Improving the Stability of Lycopene from Chemical Degradation in Model Beverage Emulsions: Impact of Hydrophilic Group Size of Emulsifier and Antioxidant Polarity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulsion Preparation
2.3. Droplet Size Measurement
2.4. Measurement of Lycopene Concentration
2.5. DPPH Radical Scavenging Activity
2.6. Ferric Reducing Antioxidant Power (FRAP) Assay
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of pH on the Stability of Lycopene in Emulsions
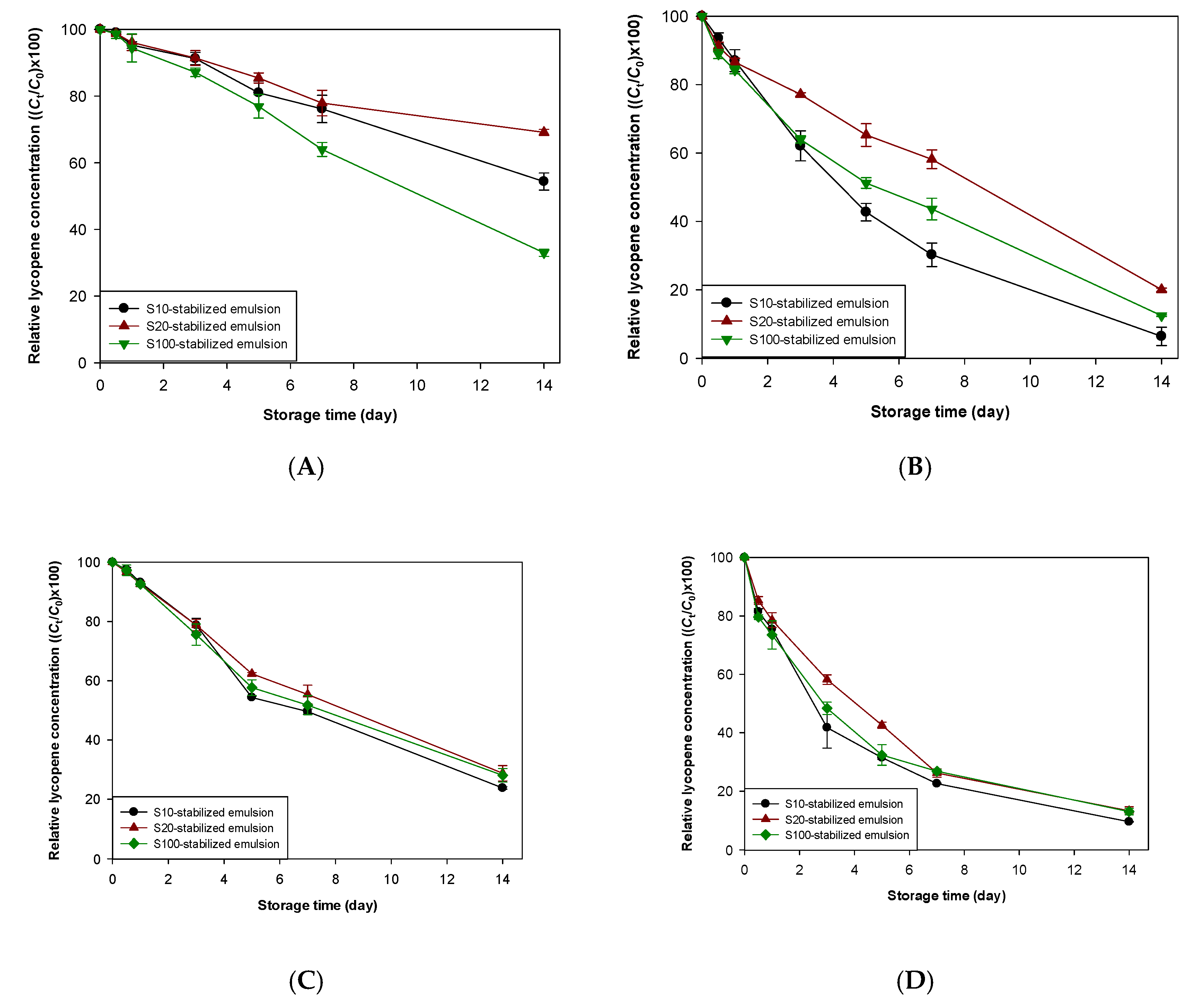
3.2. Impact of Free Radicals on Lycopene Stability in Emulsions
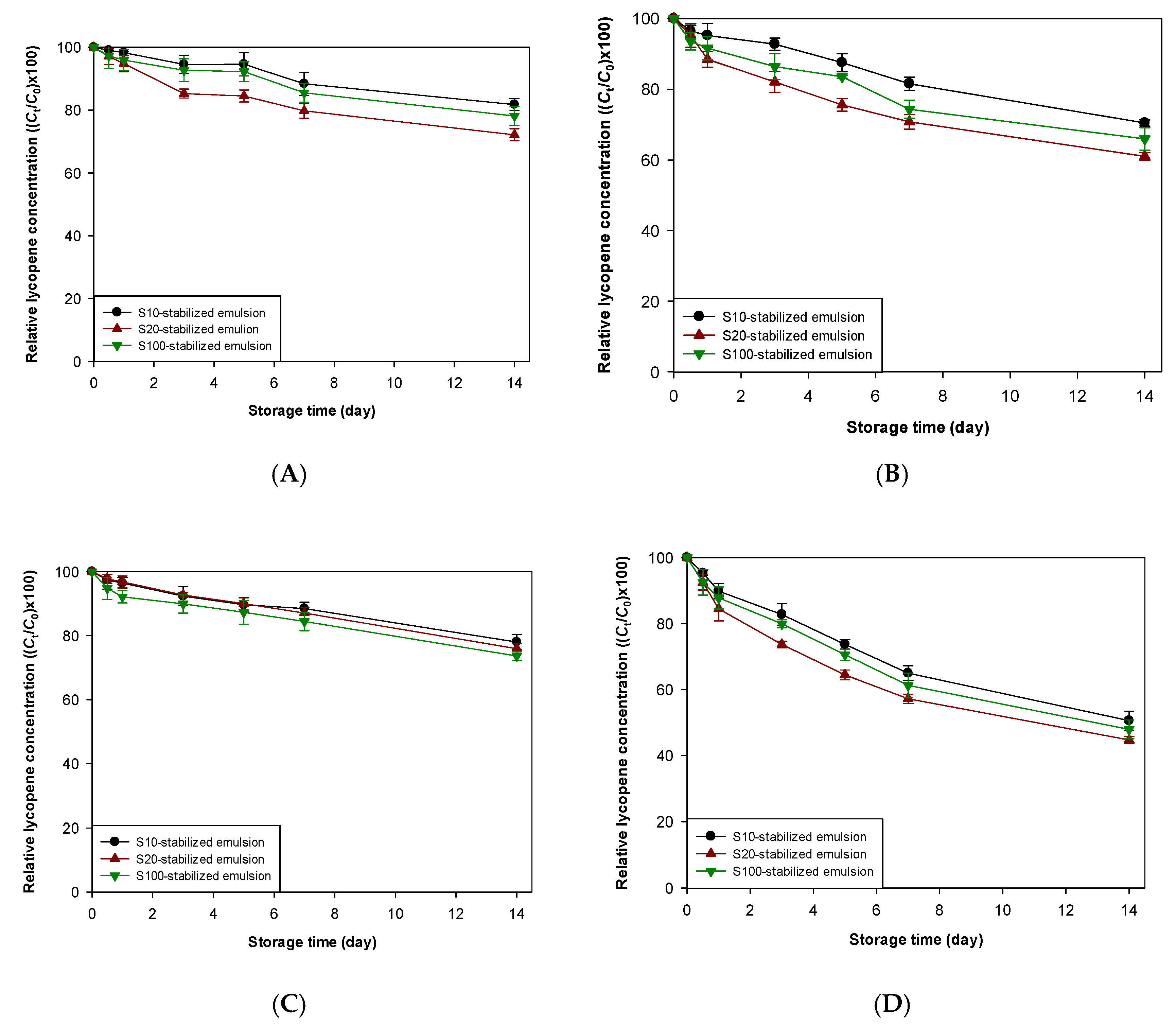
3.3. Impact of Antioxidants on Lycopene Stability in Emulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Olson, J.A.; Krinsky, N.I. Introduction: The colorful fascinating world of the carotenoids: Important physiologic modulators. FASEB J. 1995, 9, 1547–1550. [Google Scholar] [CrossRef]
- Maiani, G.; Periago Castón, M.J.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef] [PubMed]
- Britton, G. Structure and properties of carotenoids in relation to function. FASEB J. 1995, 9, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrition 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Di, M.P.; Kaiser, S.; Sies, H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989, 274, 532–538. [Google Scholar]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidants activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Giovannucci, E. Tomato products, lycopene, and prostate cancer: A review of the epidemiological literature. J. Nutr. 2005, 135, 2030S–2031S. [Google Scholar] [CrossRef]
- Wilcox, J.; Catignani, G.; Lazarus, S. Tomatoes and cardiovascular health. Crit. Rev. Food Sci. Nutr. 2003, 43, 1–18. [Google Scholar] [CrossRef]
- Kun, Y.; Lule, U.S.; Xiao-Lin, A.D. Lycopene: Its properties and relationship to human health. Food Rev. Int. 2006, 22, 309–333. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef]
- Mao, L.; Miao, S. Structuring food emulsions to improve nutrient delivery during digestion. Food Eng. Rev. 2015, 74, 439–451. [Google Scholar] [CrossRef]
