The Strength of the Nutrient Solution Modulates the Functional Profile of Hydroponically Grown Lettuce in a Genotype-Dependent Manner
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Grow Conditions and Experimental Design
2.2. Sample Preparation
2.3. Total Phenolic and Total Flavonoid Contents
2.4. UHPLC-QTOF Mass Spectrometry Profiling
2.5. Determination of Antioxidant and Enzyme Inhibitory Effects
2.6. Statistical Analysis
3. Results
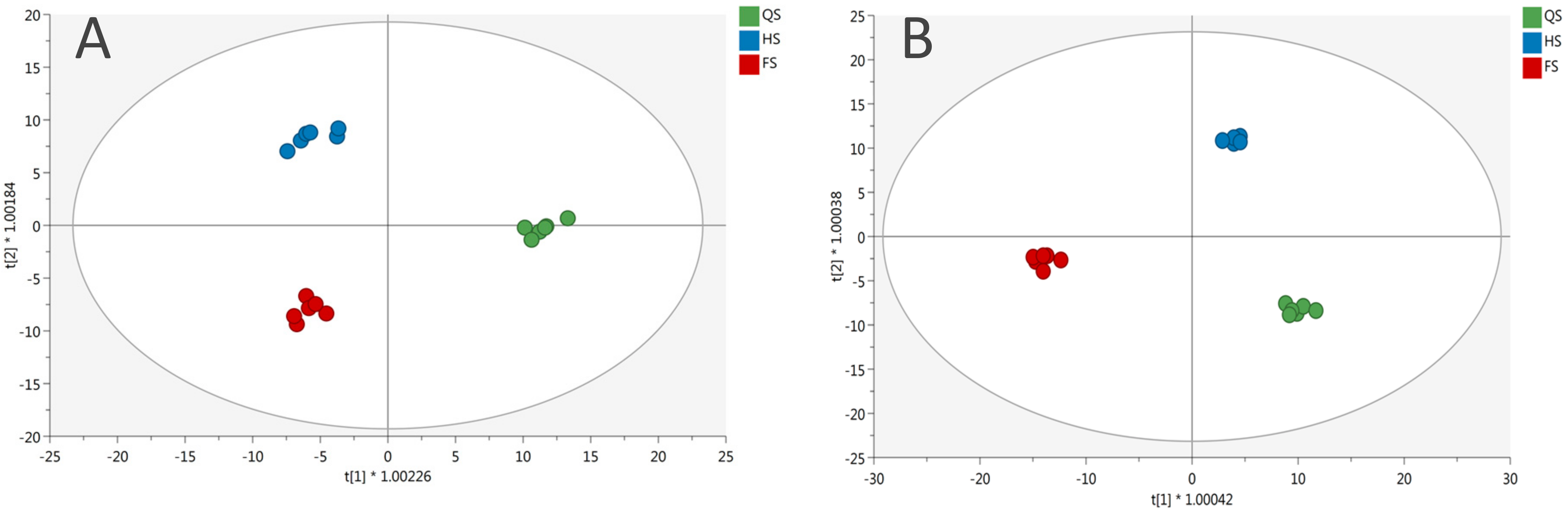
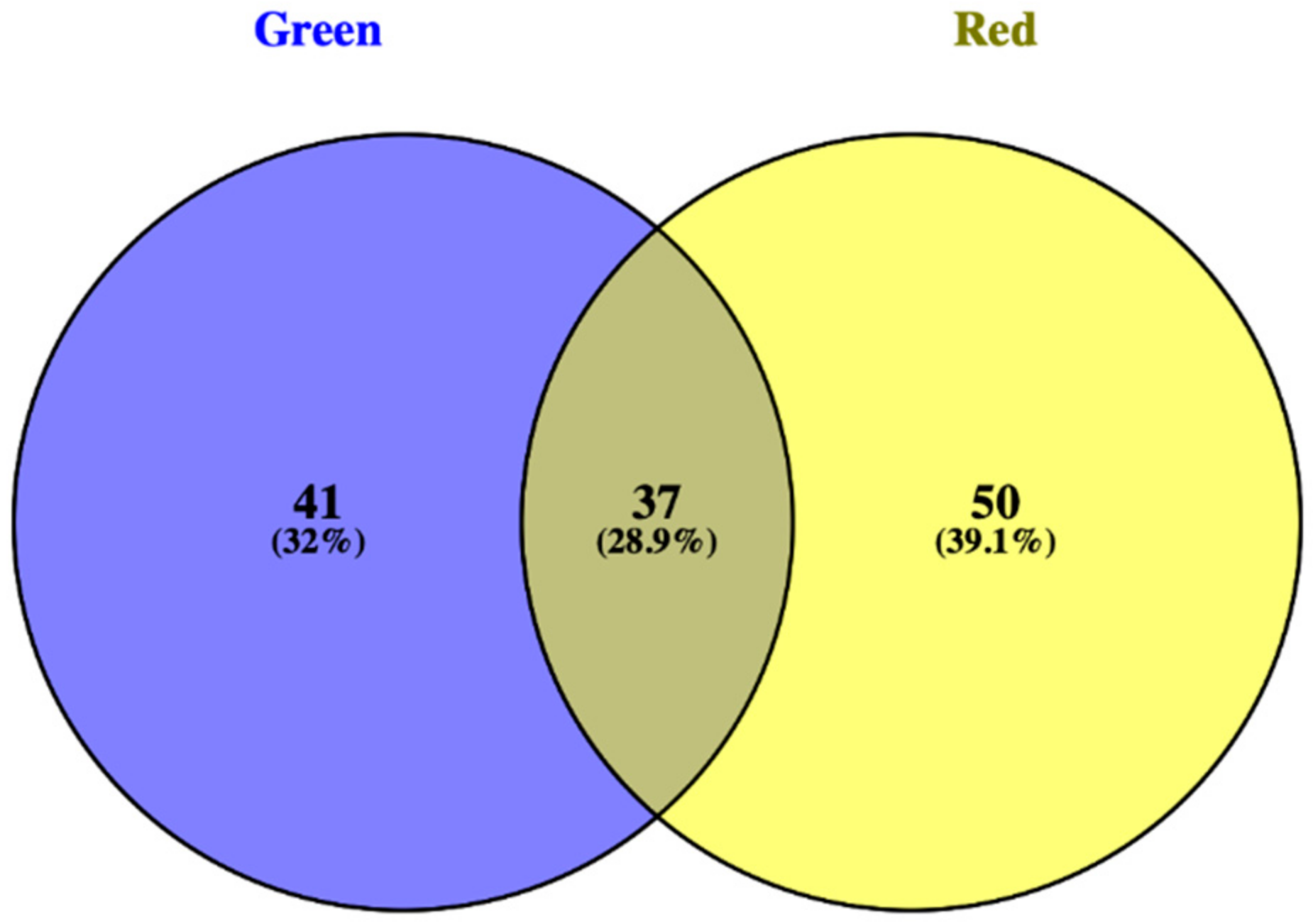
3.1. Phenolic Profile of Salanova Lettuce
3.2. In Vitro Antioxidant Capacity
3.3. Enzyme Inhibitory Activity
3.4. Pearson’s Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fageria, N.K.; Baligar, V.C.; Jones, C.A. Growth and Mineral Nutrition of Field Crops; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants; Academic Press: Waltham, MA, USA, 2011. [Google Scholar]
- White, P.; Brown, P. Plant nutrition for sustainable development and global health. Ann. Bot. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.D. Plant metabolomics in a nutshell: Potential and future challenges. Annu. Plant. Rev. Online 2018, 43, 1–24. [Google Scholar] [CrossRef]
- Nikiforova, V.J.; Kopka, J.; Tolstikov, V.; Fiehn, O.; Hopkins, L.; Hawkesford, M.J.; Hesse, H.; Hoefgen, R. Systems rebalancing of metabolism in response to sulfur deprivation, as revealed by metabolome analysis of arabidopsis plants. Plant. Physiol. 2005, 138, 304–318. [Google Scholar] [CrossRef]
- Takahashi, H.; Imamura, T.; Miyagi, A.; Uchimiya, H. Comparative metabolomics of developmental alterations caused by mineral deficiency during in vitro culture of gentiana triflora. Metabolomics 2012, 8, 154–163. [Google Scholar] [CrossRef]
- Ding, Z.; Jia, S.; Wang, Y.; Xiao, J.; Zhang, Y. Phosphate stresses affect ionome and metabolome in tea plants. Plant. Physiol. Biochem. 2017, 120, 30–39. [Google Scholar] [CrossRef]
- Schauer, N.; Fernie, A.R. Plant metabolomics: Towards biological function and mechanism. Trends Plant. Sci. 2006, 11, 508–516. [Google Scholar] [CrossRef]
- Rouached, H.; Rhee, S.Y. System-level understanding of plant mineral nutrition in the big data era. Curr. Opin. Syst. Biol. 2017, 4, 71–77. [Google Scholar] [CrossRef]
- Maucieri, C.; Nicoletto, C.; Os, E.V.; Anseeuw, D.; Havermaet, R.V.; Junge, R. Hydroponic technologies. In Aquaponics Food Production Systems: Combined Aquaculture and Hydroponic Production Technologies for the Future; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 77–110. [Google Scholar] [CrossRef]
- Epstein, E. Mineral Nutrition of Plants: Principles and Perspectives; CABI Publishing: Wallingford Oxfordshire, UK, 1972. [Google Scholar]
