Pyropheophytin a in Soft Deodorized Olive Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Qualitative Analysis of Chlorophyll Pigments
2.4. Quantitative Analysis of Chlorophyll Pigments
2.5. Sensitivity and Method Repeatability
- -
- Sr: Standard deviation of the repeatability =
- -
- r: Repeatability (2.8√Sr2) or intra-laboratory variance.
- -
- RSDr%: Relative standard deviation of the repeatability = 100 × Sr/mean.
- -
- CI: Confidence interval (95%).
2.6. Olive Oil Soft Neutralization Process
2.7. Olive Oil Mild Deodorization Procedure
- Temperature controlled shaker.
- Kitasato flask to prevent the sucking back of the sample.
- Beaker with glycerine as thermal liquid and stirring magnet.
- 60 mL Sample container with bubbler.
- Thermostat.
- Rotameter.
- Stripping gas intake system.
- Vacuum gauge.
- Vacuum pump with vacuum control.
- Deodorization time (using all four varieties: Treatment #1).
- Deodorization temperature (hojiblanca and manzanilla 1 and 2 varieties: Treatment #2).
- Free acidity (using picual variety).
- Stripping gas (using hojiblanca variety: Treatment #3).
3. Results and Discussion
3.1. Sensitivity and Method Repeatability
3.2. Qualitative Analysis of Chlorophyll Pigments
3.3. Quantitative Analysis of Chlorophyll Pigments
3.4. Olive Oil Mild Deodorization Procedure
3.4.1. Effect of Deodorization Time
3.4.2. Effect of Deodorization Temperature
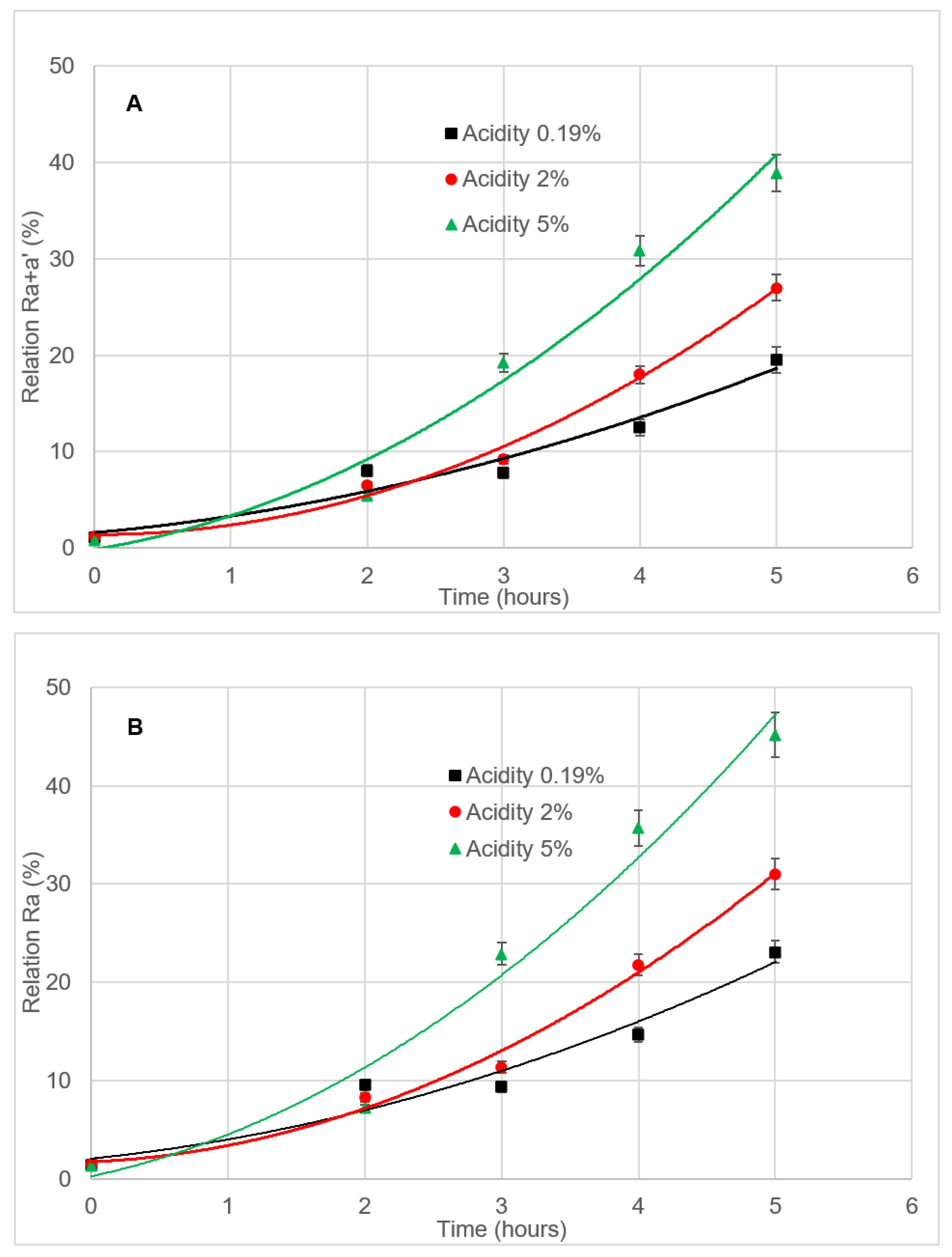
3.4.3. Effect of Free Acidity
3.4.4. Effect of the Stripping Gas
3.4.5. Effect of Neutralization Plus Soft Deodorization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- International Olive Council. Trade Standard Applying to Olive Oils and Olive Pomace Oils; COI/T. 15/NC No 3/Rev. 12; International Olive Council: Madrid, Spain, 2018; pp. 1–17. [Google Scholar]
- European Council Regulation. Regulation No 136/66/EEC of 22 September 1966 on the establishment of a common organization of the market in oils and fats. Off. J. Eur. Commun. 1966, 66, 1–11. [Google Scholar]
- European Commission Regulation. Commission Implementing Regulation (EU) 2019/1604 of 27 September 2019 amending Regulation (EEC) No 2568/91 on the characteristics of olive oil and olive-residue oil and on the relevant methods of analysis. Off. J. Eur. Commun. 2019, 14, 1. [Google Scholar]
- European Commission Regulation. Commission Implementing Regulation (EU) No 29/2012 of 13 January 2012 on marketing standards for olive oil, and successive amendments. Off. J. Eur. Commun. 2012, 12, 14. [Google Scholar]