- Neves, M.A.; Hashemi, J.; Prentice, C. Development of novel bioactives delivery systems by micro/nanotechnology. Curr. Opin. Food Sci. 2015, 1, 7–12. [Google Scholar] [CrossRef]
- Song, H.Y.; Moon, T.W.; Choi, S.J. Impact of antioxidant on the stability of β-carotene in model beverage emulsions: Role of emulsion interfacial membrane. Food Chem. 2019, 279, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Salvia-Trujillo, L.; Qian, C.; Martín-Belloso, O.; McClements, D.J. Modulating β-carotene bioaccessibility by controlling oil composition and concentration in edible nanoemulsions. Food Chem. 2013, 139, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, R.; McClements, D.J.; Li, F.; Liu, H.; Cao, Y.; Xiao, H. Nanoemulsion-based delivery systems for nutraceuticals: Influence of long-chain triglyceride (LCT) type on in vitro digestion and astaxanthin bioaccessibility. Food Biophys. 2018, 13, 412–421. [Google Scholar] [CrossRef]
- Song, H.Y.; Moon, T.W.; Choi, S.J. Storage stability of β-carotene in model beverage emulsions: Implication of interfacial thickness. Eur. J. Lipid Sci. Technol. 2018, 120, 1800127. [Google Scholar] [CrossRef]
- McClements, D.J. Nanoemulsion-based oral delivery systems for lipophilic bioactive components: Nutraceuticals and pharmaceuticals. Ther. Deliv. 2013, 4, 841–857. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Physical and chemical stability of β-carotene-enriched nanoemulsions: Influence of pH, ionic sterngth, temperature, and emulsifier type. Food Chem. 2012, 132, 1221–1229. [Google Scholar] [CrossRef]
- Qian, C.; Decker, E.A.; Xiao, H.; McClements, D.J. Inhibition of β-carotene degradation in oil-in-water nanoemulsions: Influence of oil-soluble and water-soluble antioxidants. Food Chem. 2012, 135, 1036–1043. [Google Scholar] [CrossRef]
- Guan, Y.; Wu, J.; Zhong, Q. Eugenol improves physical and chemical stabilities of nanoemulsions loaded with β-carotene. Food Chem. 2016, 194, 787–796. [Google Scholar] [CrossRef]
