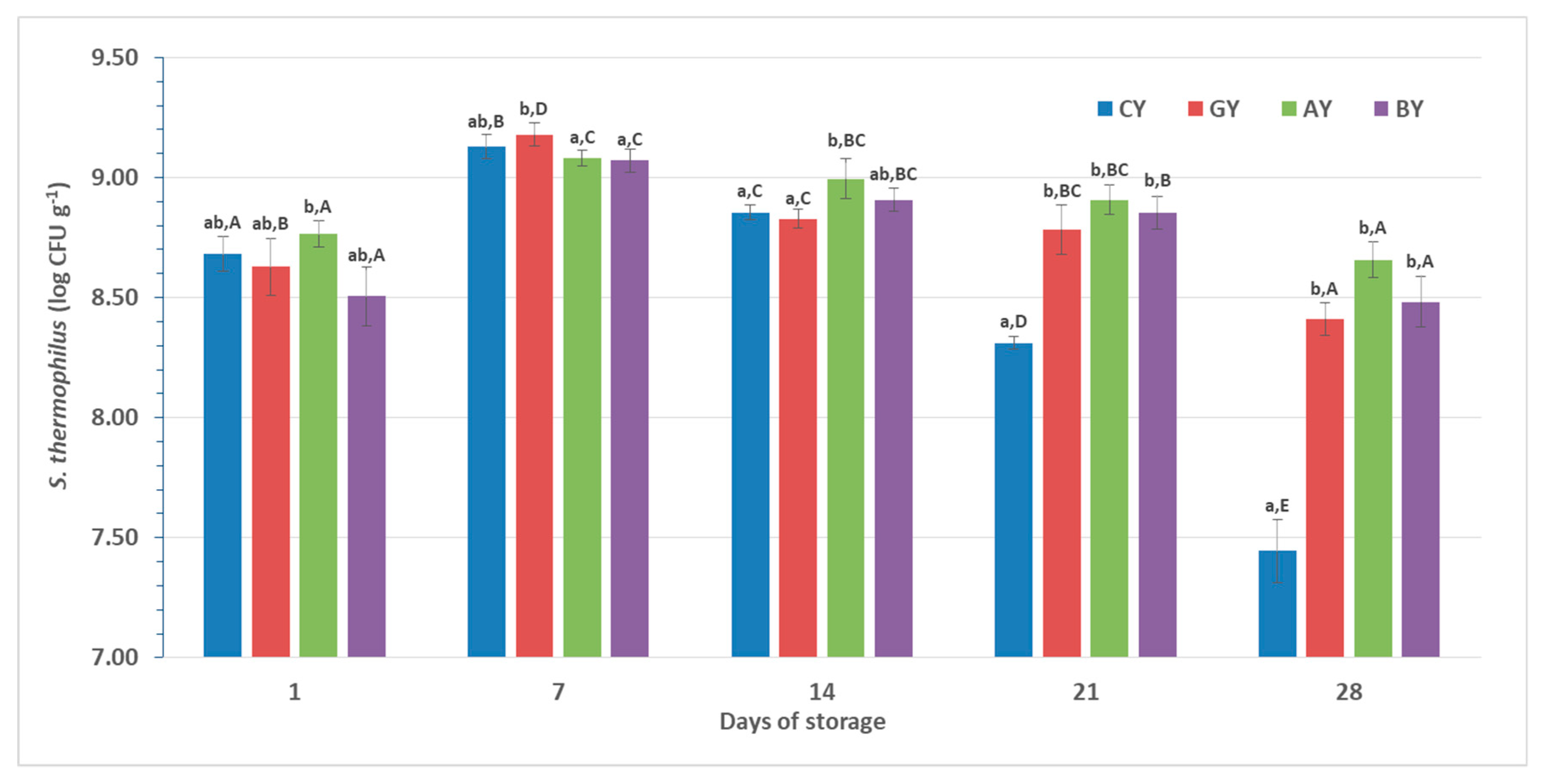
3.4. Starter Culture Viability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bimbo, F.; Bonanno, A.; Nocella, G.; Viscecchia, R.; Nardone, G.; De Devitiis, B.; Carlucci, D. Consumers’ acceptance and preferences for nutrition-modified and functional dairy products: A systematic review. Appetite 2017, 113, 141–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özer, B.H.; Kirmaci, H.A. Functional milks and dairy beverages. Int. J. Dairy Technol. 2010, 63, 1–15. [Google Scholar] [CrossRef]
- Shiby, V.K.; Mishra, H.N. Fermented milks and milk products as functional foods—A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 482–496. [Google Scholar] [CrossRef]
- Hati, S.; Das, S.; Mandal, S. Technological advancement of functional fermented dairy beverages. In Engineering Tools in the Beverage Industry; The Science of Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; Volume 3, pp. 101–136. [Google Scholar]
- Beltrán-Barrientos, L.M.; Hernández-Mendoza, A.; Torres-Llanez, M.J.; González-Córdova, A.F.; Vallejo-Córdoba, B. Invited review: Fermented milk as antihypertensive functional food. J. Dairy Sci. 2016, 99, 4099–4110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munekata, P.E.; Domínguez, R.; Budaraju, S.; Roselló-Soto, E.; Barba, F.J.; Mallikarjunan, K.; Roohinejad, S.; Lorenzo, J.M. Effect of innovative food processing technologies on the physicochemical and nutritional properties and quality of non-dairy plant-based beverages. Foods 2020, 9, 288. [Google Scholar] [CrossRef] [Green Version]
- Szilagyi, A.; Ishayek, N. Lactose intolerance, dairy avoidance, and treatment options. Nutrients 2018, 10, 1994. [Google Scholar] [CrossRef] [Green Version]
- Fiocchi, A.; Schünemann, H.J.; Brozek, J.; Restani, P.; Beyer, K.; Troncone, R.; Martelli, A.; Terracciano, L.; Bahna, S.L.; Ebisawa, M.; et al. Diagnosis and rationale for action against cow’s milk allergy (DRACMA): A summary report. J. Allergy Clin. Immunol. 2010, 126, 1119–1128. [Google Scholar] [CrossRef]
- Roth-Walter, F.; Pacios, L.F.; Gomez-Casado, C.; Hofstetter, G.; Roth, G.A.; Singer, J.; Diaz-Perales, A.; Jensen-Jarolim, E. The major cow milk allergen Bos d 5 manipulates T-helper cells depending on its load with siderophore-bound iron. PLoS ONE 2014, 9, e104803. [Google Scholar] [CrossRef]
- Downer, M.K.; Batista, J.L.; Mucci, L.A.; Stampfer, M.J.; Epstein, M.M.; Håkansson, N.; Wolk, A.; Johansson, J.E.; Andrén, O.; Fall, K.; et al. Dairy intake in relation to prostate cancer survival. Int. J. Cancer 2017, 140, 2060–2069. [Google Scholar] [CrossRef]
- Aryana, K.J.; Olson, D.W. A 100-Year Review: Yogurt and other cultured dairy products. J. Dairy Sci. 2017, 100, 9987–10013. [Google Scholar] [CrossRef] [Green Version]