- Frankel, E.N. Chemistry of extra virgin olive oil: Adulteration, oxidative stability, and antioxidants. J. Agric. Food Chem. 2010, 58, 5991–6006. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Coca, R.B.; Pérez-Camino, M.C.; Martínez-Rivas, J.M.; Bendini, A.; Gallina-Toschi, T.; Moreda, W. Olive oil mixtures. Part one: Decisional trees or how to verify the olive oil percentage in declared blends. Food Chem. 2020, 315, 126235. [Google Scholar] [CrossRef] [PubMed]
- International Olive Council. Determination of the Difference Between Actual and Theoretical Content of Triacylglycerols with ECN 42; COI/T.20/Doc. No 20/Rev. 4; International Olive Council: Madrid, Spain, 2017; pp. 1–22. [Google Scholar]
- Cert, A.; Moreda, W. Algorithms for detection of hazelnut oil in olive oil. Grasas Aceit. 2000, 51, 143–149. [Google Scholar] [CrossRef]
- Azarmad-Darmichi, S.; Dutta, P.C. Novel solid-phase extraction method to separate 4-desmethyl-, 4-monomethyl- and 4,4-dimethylsterols in vegetable oils. J. Chromatogr. A 2006, 1108, 183–187. [Google Scholar] [CrossRef]
- Azarmad-Darmichi, S.; Dutta, P.C. Free and sterified 4,4-dimethylsterols in hazelnut oil and their retention during refining processes. J. Am. Oil Chem. Soc. 2007, 84, 297–304. [Google Scholar] [CrossRef]
- Boskou, D. Olive oil. In Vegetable Oils in Food Technology. Composition, Properties and Uses, 2nd ed.; Gunstone, F.D., Ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 243–272. [Google Scholar]
- International Olive Council. Determination of Waxes, Fatty Acid Methyl Esters and Fatty Acid Ethyl Esters by Capillary Column Gas Chromatography; COI/T.20/Doc. No 28/Rev. 2; International Olive Council: Madrid, Spain, 2017; pp. 1–19. [Google Scholar]
- International Olive Council. Determination of the Sterol Composition and Content and Aliphatic Alcoholic Compounds by Capillary Gas Chromatography; COI/T.20/Doc. No 26/Rev. 4; International Olive Council: Madrid, Spain, 2018; pp. 1–31. [Google Scholar]
- Ranalli, A.; Ferrante, M.L.; De Mattia, G.; Constantini, N. Analytical evaluation of virgin olive oil of first and second extraction. J. Agric. Food Chem. 1999, 47, 417–424. [Google Scholar] [CrossRef]
- Pérez-Camino, M.C.; Cert, A.; Romero-Segura, A.; Cert-Trujillo, R.; Moreda, W. Alkyl esters of fatty acids a useful tool to detect soft deodorized olive oils. J. Agric. Food Chem. 2008, 56, 6740–6744. [Google Scholar] [CrossRef]
- Lanzón, A.; Cert, A.; Albi, T. Detección de la presencia de aceite de olive refinado en aceite de olive virgen. Grasas Aceit. 1989, 40, 385–388. [Google Scholar]
- Cert, A.; Lanzón, A.; Carelli, A.A.; Albi, T. Formation of stifmasta-3,5-diene in vegetable oils. Food Chem. 1994, 49, 287–293. [Google Scholar] [CrossRef]