- Bou, R.; Boon, C.; Kweku, A.; Hidalgo, D.; Decker, E.A. Effect of different antioxidants on lycopene degradation in oil-in-water emulsions. Eur. J. Lipid Sci. Technol. 2011, 113, 724–729. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.-W.; Kanner, J.; German, J.B. Interfacial phenomena in the evaluation of antioxidants: Bulk oils vs. emulsions. J. Agric. Food Chem. 1994, 42, 1054–1059. [Google Scholar] [CrossRef]
- Huang, S.-W.; Frankel, E.N.; Aeschbach, R.; German, J.B. Partition of selected antioxidants in corn oil-water model systems. J. Agric. Food Chem. 1997, 45, 1991–1994. [Google Scholar] [CrossRef]
- Jacobsen, C.; Schwarz, K.; Stöckmann, H.; Meyer, A.S.; Alder-Nissen, J. Partioning of selected antioxidants in mayonnaise. J. Agric. Food Chem. 1999, 47, 3601–3610. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barreiro, S.; Bravo-Díaz, C.; Paiva-Martins, F.; Romsted, L.S. Maxima in antioxidant distributions and efficiencies with increasing hydrophobicity of gallic acid and its alkyl esters. The pseudophase model interpretation of the “cutoff effect”. J. Agric. Food Chem. 2013, 61, 6533–6543. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.; Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Modulating the interfacial concentration of gallates to improve the oxidative stability of fish oil-in-water emulsions. Food Res. Int. 2018, 112, 192–198. [Google Scholar] [CrossRef]
- Han, S.W.; Song, H.Y.; Moon, T.W.; Choi, S.J. Influence of emulsion interfacial membrane characteristics on Ostwald ripening in a model emulsion. Food Chem. 2018, 242, 91–97. [Google Scholar] [CrossRef]
- Niki, E. Free radical initiators as source of water- or lipid-soluble peroxyl radicals. Methods Enzymol. 1990, 186, 100–108. [Google Scholar]
- Barba, A.O.; Hurtado, M.C.; Mata, M.S.; Ruiz, V.F.; de Tejada, M.L.S. Application of a UV–vis detection-HPLC method for a rapid determination of lycopene and β-carotene in vegetables. Food Chem. 2006, 95, 328–336. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determination by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Ultrasonic determination of depletion flocculation in oil-in-water emulsions containing a non-ionic surfactant. Colloid Surf. A Physicochem. Eng. Asp. 1994, 90, 24–35. [Google Scholar] [CrossRef]