- Kandylis, P.; Pissaridi, K.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A. Dairy and non-dairy probiotic beverages. Curr. Opin. Food Sci. 2016, 7, 58–63. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasines-Perea, Z.; Teissedre, P.L. Grape polyphenols’ effects in human cardiovascular diseases and diabetes. Molecules 2017, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Jurikova, T.; Mlcek, J.; Skrovankova, S.; Sumczynski, D.; Sochor, J.; Hlavacova, I.; Snopek, L.; Orsavova, J. Fruits of black chokeberry Aronia melanocarpa in the prevention of chronic diseases. Molecules 2017, 22, 944. [Google Scholar] [CrossRef]
- Liu, B.; Fang, Y.; Yi, R.; Zhao, X. Preventive effect of blueberry extract on liver injury induced by carbon tetrachloride in mice. Foods 2019, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Rocha, D.M.U.P.; Caldas, A.P.S.; da Silva, B.P.; Hermsdorff, H.H.M.; Alfenas, R.D.C.G. Effects of blueberry and cranberry consumption on type 2 diabetes glycemic control: A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1816–1828. [Google Scholar] [CrossRef]
- Kandylis, P.; Kokkinomagoulos, E. Food applications and potential health benefits of pomegranate and its derivatives. Foods 2020, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Öztürk, B.A.; Öner, M.D. Production and evaluation of yogurt with concentrated grape juice. J. Food Sci. 1999, 64, 530–532. [Google Scholar] [CrossRef]
- Kiros, E.; Seifu, E.; Bultosa, G.; Solomon, W.K. Effect of carrot juice and stabilizer on the physicochemical and microbiological properties of yoghurt. LWT-Food Sci. Technol. 2016, 69, 191–196. [Google Scholar] [CrossRef]
- Cui, Y.; Jiang, X.; Hao, M.; Qu, X.; Hu, T. New advances in exopolysaccharides production of Streptococcus thermophilus. Arch. Microbiol. 2017, 199, 799–809. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Liu, J. Rheological and physical characteristics of non-fat set yogurt prepared with EPS-producing Streptococcus thermophilus and an H+-ATPase-defective mutant Lactobacillus delbrueckii subsp. bulgaricus. Int. J. Food Prop. 2017, 20, 745–753. [Google Scholar] [CrossRef] [Green Version]
- Ramchandran, L.; Shah, N.P. Characterization of functional, biochemical and textural properties of synbiotic low-fat yogurts during refrigerated storage. LWT-Food Sci. Technol. 2010, 43, 819–827. [Google Scholar] [CrossRef]
- Amatayakul, T.; Sherkat, F.; Shah, N.P. Syneresis in set yogurt as affected by EPS starter cultures and levels of solids. Int. J. Dairy Technol. 2006, 59, 216–221. [Google Scholar] [CrossRef]