- Serra Bonveh, J.; Soliva Torrent, M.; Ventura Coll, F. A laboratory study of the bleaching process in stigmasta-3,5-diene concentration in olive oils. J. Am. Oil Chem. Soc. 2001, 78, 305–310. [Google Scholar] [CrossRef]
- Gertz, C.; Klostermann, S. A new analytical procedure to differentiate virgin or non-refined from refined vegetable fats and oils. Eur. J. Lipid Sci. Technol. 2000, 102, 329–336. [Google Scholar] [CrossRef]
- Gómez-Coca, R.B.; Pérez-Camino, M.C.; Bendini, A.; Gallina-Toschi, T.; Moreda, W. Olive oil mixtures. Part two: Detection of soft deodorized oil in extra virgin olive oil through diacylglycerol determination. Relationship with free acidity. Food Chem. 2020, 330, 127226. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Romero, I.; García-González, D.L.; Oliver-Pozo, C.; Aparicio, R. Soft-deodorization of virgin olive oil: Study of the changes of quality and chemical composition. Food Chem. 2017, 220, 42–50. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Valli, E.; Lercker, G.; Mazzini, C. Application of analytical methods to determine mildly deodorized olive oils in commercial extra virgin olive oils. Ind. Alim. Italy 2009, 48, 46–51. [Google Scholar]
- Gómez-Coca, R.B.; Moreda, W.; Pérez-Camino, M.C. Fatty acid alkyl esters in olive oil vs. organoleptic assessment. Food Chem. 2012, 135, 1205–1209. [Google Scholar] [CrossRef]
- Bendini, A.; Valli, E.; Cerretani, L.; Chiavaro, E.; Lercker, G. Study on the effects of heating of virgin olive oil blended with mildly deodorized olive oil: Focus on the hydrolytic and oxidative state. J. Agric. Food Chem. 2009, 57, 10055–10062. [Google Scholar] [CrossRef]
- Mínguez-Mosquera, M.I.; Garrido-Fernández, J. Chlorophyll and carotenoid presence in olive fruit (Olea europaea). J. Agric. Food Chem. 1989, 37, 1–7. [Google Scholar] [CrossRef]
- Mínguez-Mosquera, M.I.; Gandul-Rojas, B.; Garrido-Fernández, J.; Gallardo-Guerrero, L. Pigments present in virgin olive oil. J. Am. Oil Chem. Soc. 1990, 67, 192–196. [Google Scholar] [CrossRef]
- Aparicio-Ruiz, R.; Mínguez-Mosquera, M.I.; Gandul-Rojas, B. Thermal degradation kinetics of chlorophyll pigments in virgin olive oils. 1. Compounds of series a. J. Agric. Food Chem. 2010, 58, 6200–6208. [Google Scholar] [CrossRef] [PubMed]
- Serani, A.; Piacenti, D. Sistema analitico per l’identificazione di oli deodorati in oli vergini di olive. Nota 1—Analisi dei pigmenti clorofilliani in oli vergini di oliva. Riv. Ital. Sostanze Grasse 2001, 78, 459–463. [Google Scholar]