- Jones, J.B., Jr. Hydroponics: A practical Guide for the Soilless Grower; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- López-Millán, A.F.; Sagardoy, R.; Solanas, M.; Abadía, A.; Abadía, J. Cadmium toxicity in tomato (Lycopersicon esculentum) plants grown in hydroponics. Environ. Exp. Bot. 2009, 65, 376–385. [Google Scholar] [CrossRef]
- Chatzigianni, M.; Ntatsi, G.; Theodorou, M.; Stamatakis, A.; Livieratos, I.; Rouphael, Y.; Savvas, D. Functional quality, mineral composition and biomass production in hydroponic spiny chicory (Cichorium spinosum L.) are modulated interactively by ecotype, salinity and nitrogen supply. Front. Plant Sci. 2019, 10, 1040. [Google Scholar] [CrossRef]
- Sagardoy, R.; Morales, F.; López-Millán, A.F.; Abadía, A.; Abadía, J. Effects of zinc toxicity on sugar beet (Beta vulgaris l.) plants grown in hydroponics. Plant. Biol. 2009, 11, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Walters, K.J.; Behe, B.K.; Currey, C.J.; Lopez, R.G. Historical, current, and future perspectives for controlled environment hydroponic food crop production in the united states. HortScience 2020, 55, 758–767. [Google Scholar] [CrossRef]
- Taiz, L.; Zeiger, E. Plant Physiology; Sinauer Associates: Sunderland, MA, USA, 2010. [Google Scholar]
- Sanchez, D.H.; Siahpoosh, M.R.; Roessner, U.; Udvardi, M.; Kopka, J. Plant metabolomics reveals conserved and divergent metabolic responses to salinity. Physiol. Plant. 2008, 132, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Baron, D.; Amaro, A.C.; Campos, F.G.; Boaro, C.S.; Ferreira, G. Plant physiological responses to nutrient solution: An overview. In Plant Metabolites and Regulation Under Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2018; pp. 415–425. [Google Scholar]
- Kumar, P.; Saini, S. Nutrients for hydroponic systems in fruit crops. In Hydrocultural and Hydroponics Systems; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Tyler, G.; Olsson, T. Concentrations of 60 elements in the soil solution as related to the soil acidity. Eur. J. Soil Sci. 2001, 52, 151–165. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Carillo, P.; Pizzolongo, F.; Romano, R.; Sifola, M.I. Chemical eustress elicits tailored responses and enhances the functional quality of novel food perilla frutescens. Molecules 2019, 24, 185. [Google Scholar] [CrossRef]
- Pannico, A.; El Nakhel, C.; Kyriacou, M.C.; Giordano, M.; Stazi, S.R.; De Pascale, S.; Rouphael, Y. Combating micronutrient deficiency and enhancing food functional quality through selenium fortification of select lettuce genotypes grown in a closed soilless system. Front. Plant Sci. 2019, 10, 1495. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C. Enhancing quality of fresh vegetables through salinity eustress and biofortification applications facilitated by soilless cultivation. Front. Plant Sci. 2018, 9, 1254. [Google Scholar] [CrossRef]