- Boycheva, S.; Dimitrov, T.; Naydenova, N.; Mihaylova, G. Quality characteristics of yogurt from goat’s milk, supplemented with fruit juice. Czech J. Food Sci. 2011, 29, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Calvo, M.M.; Diez, O.; Cobos, A. Use of rectified grape juice in yogurt edulcoration. J. Food Sci. 2002, 67, 3140–3143. [Google Scholar] [CrossRef]
- Barat, A.; Ozcan, T. Growth of probiotic bacteria and characteristics of fermented milk containing fruit matrices. Int. J. Dairy Technol. 2018, 71, 120–129. [Google Scholar] [CrossRef]
- Nguyen, L.; Hwang, E.S. Quality characteristics and antioxidant activity of yogurt supplemented with aronia (Aronia melanocarpa) juice. Prev. Nutr. Food Sci. 2016, 21, 330. [Google Scholar] [CrossRef]
- Sidira, M.; Santarmaki, V.; Kiourtzidis, M.; Argyri, A.A.; Papadopoulou, O.S.; Chorianopoulos, N.; Tassou, C.; Kaloutsas, S.; Galanis, A.; Kourkoutas, Y. Evaluation of immobilized Lactobacillus plantarum 2035 on whey protein as adjunct probiotic culture in yoghurt production. LWT-Food Sci. Technol. 2017, 75, 137–146. [Google Scholar] [CrossRef]
- Sah, B.N.P.; Vasiljevic, T.; McKechnie, S.; Donkor, O.N. Physicochemical, textural and rheological properties of probiotic yogurt fortified with fibre-rich pineapple peel powder during refrigerated storage. LWT-Food Sci. Technol. 2016, 65, 978–986. [Google Scholar] [CrossRef]
- Shori, A.B.; Rashid, F.; Baba, A.S. Effect of the addition of phytomix-3+ mangosteen on antioxidant activity, viability of lactic acid bacteria, type 2 diabetes key-enzymes, and sensory evaluation of yogurt. LWT 2018, 94, 33–39. [Google Scholar] [CrossRef]
- Arnous, A.; Makris, D.P.; Kefalas, P. Correlation of pigment and flavanol content with antioxidant properties in selected aged regional wines from Greece. J. Food Compost. Anal. 2002, 15, 655–665. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- ISO. Yogurt—Enumeration of Characteristics Microorganisms—Colony Count Technique at 37 °C; ISO 7889:2003(E); IDF 117:2003(E); International Organization for Standardization: Geneva, Switzerland, 2003. [Google Scholar]
- Dimitrellou, D.; Kandylis, P.; Kourkoutas, Y. Assessment of freeze-dried immobilized Lactobacillus casei as probiotic adjunct culture in yogurts. Foods 2019, 8, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, J.B.; Sutton, E.H.; Hancock, N. Sugar reduction in yogurt products sold in the UK between 2016 and 2019. Nutrients 2020, 12, 171. [Google Scholar] [CrossRef] [Green Version]
- European Parliament Council of the European Union. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Off. J. Eur. Union 2006, L404, 9–25. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2006:404:0009:0025:EN:PDF (accessed on 14 August 2020).