- Gertz, C.; Fiebig, H.J. Pyropheophytin a—Determination of thermal degradation products of chlorophyll a in virgin olive oil. Eur. J. Lipid Sci. Technol. 2006, 108, 1062–1065. [Google Scholar] [CrossRef]
- Hornero-Méndez, D.; Gandul-Rojas, B.; Mínguez-Mosquera, M.I. Routine and sensitive SPE-HPLC method for quantitative determination of pheophytin a and pyropheophytin a in olive oils. Food Res. Int. 2005, 38, 1067–1072. [Google Scholar] [CrossRef]
- Li, X.; Woodman, M.; Wang, S.C. High-performance liquid chromatography with fluorescence detection for the rapid analysis of pheophytins and pyropheophytins in virgin olive oil. J. Sep Sci. 2015, 38, 2813–2818. [Google Scholar] [CrossRef]
- International Organization for Standardization. Animal and Vegetable Fats and Oils—Determination of the Degradation Products of Chlorophylls A (Phepophytins and Pyropheophytins); ISO/DIS 29841; International Organization for Standardization: Geneva, Switzerland, 2008; pp. 1–10. [Google Scholar]
- German Society for Fat Science. Determination of Pyropheophytin A in Olive Oil; Draft 4.10.2005; German Society for Fat Science: Berlin, Germany, 2005. [Google Scholar]
- International Union of Pure and Applied Chemistry. Nomenclature in evaluation of analytical methods including detection and quantification capabilities. Pure Appl. Chem. 1995, 67, 1699–1723. [Google Scholar] [CrossRef]
- International Organization for Standardization. Precision of test methods. Determination of repeatability and reproducibility of a standard test method by inter-laboratory tests. ISO 1986, 5725, 1–49. [Google Scholar]
- Association of Official Agricultural Chemists. Collaborative study guideline. J. Assoc. Off. Anal. Chem. Int. 1995, 78, 143A–160A. [Google Scholar]
- International Olive Council. Determination of Free Fatty Acids, Cold Method; COI/T.20/Doc. No 34/Rev. 1; International Olive Council: Madrid, Spain, 2007; pp. 1–4. [Google Scholar]
- Giuffrida, D.; Salvo, F.; Salvo, A.; Cossignani, L.; Dugo, G. Pigments profile in monovarietal virgin olive oils from various Italian olive varieties. Food Chem. 2011, 124, 1119–1123. [Google Scholar] [CrossRef]
- Guillaume, C.; Gertz, C.; Ravetti, L. Pyropheophytin a and 1,2-diacyl-glycerols over time under different storage conditions in natural olive oils. J. Am. Oil Chem. Soc. 2014, 91, 697–709. [Google Scholar] [CrossRef]
- Standards Australia. AS5264-2011: Olive Oils and Olive-Pomace Oils; Committee FT-034; Standards Australia: Sydney, Australia, 2011. [Google Scholar]
- California Department of Food and Agriculture. Grade and Labelling Standards for Olive Oil, Refined-Olive Oil and Olive-Pomace Oil; California Department of Food and Agriculture: Fresno, CA, USA, 2014.