- Mulabagal, V.; Ngouajio, M.; Nair, A.; Zhang, Y.; Gottumukkala, A.L.; Nair, M.G. In vitro evaluation of red and green lettuce (Lactuca sativa) for functional food properties. Food Chem. 2010, 118, 300–306. [Google Scholar] [CrossRef]
- Kim, M.J.; Moon, Y.; Tou, J.C.; Mou, B.; Waterland, N.L. Nutritional value, bioactive compounds and health benefits of lettuce (Lactuca sativa L.). J. Food Compos. Anal. 2016, 49, 19–34. [Google Scholar] [CrossRef]
- Jurić, S.; Sopko Stracenski, K.; Król-Kilińska, Ż.; Žutić, I.; Uher, S.F.; Đermić, E.; Topolovec-Pintarić, S.; Vinceković, M. The enhancement of plant secondary metabolites content in Lactuca sativa L. by encapsulated bioactive agents. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; De Pascale, S.; Rouphael, Y. Macronutrient deprivation eustress elicits differential secondary metabolites in red and green-pigmented butterhead lettuce grown in a closed soilless system. J. Sci. Food Agric. 2019, 99, 6962–6972. [Google Scholar] [CrossRef]
- Złotek, U.; Świeca, M.; Jakubczyk, A. Effect of abiotic elicitation on main health-promoting compounds, antioxidant activity and commercial quality of butter lettuce (Lactuca sativa L.). Food Chem. 2014, 148, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Gou, W.; Xue, X.; Wang, L.; Qiao, X. Growth and quality responses of “Green Oak Leaf” lettuce as affected by monochromic or mixed radiation provided by fluorescent lamp (FL) and light-emitting diode (LED). Sci. Hortic. 2014, 172, 168–175. [Google Scholar] [CrossRef]
- Oh, M.M.; Carey, E.E.; Rajashekar, C.B. Environmental stresses induce health-promoting phytochemicals in lettuce. Plant Physiol. Biochem. 2009, 47, 578–583. [Google Scholar] [CrossRef] [PubMed]
- El-Nakhel, C.; Petropoulos, S.A.; Pannico, A.; Kyriacou, M.C.; Giordano, M.; Colla, G.; Troise, A.D.; Vitaglione, P.; De Pascale, S.; Rouphael, Y. The bioactive profile of lettuce produced in a closed soilless system as configured by combinatorial effects of genotype and macrocation supply composition. Food Chem. 2020, 309, 125713. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Chin-Wo, S.; Wang, Z.; Yang, X.; Kozik, A.; Arikit, S.; Song, C.; Xia, L.; Froenicke, L.; Lavelle, D.O.; Truco, M.-J. Genome assembly with in vitro proximity ligation data and whole-genome triplication in lettuce. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef]
- Zengin, G.; Karanfil, A.; Uren, M.C.; Kocak, M.S.; Sarikurkcu, C.; Gungor, H.; Nancy Picot, C.M.; Mahomoodally, M.F. Phenolic content, antioxidant and enzyme inhibitory capacity of two: Trametes species. RSC Adv. 2016, 6, 73351–73357. [Google Scholar] [CrossRef]
- Rocchetti, G.; Chiodelli, G.; Giuberti, G.; Lucini, L. Bioaccessibility of phenolic compounds following in vitro large intestine fermentation of nuts for human consumption. Food Chem. 2018, 245, 633–640. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 1–8. [Google Scholar] [CrossRef]