- Pang, Z.; Deeth, H.; Sharma, R.; Bansal, N. Effect of addition of gelatin on the rheological and microstructural properties of acid milk protein gels. Food Hydrocoll. 2015, 43, 340–351. [Google Scholar] [CrossRef] [Green Version]
- O’connell, J.E.; Fox, P.F. Significance and applications of phenolic compounds in the production and quality of milk and dairy products: A review. Int. Dairy J. 2001, 11, 103–120. [Google Scholar] [CrossRef]
- Trigueros, L.; Wojdyło, A.; Sendra, E. Antioxidant activity and protein-polyphenol interactions in a pomegranate (Punica granatum L.) yogurt. J. Agric. Food Chem. 2014, 62, 6417–6425. [Google Scholar] [CrossRef]
- Pan, L.H.; Liu, F.; Luo, S.Z.; Luo, J.P. Pomegranate juice powder as sugar replacer enhanced quality and function of set yogurts: Structure, rheological property, antioxidant activity and in vitro bioaccessibility. LWT 2019, 115, 108479. [Google Scholar] [CrossRef]
- Karaaslan, M.; Ozden, M.; Vardin, H.; Turkoglu, H. Phenolic fortification of yogurt using grape and callus extracts. LWT-Food Sci. Technol. 2011, 44, 1065–1072. [Google Scholar] [CrossRef]
- Vital, A.C.P.; Goto, P.A.; Hanai, L.N.; Gomes-da-Costa, S.M.; de Abreu Filho, B.A.; Nakamura, C.V.; Matumoto-Pintro, P.T. Microbiological, functional and rheological properties of low fat yogurt supplemented with Pleurotus ostreatus aqueous extract. LWT-Food Sci. Technol. 2015, 64, 1028–1035. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein-phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Gonnet, J.F. Colour effects of co-pigmentation of anthocyanins revisited—2. A colorimetric look at the solutions of cyanin co-pigmented byrutin using the CIELAB scale. Food Chem. 1999, 66, 387–394. [Google Scholar] [CrossRef]
- Wang, S.Y.; Camp, M.J. Temperatures after bloom affect plant growth and fruit quality of strawberry. Sci. Hortic. 2000, 85, 183–199. [Google Scholar] [CrossRef]
- Wallace, T.C.; Giusti, M.M. Determination of color, pigment, and phenolic stability in yogurt systems colored with nonacylated anthocyanins from Berberis boliviana L. as compared to other natural/synthetic colorants. J. Food Sci. 2008, 73, C241–C248. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. Probiotic viability and physico-chemical and sensory properties of plain and stirred fruit yogurts made from goat’s milk. Food Chem. 2012, 135, 1411–1418. [Google Scholar] [CrossRef]
- FAO/WHO. Codex standard for fermented milks 243. In Codex Alimentarius Commission: Milk and Milk Products, 2nd ed.; CODEX STAN 243-2003; World Health Organization & Food and Agriculture Organization of the United Nations: Rome, Italy, 2011; pp. 6–16. [Google Scholar]