- Cho, Y.-J.; McClements, D.J.; Decker, E.A. Ability of surfactant micelles to alter the physical location and reactivity of iron in oil-in-water emulsion. J. Agric. Food Chem. 2002, 50, 5704–5710. [Google Scholar] [CrossRef] [PubMed]
- Berton-Carabin, C.C.; Ropers, M.-H.; Genot, C. Lipid oxidation in oil-in-water emulsions: Involvement of the interfacial layer. Compr. Rev. Food. Sci. Food Saf. 2014, 13, 945–977. [Google Scholar] [CrossRef]
- Lee, H.Y.; Song, H.Y.; Choi, S.J. Lipid hydroperoxide decomposition in model emulsions stabilized with emulsifiers having various sizes of hydrophilic heads. Food Sci. Biotechnol. 2019, 28, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Role of iron and hydroperoxides in the degradation of lycopene in oil-in-water emulsions. J. Agric. Food Chem. 2009, 57, 2993–2998. [Google Scholar] [CrossRef]
- Jeevarajan, A.S.; Wei, C.-C.; Kispert, L.D. Geometrical isomerization of carotenoids in dichloromethan. J. Chem. Soc. Perkin Trans. 2 1994, 861–869. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.M. Antioxidant and prooxidant properties of carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef]
- Woodall, A.A.; Lee, S.W.-M.; Weesie, R.J.; Jackson, M.J.; Britton, G. Oxidation of carotenoids by free radicals: Relationship between structure and reactivity. Biochim. Biophys. Acta 1997, 1336, 33–42. [Google Scholar] [CrossRef]
- Liebler, D.C.; McClure, T.D. Antioxidant reactions of β-carotene: Identification of carotenoid-radical adducts. Chem. Res. Toxicol. 1996, 9, 8–11. [Google Scholar] [CrossRef]
- Silvestre, M.P.C.; Chaiyasit, W.; Brannan, R.G.; McClements, D.J.; Decker, E.A. Ability of surfactant headgroup size to alter lipid and antioxidant oxidation in oil-in-water emulsions. J. Agric. Food Chem. 2000, 48, 2057–2061. [Google Scholar] [CrossRef] [PubMed]
- Waraho, T.; McClements, D.J.; Decker, E.A. Mechanisms of lipid oxidation in food dispersions. Trends Food Sci. Technol. 2011, 22, 3–13. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A. Lipid oxidation in oil-in-water emulsions: Impact of molecular environment on chemical reactions in heterogeneous food systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Makahleh, A.; Saad, B.; Bari, M.F. Synthetic phenolics as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 51–78. [Google Scholar]