- Salek, R.M.; Steinbeck, C.; Viant, M.R.; Goodacre, R.; Dunn, W.B. The role of reporting standards for metabolite annotation and identification in metabolomic studies. Gigascience 2013, 2, 2–4. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; Vandergheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Rocchetti, G.; Lucini, L.; Rodriguez, J.M.L.; Barba, F.J.; Giuberti, G. Gluten-free flours from cereals, pseudocereals and legumes: Phenolic fingerprints and in vitro antioxidant properties. Food Chem. 2019, 271, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Sambo, P.; Nicoletto, C.; Giro, A.; Pii, Y.; Valentinuzzi, F.; Mimmo, T.; Lugli, P.; Orzes, G.; Mazzetto, F.; Astolfi, S.; et al. Hydroponic solutions for soilless production systems: Issues and opportunities in a smart agriculture perspective. Front. Plant Sci. 2019, 10, 923. [Google Scholar] [CrossRef]
- Savvas, D. Hydroponics: A modern technology supporting the application of integrated crop management in greenhouse. Food Agric. Environ. 2003, 1, 80–86. [Google Scholar]
- Gruda, N. Do soilless culture systems have an influence on product quality of vegetables. J. Appl. Bot. Food Qual. 2009, 82, 141–147. [Google Scholar]
- Krauss, S.; Schnitzler, W.H.; Grassman, J.; Woitke, M. The influence of different electrical conductivity values in a simplified recirculating soilless system on inner and outer fruit quality characteristics of tomato. J. Agric. Food Chem. 2006, 54, 441–448. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C.; Petropoulos, S.A.; De Pascale, S.; Colla, G. Improving vegetable quality in controlled environments. Sci. Hort. 2018, 234, 275–289. [Google Scholar] [CrossRef]
- Fallovo, C.; Rouphael, Y.; Rea, E.; Battistelli, A.; Colla, G. Nutrient solution concentration and growing season affect yield and quality of Lactuca sativa L. var. acephala in floating raft culture. J. Sci. Food Agric. 2009, 89, 1682–1689. [Google Scholar] [CrossRef]
- Grochowski, D.M.; Uysal, S.; Aktumsek, A.; Granica, S.; Zengin, G.; Ceylan, R.; Locatelli, M.; Tomczyk, M. In vitro enzyme inhibitory properties, antioxidant activities, and phytochemical profile of Potentilla thuringiaca. Phytochem. Lett. 2017, 20, 365–372. [Google Scholar] [CrossRef]
- Lucini, L.; Rocchetti, G.; Trevisan, M. Extending the concept of terroir from grapes to other agricultural commodities: An overview. Curr. Opin. Food Sci. 2020, 31, 88–95. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources Bioavailab. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Bolvig, A.K.; Adlercreutz, H.; Theil, P.K.; Jorgensen, H.; Knudsen, K.E.B. Absorption of plant lignans from cereals in an experimental pig model. Br. J. Nutr. 2016, 115, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Kuijsten, A.; Arts, I.C.W.; Van’t Veer, P.; Hollman, P.C.H. The relative bioavailability of enterolignans in humans is enhanced by milling and crushing of flaxseed. J. Nutr. 2005, 135, 2812–2816. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Borriello, S.P.; Gordon, H.; Lawson, A.M.; Harkness, R.; Morgan, D.M.L.; Kirk, D.N.; Adlercreutz, H.; Anderson, L.C.; Axelson, M. Lignan formation in man-microbial involvement and possible roles in relation to cancer. Lancet 1981, 318, 4–7. [Google Scholar] [CrossRef]
- Mousavi, Y.; Adlercreutz, H. Enterolactone and estradiol inhibit each other’s proliferative effect on MCF-7 breast cancer cells in culture. J. Steroid Biochem. Mol. Biol. 1992, 41, 615–619. [Google Scholar] [CrossRef]
- Sammartino, A.; Tommaselli, G.A.; Gargano, V.; Di Carlo, C.; Attianese, W.; Nappi, C. Short-term effects of a combination of isoflavones, lignans and Cimicifuga racemosa on climacteric-related symptoms in postmenopausal women: A double-blind, randomized, placebo-controlled trial. Gynecol. Endocrinolgy 2006, 22, 646–650. [Google Scholar] [CrossRef]
- Jiang, K.; Song, Q.Y.; Peng, S.J.; Zhao, Q.Q.; Li, G.-D.; Li, Y.; Gao, K. New lignans from the roots of Schisandra sphenanthera. Fitoterapia 2015, 103, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Sharififar, F.; Dehghn-Nudeh, G.; Mirtajaldini, M. Major flavonoids with antioxidant activity from Teucrium polium L. Food Chem. 2009, 112, 885–888. [Google Scholar] [CrossRef]
- Martijn, P. Absorption of dietary quercetin healthy ileostomy volunteers13 glycosides. Am. J. Clin. Nutr. 2018, 1276–1282. [Google Scholar] [CrossRef]
- Morand, C.; Manach, C.; Crespy, V.; Remesy, C. Quercetin 3-o-β-glucoside is better absorbed than other quercetin forms and is not present in rat plasma. Free Radic. Res. 2000, 33, 667–676. [Google Scholar] [CrossRef]
- Villanueva-Suárez, M.J.; Mateos-Aparicio, I.; Pérez-Cózar, M.L.; Yokoyama, W.; Redondo-Cuenca, A. Hypolipidemic effects of dietary fibre from an artichoke by-product in Syrian hamsters. J. Funct. Foods 2019, 56, 156–162. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Lucini, L. Interactions between phenolic compounds, amylolytic enzymes and starch: An updated overview. Curr. Opin. Food Sci. 2020. [Google Scholar] [CrossRef]
- Gopal, S.S.; Lakshmi, M.J.; Sharavana, G.; Sathaiah, G.; Sreerama, Y.N.; Baskaran, V. Lactucaxanthin-a potential anti-diabetic carotenoid from lettuce (Lactuca sativa) inhibits α-amylase and α-glucosidase activity in vitro and in diabetic rats. Food Funct. 2017, 8, 1124–1131. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.X.; Duan, F.F.; Jia, S.S.; Cheng, F.R.; Yuan, K. Antioxidant and tyrosinase inhibitory activities of seed oils from torreya grandis Fort. ex Lindl. Biomed. Res. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Heimler, D.; Romani, A.; Ieri, F. Plant polyphenol content, soil fertilization and agricultural management: A review. Eur. Food Res. Technol. 2017, 243, 1107–1115. [Google Scholar] [CrossRef]