- Varga, L.; Süle, J.; Nagy, P. Short communication: Survival of the characteristic microbiota in probiotic fermented camel, cow, goat, and sheep milks during refrigerated storage. J. Dairy Sci. 2014, 97, 2039–2044. [Google Scholar] [CrossRef] [Green Version]
- Dimitrellou, D.; Salamoura, C.; Kontogianni, A.; Katsipi, D.; Kandylis, P.; Zakynthinos, G.; Varzakas, T. Effect of milk type on the microbiological, physicochemical and sensory characteristics of probiotic fermented milk. Microorganisms 2019, 7, 274. [Google Scholar] [CrossRef] [Green Version]
- Vinderola, C.G.; Costa, G.A.; Regenhardt, S.; Reinheimer, J.A. Influence of compounds associated with fermented dairy products on the growth of lactic acid starter and probiotic bacteria. Int. Dairy J. 2002, 12, 579–589. [Google Scholar] [CrossRef]
- Güler-Akın, M.B.; Akın, M.S. Effects of cysteine and different incubation temperatures on the microflora, chemical composition and sensory characteristics of bio-yogurt made from goat’s milk. Food Chem. 2007, 100, 788–793. [Google Scholar] [CrossRef]
- Birollo, G.A.; Reinheimer, J.A.; Vinderola, C.G. Viability of lactic acid microflora in different types of yoghurt. Food Res. Int. 2000, 33, 799–805. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [Green Version]
- Coman, M.M.; Oancea, A.M.; Verdenelli, M.C.; Cecchini, C.; Bahrim, G.E.; Orpianesi, C.; Cresci, A.; Silvi, S. Polyphenol content and in vitro evaluation of antioxidant, antimicrobial and prebiotic properties of red fruit extracts. Eur. Food Res. Technol. 2018, 244, 735–745. [Google Scholar] [CrossRef]
- Rodríguez-Costa, S.; Cardelle-Cobas, A.; Roca-Saavedra, P.; Porto-Arias, J.J.; Miranda, J.M.; Cepeda, A. In vitro evaluation of the prebiotic effect of red and white grape polyphenolic extracts. J. Physiol. Biochem. 2018, 74, 101–110. [Google Scholar] [CrossRef]
- Uriot, O.; Denis, S.; Junjua, M.; Roussel, Y.; Dary-Mourot, A.; Blanquet-Diot, S. Streptococcus thermophilus: From yogurt starter to a new promising probiotic candidate? J. Funct. Foods 2017, 37, 74–89. [Google Scholar] [CrossRef]
- Tarrah, A.; Castilhos, J.D.; Rossi, R.C.; Duarte, V.D.S.; Ziegler, D.R.; Corich, V.; Giacomini, A. In vitro probiotic potential and anti-cancer activity of newly isolated folate-producing Streptococcus thermophilus strains. Front. Microbiol. 2018, 9, 2214. [Google Scholar] [CrossRef]
- Mater, D.D.; Bretigny, L.; Firmesse, O.; Flores, M.J.; Mogenet, A.; Bresson, J.L.; Corthier, G. Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus survive gastrointestinal transit of healthy volunteers consuming yogurt. FEMS Microbiol. Lett. 2005, 250, 185–187. [Google Scholar] [CrossRef] [Green Version]