- Huang, S.-W.; Hopia, A.; Schwarz, K.; Frankel, E.N.; German, J.B. Antioxidant activity of α-tocopherol and trolox in different lipid substrates: Bulk oils vs. oil-in-water emulsions. J. Agric. Food Chem. 1996, 44, 444–452. [Google Scholar] [CrossRef]
- Huang, S.-W.; Frankel, E.N.; Schwarz, K.; Aeschbach, R.; German, J.B. Antioxidant activity of carnosic acid and methyl carnosate in bulk oils and oil-in-water emulsions. J. Agric. Food Chem. 1996, 44, 2951–2956. [Google Scholar] [CrossRef]
- Freiría-Gándara, J.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Enhancement of the antioxidant efficiency of gallic acid derivatives in intact fish oil-in-water emulsions through optimization of their interfacial concentrations. Food Funct. 2018, 9, 4429–4442. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Losada-Barreiro, S.; Bravo-Díaz, C.; Vicente, A.A.; Monteiro, L.S.; Paiva-Martins, F. Influence of AO chain length, droplet size and oil to water ratio on the distribution and on the activity of gallates infish oil-in-water emulsified systems: Emulsion and nanoemulsion comparison. Food Chem. 2020, 310, 125716. [Google Scholar] [CrossRef]
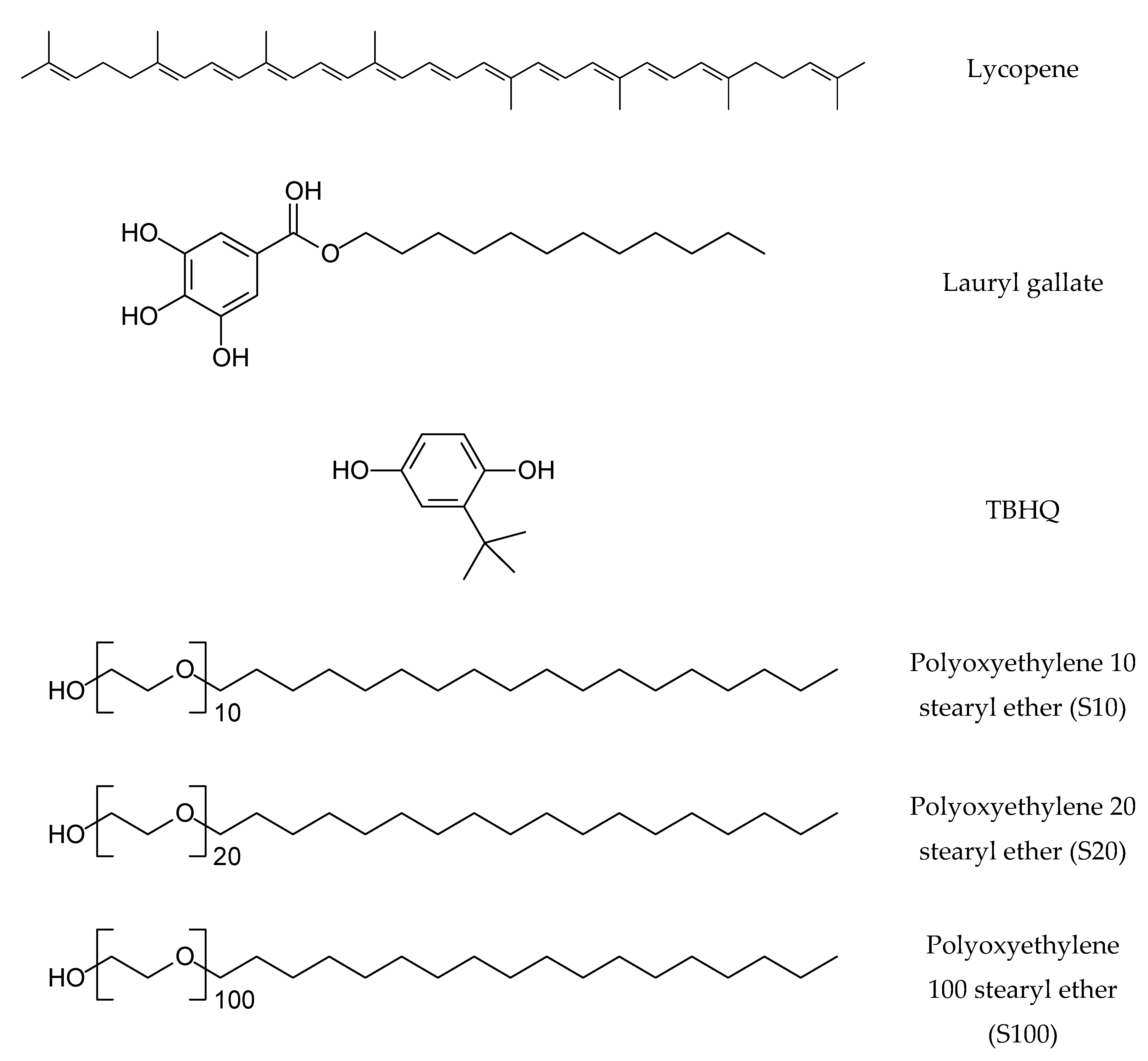

| Free Radicals | S10 | S20 | S100 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k (day−1) | S.E. | r2 | k (day−1) | S.E. | r2 | k (day−1) | S.E. | r2 | ||
| pH 7 | Absent | C 0.0435 b | 0.0015 | 0.994 | D 0.0273 c | 0.0021 | 0.972 | C 0.0788 a | 0.0051 | 0.980 |
| Present | B 0.1048 a | 0.0045 | 0.991 | C 0.0894 b | 0.0018 | 0.998 | B 0.0923 b | 0.0034 | 0.993 | |
| pH 3 | Absent | A 0.1956 a | 0.0062 | 0.995 | B 0.1086 c | 0.0096 | 0.963 | A 0.1431 b | 0.0075 | 0.987 |
| Present | A 0.1657 a | 0.0140 | 0.966 | A 0.1452 a | 0.0100 | 0.977 | A 0.1424 a | 0.0136 | 0.956 | |
| Free Radicals | S10 | S20 | S100 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k (day−1) | S.E. | r2 | k (day−1) | S.E. | r2 | k (day−1) | S.E. | r2 | ||