- Nguyen, P.M.; Niemeyer, E.D. Effects of nitrogen fertilization on the phenolic composition and antioxidant properties of basil (Ocinum basilicum L.). J. Agric. Food Chem. 2008, 56, 8685–8691. [Google Scholar] [CrossRef]
- Erdogan Orhan, I.; Senol, F.S.; Ozturk, N.; Celik, S.A.; Pulur, A.; Kan, Y. Phytochemical contents and enzyme inhibitory and antioxidant properties of Anethum graveolens L. (dill) samples cultivated under organic and conventional agricultural conditions. Food Chem. Toxicol. 2013, 59, 96–103. [Google Scholar] [CrossRef]
- Muñoz, E.; Avila, J.G.; Alarcón, J.; Kubo, I.; Werner, E.; Céspedes, C.L. Tyrosinase inhibitors from calceolaria integrifolia s.l.: Calceolaria talcana aerial parts. J. Agric. Food Chem. 2013, 61, 4336–4343. [Google Scholar] [CrossRef]
| Source of Variance | Anthocyanins | Flavanols | Flavones | Flavonols | Lignans | Tyrosols | Phenolic Acids | Stilbenes |
|---|---|---|---|---|---|---|---|---|
| Cyanidin eq. (mg/100 g) | Catchin eq. (mg/100 g) | Luteolin eq. (mg/100 g) | Quercetin eq. (mg/100 g) | Sesamin eq. (mg/100 g) | Tyrosols eq. (mg/100 g) | Ferulic eq. (mg/100 g) | Resveratrol eq. (mg/100 g) | |
| Salanova (S) | ||||||||
| Green | 26.18 ± 1.23 b | 31.21 ± 1.83 b | 143.5 ± 9.49 b | 62.66 ± 2.85 b | 724 ± 20.05 b | 759 ± 22.66 b | 328.2 ± 14.22 b | 34.21 ± 2.37 b |
| Red | 45.61 ± 2.37 a | 49.61 ± 4.70 a | 193.8 ± 13.19 a | 106.5 ± 4.77 a | 1116 ± 109.51 a | 1227 ± 44.80 a | 778.0 ± 29.06 a | 58.22 ± 5.40 a |
| Concentration (C) | ||||||||
| Full strength | 32.52 ± 1.63 | 35.18 ± 2.74 b | 153.0 ± 14.18 b | 72.00 ± 5.30 b | 717 ± 37.71 b | 897 ± 66.19 b | 492.0 ± 51.78 b | 48.30 ± 9.39 |
| Half strength | 37.16 ± 3.96 | 36.19 ± 6.57 b | 194.0 ± 19.22 a | 85.76 ± 9.55 a | 820 ± 54.44 b | 1022 ± 96.57 a | 544.7 ± 81.41 b | 43.02 ± 3.27 |
| Quarter strength | 38.01 ± 4.72 | 49.85 ± 4.38 a | 159.0 ± 11.17 b | 95.99 ± 7.55 a | 1224 ± 147.49 a | 1059 ± 76.23 a | 622.5 ± 78.29 a | 47.32 ± 4.45 |
| C × S | ||||||||
| Green × Full strength | 29.27 ± 2.15 bc | 28.04 ± 2.53 | 121.0 ± 6.40 c | 58.06 ± 5.48 c | 729 ± 48.87 c | 718 ± 47.40 | 329.2 ± 21.76 c | 30.77 ± 1.54 |
| Green × Half strength | 25.97 ± 2.10 c | 27.11 ± 2.55 | 141.4 ± 17.00 bc | 56.68 ± 1.72 c | 679 ± 29.54 c | 728 ± 31.51 | 286.5 ± 6.08 c | 36.12 ± 5.16 |
| Green × Quarter strength | 23.29 ± 1.67 c | 38.47 ± 2.30 | 168.1 ± 19.08 bc | 73.23 ± 4.05 b | 765 ± 9.67 c | 830 ± 21.59 | 368.8 ± 29.56 c | 35.72 ± 4.97 |
| Red × Full strength | 35.76 ± 1.72 b | 42.32 ± 2.52 | 185.1 ± 20.83 b | 85.94 ± 4.01 b | 704 ± 61.70 c | 1077 ± 64.24 | 654.7 ± 27.10 b | 65.83 ± 16.20 |