| Analyses | Days | CY | GY | AY | BY |
|---|---|---|---|---|---|
| pH | 1 | 4.64 ± 0.03 c,A | 4.66 ± 0.01 d,A,B | 4.72 ± 0.01 c,B,C | 4.76 ± 0.01 c,C |
| 7 | 4.57 ± 0.00 b,c,A | 4.59 ± 0.01 c,A | 4.51 ± 0.01 b,B | 4.51 ± 0.01 b,B | |
| 14 | 4.50 ± 0.00 a,b,A | 4.48 ± 0.01 b,A | 4.50 ± 0.01 b,A | 4.49 ± 0.00 b,A | |
| 21 | 4.45 ± 0.06 a,A | 4.39 ± 0.01 a,A | 4.40 ± 0.01 a,A | 4.38 ± 0.00 a,A | |
| 28 | 4.39 ± 0.01 a,A | 4.36 ± 0.01 a,A | 4.38 ± 0.01 a,A | 4.37 ± 0.01 a,A | |
| Acidity (% w/w lactic acid) | 1 | 1.02 ± 0.00 a,B | 1.03 ± 0.01 a,b,B | 0.85 ± 0.01 a,A | 0.86 ± 0.01 a,A |
| 7 | 1.03 ± 0.02 a,A,B | 0.99 ± 0.02 a,A | 1.06 ± 0.02 b,B | 1.08 ± 0.01 c,B | |
| 14 | 1.04 ± 0.02 a,A | 1.00 ± 0.01 a,A | 1.03 ± 0.01 b,A | 1.02 ± 0.01 b,A | |
| 21 | 0.99 ± 0.02 a,A | 1.00 ± 0.00 a,A | 1.01 ± 0.04 b,A | 1.00 ± 0.01 b,A | |
| 28 | 1.03 ± 0.01 a,A | 1.07 ± 0.01 b,A | 0.99 ± 0.04 b,A | 1.00 ± 0.00 b,A | |
| Reducing sugars (% w/w lactose) | 1 | 5.00 ± 0.10 a,B | 6.18 ± 0.16 a,C | 4.22 ± 0.12 a,A | 4.35 ± 0.03 a,A |
| 7 | 4.19 ± 0.39 b,A,B | 5.10 ± 0.07 b,B | 3.64 ± 0.10 a,b,A | 3.55 ± 0.11 b,A | |
| 14 | 3.49 ± 0.00 b,c,A | 4.39 ± 0.14 c,B | 3.48 ± 0.24 a,b,A | 3.66 ± 0.26 b,A | |
| 21 | 3.30 ± 0.08 c,A | 4.20 ± 0.02 c,d,B | 3.64 ± 0.14 b,A | 3.56 ± 0.00 b,A | |
| 28 | 3.39 ± 0.00 c,A | 3.98 ± 0.00 d,B | 3.40 ± 0.13 b,A | 3.28 ± 0.23 b,A |
| Analyses | Days | CY | GY | AY | BY |
|---|---|---|---|---|---|
| WHC (%) | 1 | 69.6 ± 3.0 a | 72.2 ± 4.2 a | 67.6 ± 0.1 a,b | 66.6 ± 0.1 a,b |
| 7 | 65.4 ± 0.1 a | 66.4 ± 3.5 a | 68.9 ± 3.3 b | 66.9 ± 1.1 b | |
| 14 | 65.9 ± 0.5 a | 67.7 ± 2.1 a | 68.9 ± 0.7 b | 69.1 ± 1.5 b | |
| 21 | 61.9 ± 3.6 a | 64.3 ± 1.4 a | 64.6 ± 0.4 a | 66.2 ± 2.3 a,b | |
| 28 | 62.0 ± 0.1 a | 62.5 ± 0.1 a | 61.9 ± 1.7 a | 61.2 ± 1.1 a | |
| Syneresis (%) | 1 | 30.0 ± 0.0 a | 31.0 ± 1.4 a | 38.0 ± 2.1 a | 38.3 ± 4.6 a |
| 7 | 35.5 ± 0.7 a | 38.5 ± 0.7 a | 37.5 ± 2.1 a | 37.5 ± 2.1 c | |
| 14 | 33.8 ± 3.2 a | 33.0 ± 3.5 a | 33.0 ± 1.4 a | 37.0 ± 4.2 b | |
| 21 | 38.5 ± 3.5 a | 31.4 ± 2.8 a | 37.0 ± 4.2 a | 34.0 ± 2.8 b | |
| 28 | 34.6 ± 0.9 a | 33.8 ± 3.9 a | 39.0 ± 1.4 a | 40.5 ± 3.5 b |
| Yogurt | DPPH● | Total Phenolic Content μg GAE g−1 | |
|---|---|---|---|
| % | μmol TE 100 g−1 | ||
| CY | 17.5 ± 1.2 a | 14.7 ± 0.9 a | 43.3 ± 0.2 a |
| GY | 27.1 ± 2.6 b | 21.5 ± 1.8 b | 57.6 ± 0.3 b |
| AY | 39.2 ± 1.1 c | 30.0 ± 0.8 c | 56.5 ± 1.1 b |
| BY | 43.5 ± 0.2 d | 33.0 ± 0.2 d | 64.6 ± 0.6 c |