| pH 7 | Absent | C 0.0145 b | 0.0011 | 0.972 | C 0.0227 a | 0.0030 | 0.919 | C 0.0169 ab | 0.0014 | 0.969 |
| Present | C 0.0197 b | 0.0010 | 0.988 | BC 0.0301 a | 0.0038 | 0.928 | B 0.0251 a | 0.0021 | 0.967 | |
| pH 3 | Absent | B 0.0244 b | 0.0011 | 0.989 | B 0.0339 a | 0.0042 | 0.930 | B 0.0282 ab | 0.0029 | 0.951 |
| Present | A 0.0550 b | 0.0068 | 0.929 | A 0.0730 ab | 0.0070 | 0.956 | A 0.0866 a | 0.0064 | 0.974 | |
| Free Radicals | S10 | S20 | S100 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k (day−1) | S.E. | r2 | k (day−1) | S.E. | r2 | k (day−1) | S.E. | r2 | ||
| pH 7 | Absent | C 0.0167 b | 0.0009 | 0.985 | C 0.0189 a | 0.0005 | 0.996 | D 0.0194 ab | 0.0017 | 0.962 |
| Present | B 0.0196 b | 0.0009 | 0.990 | B 0.0296 a | 0.0037 | 0.927 | C 0.0248 a | 0.0020 | 0.968 | |
| pH 3 | Absent | A 0.0482 a | 0.0030 | 0.981 | A 0.0558 a | 0.0062 | 0.943 | B 0.0513 a | 0.0040 | 0.970 |
| Present | A 0.0540 b | 0.0067 | 0.929 | A 0.0664 ab | 0.0076 | 0.939 | A 0.0855 a | 0.0062 | 0.974 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Choi, S.J. Improving the Stability of Lycopene from Chemical Degradation in Model Beverage Emulsions: Impact of Hydrophilic Group Size of Emulsifier and Antioxidant Polarity. Foods 2020, 9, 971. https://doi.org/10.3390/foods9080971
Kim J, Choi SJ. Improving the Stability of Lycopene from Chemical Degradation in Model Beverage Emulsions: Impact of Hydrophilic Group Size of Emulsifier and Antioxidant Polarity. Foods. 2020; 9(8):971. https://doi.org/10.3390/foods9080971
Chicago/Turabian StyleKim, Jinhyuk, and Seung Jun Choi. 2020. "Improving the Stability of Lycopene from Chemical Degradation in Model Beverage Emulsions: Impact of Hydrophilic Group Size of Emulsifier and Antioxidant Polarity" Foods 9, no. 8: 971. https://doi.org/10.3390/foods9080971
APA StyleKim, J., & Choi, S. J. (2020). Improving the Stability of Lycopene from Chemical Degradation in Model Beverage Emulsions: Impact of Hydrophilic Group Size of Emulsifier and Antioxidant Polarity. Foods, 9(8), 971. https://doi.org/10.3390/foods9080971