| Red × Half strength | 48.35 ± 3.80 a | 45.27 ± 12.26 | 246.6 ± 15.14 a | 114.8 ± 7.75 a | 962 ± 64.65 b | 1316 ± 73.80 | 803.0 ± 49.45 a | 49.92 ± 1.12 |
| Red × Quarter strength | 52.73 ± 2.95 a | 61.23 ± 5.24 | 149.8 ± 12.29 bc | 118.8 ± 5.20 a | 1682 ± 107.47 a | 1288 ± 63.68 | 876.3 ± 18.27 a | 58.92 ± 2.92 |
| Significance | ||||||||
| Salanova (S) | *** | *** | ** | *** | *** | *** | *** | *** |
| Concentration (C) | ns | * | * | *** | *** | * | *** | ns |
| S × C | *** | ns | ** | * | *** | ns | ** | ns |
| Source of Variance | Total Phenolic Content | Total Flavonoid Content | DPPH | ABTS | CUPRAC | FRAP | Phosphomolydenum | Metal Chelating |
|---|---|---|---|---|---|---|---|---|
| (mg GAE) | (mg RE) | (mg TE) | (mg TE) | (mg TE) | (mg TE) | (mmol TE) | (mg EDTAE) | |
| Salanova (S) | ||||||||
| Green | 12.41 ± 0.28 b | 22.17 ± 1.39 a | 3.31 ± 0.86 b | 12.28 ± 0.81 b | 30.41 ± 1.47 b | 12.73 ± 0.58 b | 0.74 ± 0.02 b | 15.61 ± 0.57 b |
| Red | 22.31 ± 1.42 a | 12.80 ± 1.80 b | 22.64 ± 2.78 a | 30.94 ± 2.34 a | 69.33 ± 5.31 a | 27.30 ± 2.00 a | 1.11 ± 0.05 a | 19.52 ± 0.54 a |
| Concentration (C) | ||||||||
| Full strength | 14.87 ± 0.88 b | 21.18 ± 1.57 a | 5.71 ± 1.30 b | 16.73 ± 1.70 c | 38.98 ± 3.35 c | 15.36 ± 1.17 b | 0.85 ± 0.04 b | 16.91 ± 0.88 b |
| Half strength | 20.67 ± 2.75 a | 14.16 ± 3.02 b | 17.08 ± 5.04 a | 26.45 ± 4.83 a | 60.49 ± 10.23 a | 23.46 ± 3.82 a | 1.02 ± 0.10 a | 19.36 ± 0.93 a |
| Quarter strength | 16.55 ± 1.25 b | 17.11 ± 2.04 ab | 16.14 ± 3.26 a | 21.64 ± 2.74 b | 50.14 ± 6.05 b | 21.23 ± 2.33 a | 0.91 ± 0.07 b | 16.42 ± 0.61 b |
| C × S | ||||||||
| Green × Full strength | 12.12 ± 0.39 c | 23.07 ± 0.59 a | 1.89 ± 0.64 d | 12.33 ± 2.10 d | 28.90 ± 2.09 d | 11.71 ± 0.55 d | 0.79 ± 0.05 cd | 14.92 ± 1.02 |
| Green × Half strength | 12.57 ± 0.57 c | 23.90 ± 1.46 a | 1.70 ± 0.23 d | 11.18 ± 0.36 d | 29.58 ± 1.95 d | 12.04 ± 0.44 d | 0.74 ± 0.02 cd | 16.83 ± 1.01 |
| Green × Quarter strength | 12.54 ± 0.56 c | 19.54 ± 3.89 ab | 6.36 ± 2.07 cd | 13.34 ± 1.30 d | 32.74 ± 3.51 d | 14.45 ± 1.42 cd | 0.69 ± 0.05 d | 15.08 ± 0.89 |
| Red × Full strength | 17.61 ± 0.49 b | 19.30 ± 3.02 ab | 9.53 ± 1.08 c | 21.14 ± 0.74 c | 49.05 ± 2.10 c | 19.01 ± 0.58 c | 0.90 ± 0.05 c | 18.91 ± 0.88 |
| Red × Half strength | 28.77 ± 2.57 a | 4.42 ± 0.10 c | 32.46 ± 4.16 a | 41.73 ± 3.00 a | 91.41 ± 8.64 a | 34.87 ± 3.43 a | 1.30 ± 0.09 a | 21.89 ± 0.47 |
| Red × Quarter strength | 20.55 ± 0.44 b | 14.67 ± 0.84 b | 25.92 ± 2.07 b | 29.95 ± 1.95 b | 67.53 ± 5.28 b | 28.01 ± 1.84 b | 1.12 ± 0.03 b | 17.77 ± 0.36 |
| Significance | ||||||||
| Salanova (S) | *** | *** | *** | *** | *** | *** | *** | *** |
| Concentration (C) | *** | * | *** | *** | *** | *** | *** | ** |
| S × C | *** | ** | *** | *** | *** | *** | *** | ns |
| Source of Variance | AChE | BChE | Tyrosinase | Amylase | Glucosidase |