| Analyses | Days | CY | GY | AY | BY |
|---|---|---|---|---|---|
| L* | 1 | 81.43 ± 7.13 a | 72.01 ± 3.23 a | 81.59 ± 1.87 b | 70.94 ± 2.69 a |
| 7 | 81.46 ± 7.22 a | 71.99 ± 3.30 a | 83.32 ± 7.22 b | 76.76 ± 2.39 c | |
| 14 | 81.83 ± 2.49 a | 73.79 ± 5.67 a | 73.00 ± 2.58 a | 66.21 ± 3.18 b | |
| 21 | 81.11 ± 6.34 a | 77.85 ± 3.83 a | 73.85 ± 0.26 a | 71.69 ± 0.82 a | |
| 28 | 87.04 ± 5.48 a | 77.07 ± 0.03 a | 73.22 ± 0.22 a | 71.98 ± 0.15 a | |
| a* | 1 | −2.78 ± 0.26 a | 1.64 ± 0.05 a | 1.21 ± 0.25 a | 7.35 ± 0.17 a |
| 7 | −2.71 ± 0.37 a | 1.62 ± 0.10 a | 1.89 ± 0.30 b,c | 8.74 ± 0.27 c | |
| 14 | −2.64 ± 0.16 a | 1.48 ± 0.16 a | 1.73 ± 0.15 b | 7.61 ± 0.51 a,b | |
| 21 | −2.72 ± 0.31 a | 1.48 ± 0.06 a | 1.98 ± 0.03 b,c | 8.02 ± 0.11 b | |
| 28 | −2.71 ± 0.22 a | 1.51 ± 0.07 a | 2.09 ± 0.03 c | 7.94 ± 0.06 b | |
| b* | 1 | 5.95 ± 0.49 a | 2.78 ± 0.01 a | 5.09 ± 0.04 c | 0.96 ± 0.03 a |
| 7 | 5.81 ± 0.77 a | 2.82 ± 0.01 a,b | 4.72 ± 0.45 b,c | 1.13 ± 0.05 a,b | |
| 14 | 3.59 ± 0.22 a | 2.91 ± 0.34 a,b,c | 4.17 ± 0.26 a | 0.78 ± 0.05 a | |
| 21 | 5.72 ± 0.76 a | 3.20 ± 0.20 c | 4.27 ± 0.26 a,b | 1.32 ± 0.32 b | |
| 28 | 5.60 ± 0.26 a | 3.12 ± 0.04 b,c | 4.30 ± 0.19 a,b | 1.40 ± 0.33 b | |
| C* | 1 | 6.57 ± 0.55 a | 3.23 ± 0.02 a | 5.24 ± 0.09 c | 7.41 ± 0.02 a |
| 7 | 6.41 ± 0.85 a | 3.25 ± 0.04 a | 5.08 ± 0.53 b,c | 8.82 ± 0.26 d | |
| 14 | 6.19 ± 0.26 a | 3.26 ± 0.37 a | 4.51 ± 0.30 a | 7.65 ± 0.51 a,b | |
| 21 | 6.33 ± 0.82 a | 3.53 ± 0.20 a | 4.71 ± 0.22 a,b | 8.13 ± 0.16 c | |
| 28 | 6.22 ± 0.33 a | 3.47 ± 0.07 a | 4.78 ± 0.16 a,b,c | 8.06 ± 0.10 b,c | |
| °hue | 1 | 115.0 ± 0.3 a | 59.5 ± 0.9 a | 76.7 ± 2.6 d | 7.4 ± 0.4 a,b |
| 7 | 115.0 ± 0.1 a | 60.2 ± 1.6 a | 68.3 ± 1.3 c | 7.3 ± 0.6 a,b | |
| 14 | 115.3 ± 0.5 a | 63.0 ± 0.5 b | 67.5 ± 0.5 b,c | 5.9 ± 0.8 a | |
| 21 | 115.5 ± 0.5 a,b | 65.2 ± 0.7 c | 65.1 ± 1.7 a,b | 9.3 ± 2.1 b,c | |
| 28 | 115.8 ± 0.8 b | 64.2 ± 0.8 b,c | 64.0 ± 1.1 a | 10.0 ± 2.3 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dimitrellou, D.; Solomakou, N.; Kokkinomagoulos, E.; Kandylis, P. Yogurts Supplemented with Juices from Grapes and Berries. Foods 2020, 9, 1158. https://doi.org/10.3390/foods9091158
Dimitrellou D, Solomakou N, Kokkinomagoulos E, Kandylis P. Yogurts Supplemented with Juices from Grapes and Berries. Foods. 2020; 9(9):1158. https://doi.org/10.3390/foods9091158
Chicago/Turabian StyleDimitrellou, Dimitra, Nikoletta Solomakou, Evangelos Kokkinomagoulos, and Panagiotis Kandylis. 2020. "Yogurts Supplemented with Juices from Grapes and Berries" Foods 9, no. 9: 1158. https://doi.org/10.3390/foods9091158