- Guillaume, C.; Ravetti, L. Shelf-life prediction of extra virgin olive oils using an empirical model based on standard quality tests. J. Chem. 2016, 2016, 1–7. [Google Scholar] [CrossRef]




| Olive Oil Variety | Treatment | Deodorization Conditions | ||
|---|---|---|---|---|
| Time (h) | Temperature (°C) | Gas | ||
| Hojiblanca | Treatment #1 | 0.5, 1.5, 2.5, 3.5, 4.5, 5.5 | 98 | N2 |
| Treatment #2 | 2 | 50, 75, 100, 130, 150 | N2 | |
| Treatment #3 | 2, 3, 4, 5 | 98 | Air/N2 | |
| Manzanilla 1 | Treatment #1 | 0.5, 2.5, 3.5, 4.5, 5.5 | 98 | N2 |
| Treatment #2 | 2 | 50, 75, 100, 130, 150 | N2 | |
| Manzanilla 2 | Treatment #1 | 0.5, 2.5, 3.5, 4.5, 5.5 | 98 | N2 |
| Treatment #2 | 2 | 50, 75, 100, 130, 150 | N2 | |
| Picual | Treatment #1 | 0.5, 2.5, 3.5, 4.5, 5.5 | 98 | N2 |
| 0.19% free acidity | 2, 3, 4, 5 | 98 | N2 | |
| 2% free acidity | 2, 3, 4, 5 | 98 | N2 | |
| 5% free acidity | 2, 3, 4, 5 | 98 | N2 | |
| Parameters | VOO Samples | ||
|---|---|---|---|
| L-1 | M-1 | H-1 | |
| Pheophytin (a+a’) | |||
| Mean (mg/kg) | 11.6 | 20.9 | 41.5 |
| Sr | 0.77 | 0.18 | 0.14 |
| r | 2.15 | 0.52 | 0.40 |
| RSDr, % (n = 3) | 6.59 | 0.89 | 0.34 |
| CI (0.05) | 0.87 | 0.21 | 0.16 |
| Pyropheophytin a | |||
| Mean (mg/kg) | 0.1 | 0.9 | 2.0 |
| Sr | 0.01 | 0.04 | 0.05 |
| r | 0.03 | 0.11 | 0.15 |
| RSDr, % (n = 3) | 10 | 4.44 | 2.50 |
| CI (0.05) | 0.01 | 0.05 | 0.06 |
| Ra+a’ | |||
| Mean (%) | 1.1 | 3.9 | 4.6 |
| Sr | 0.02 | 0.18 | 0.12 |
| r | 0.04 | 0.50 | 0.32 |
| RSDr, % (n = 3) | 1.82 | 4.62 | 2.61 |
| CI (0.05) | 0.02 | 0.20 | 0.13 |
| Olive Oil Variety | Phy a | Phy a’ | Pyphy a | Ra+a | Ra |
|---|---|---|---|---|---|
| Hojiblanca | 8.74 | 2.16 | 0.70 | 6.12 | 7.52 |
| Manzanilla 1 | 102.48 | 13.05 | 3.44 | 2.90 | 3.26 |
| Manzanilla 2 | 14.36 | 1.38 | 0.00 | 0.03 | 0.04 |
| Picual | 10.71 | 10.22 | 2.08 | 0.92 | 0.97 |
| Sample | Treatment | phy a | phy a’ | pyphy a | Ra+a’ | Ra |
|---|---|---|---|---|---|---|
| L-2 | Initial | 2.53 | 0.07 | 0.00 | 0.00 | 0.00 |
| Neutralization | 2.49 | 0.50 | 0.00 | 0.00 | 0.00 | |
| Neutralization + soft deodorization | 1.38 | 0.40 | 0.00 | 0.00 | 0.00 | |
| M-2 | Initial | 16.52 | 1.66 | 0.58 | 3.09 | 3.39 a |
| Neutralization | 12.65 | 2.36 | 0.46 | 2.97 | 3.51 a | |
| Neutralization + soft deodorization | 11.75 | 2.67 | 0.78 | 5.13 | 6.22 b | |
| H-2 | Initial | 36.30 | 4.07 | 1.22 | 2.93 | 3.25 a |
| Neutralization | 34.92 | 7.72 | 1.41 | 3.20 | 3.88 a | |
| Neutralization + soft deodorization | 17.94 | 3.85 | 2.23 | 9.28 | 11.06 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Coca, R.B.; Alassi, M.; Moreda, W.; Pérez-Camino, M.d.C. Pyropheophytin a in Soft Deodorized Olive Oils. Foods 2020, 9, 978. https://doi.org/10.3390/foods9080978
Gómez-Coca RB, Alassi M, Moreda W, Pérez-Camino MdC. Pyropheophytin a in Soft Deodorized Olive Oils. Foods. 2020; 9(8):978. https://doi.org/10.3390/foods9080978
Chicago/Turabian StyleGómez-Coca, Raquel B., Mahmoud Alassi, Wenceslao Moreda, and María del Carmen Pérez-Camino. 2020. "Pyropheophytin a in Soft Deodorized Olive Oils" Foods 9, no. 8: 978. https://doi.org/10.3390/foods9080978
APA StyleGómez-Coca, R. B., Alassi, M., Moreda, W., & Pérez-Camino, M. d. C. (2020). Pyropheophytin a in Soft Deodorized Olive Oils. Foods, 9(8), 978. https://doi.org/10.3390/foods9080978