|---|---|---|---|---|---|
| (mg GALAE) | (mg GALAE) | (mg KAE) | (mmol ACAE) | (mmol ACAE) | |
| Salanova (S) | |||||
| Green | 2.20 ± 0.14 | 5.58 ± 0.21 a | 64.77 ± 0.90 | 0.35 ± 0.01 | 0.79 ± 0.01 |
| Red | 1.88 ± 0.21 | 4.55 ± 0.20 b | 65.82 ± 1.37 | 0.35 ± 0.01 | 0.84 ± 0.04 |
| Concentration (C) | |||||
| Full strength | 1.98 ± 0.19 | 5.25 ± 0.12 a | 66.62 ± 1.45 | 0.35 ± 0.01 ab | 0.83 ± 0.02 |
| Half strength | 2.16 ± 0.25 | 5.36 ± 0.32 a | 62.54 ± 1.47 | 0.37 ± 0.02 a | 0.85 ± 0.04 |
| Quarter strength | 1.98 ± 0.20 | 4.59 ± 0.34 b | 66.72 ± 1.00 | 0.33 ± 0.01 b | 0.78 ± 0.04 |
| C × S | |||||
| Green × Full strength | 2.23 ± 0.12 | 5.30 ± 0.19 ab | 63.08 ± 1.29 | 0.35 ± 0.02 b | 0.79 ± 0.02 |
| Green × Half strength | 2.55 ± 0.05 | 6.16 ± 0.40 a | 63.85 ± 0.65 | 0.34 ± 0.01 b | 0.78 ± 0.02 |
| Green × Quarter strength | 1.79 ± 0.39 | 5.27 ± 0.36 ab | 67.38 ± 2.00 | 0.35 ± 0.02 b | 0.81 ± 0.02 |
| Red × Full strength | 1.23 ± 0.04 | 5.20 ± 0.15 ab | 70.17 ± 1.59 | 0.35 ± 0.01 b | 0.86 ± 0.02 |
| Red × Half strength | 1.90 ± 0.40 | 4.56 ± 0.18 bc | 61.23 ± 2.90 | 0.40 ± 0.02 a | 0.92 ± 0.07 |
| Red × Quarter strength | 2.18 ± 0.04 | 3.90 ± 0.44 c | 66.06 ± 0.46 | 0.31 ± 0.01 b | 0.74 ± 0.09 |
| Significance | |||||
| Salanova (S) | ns | *** | ns | ns | ns |
| Concentration (C) | ns | ** | ns | *** | ns |
| S × C | ns | * | ns | *** | ns |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senizza, B.; Zhang, L.; Miras-Moreno, B.; Righetti, L.; Zengin, G.; Ak, G.; Bruni, R.; Lucini, L.; Sifola, M.I.; El-Nakhel, C.; et al. The Strength of the Nutrient Solution Modulates the Functional Profile of Hydroponically Grown Lettuce in a Genotype-Dependent Manner. Foods 2020, 9, 1156. https://doi.org/10.3390/foods9091156
Senizza B, Zhang L, Miras-Moreno B, Righetti L, Zengin G, Ak G, Bruni R, Lucini L, Sifola MI, El-Nakhel C, et al. The Strength of the Nutrient Solution Modulates the Functional Profile of Hydroponically Grown Lettuce in a Genotype-Dependent Manner. Foods. 2020; 9(9):1156. https://doi.org/10.3390/foods9091156
Chicago/Turabian StyleSenizza, Biancamaria, Leilei Zhang, Begoña Miras-Moreno, Laura Righetti, Gokhan Zengin, Gunes Ak, Renato Bruni, Luigi Lucini, Maria Isabella Sifola, Christophe El-Nakhel, and et al. 2020. "The Strength of the Nutrient Solution Modulates the Functional Profile of Hydroponically Grown Lettuce in a Genotype-Dependent Manner" Foods 9, no. 9: 1156. https://doi.org/10.3390/foods9091156
APA StyleSenizza, B., Zhang, L., Miras-Moreno, B., Righetti, L., Zengin, G., Ak, G., Bruni, R., Lucini, L., Sifola, M. I., El-Nakhel, C., Corrado, G., & Rouphael, Y. (2020). The Strength of the Nutrient Solution Modulates the Functional Profile of Hydroponically Grown Lettuce in a Genotype-Dependent Manner. Foods, 9(9), 1156. https://doi.org/10.3390/foods9091156