Metabolomic Analysis of Germinated Brown Rice at Different Germination Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. BR and Germination
2.2. Determination of Moisture Content, Germination Rate, and Seedling Length
2.3. Sample Preparation for Metabolomic Analysis
2.4. Analysis of GBR Metabolites Using UPLC-Q-TOF MS
2.5. Analysis of GBR Metabolites Using GC-MS
2.6. Data Processing
2.7. Statistical Analysis
3. Results and Discussion
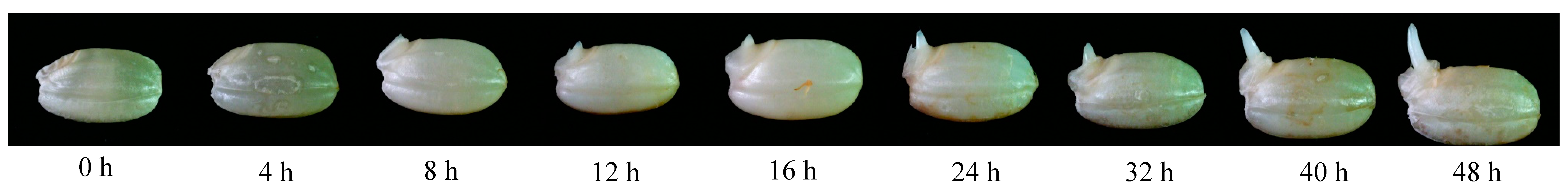
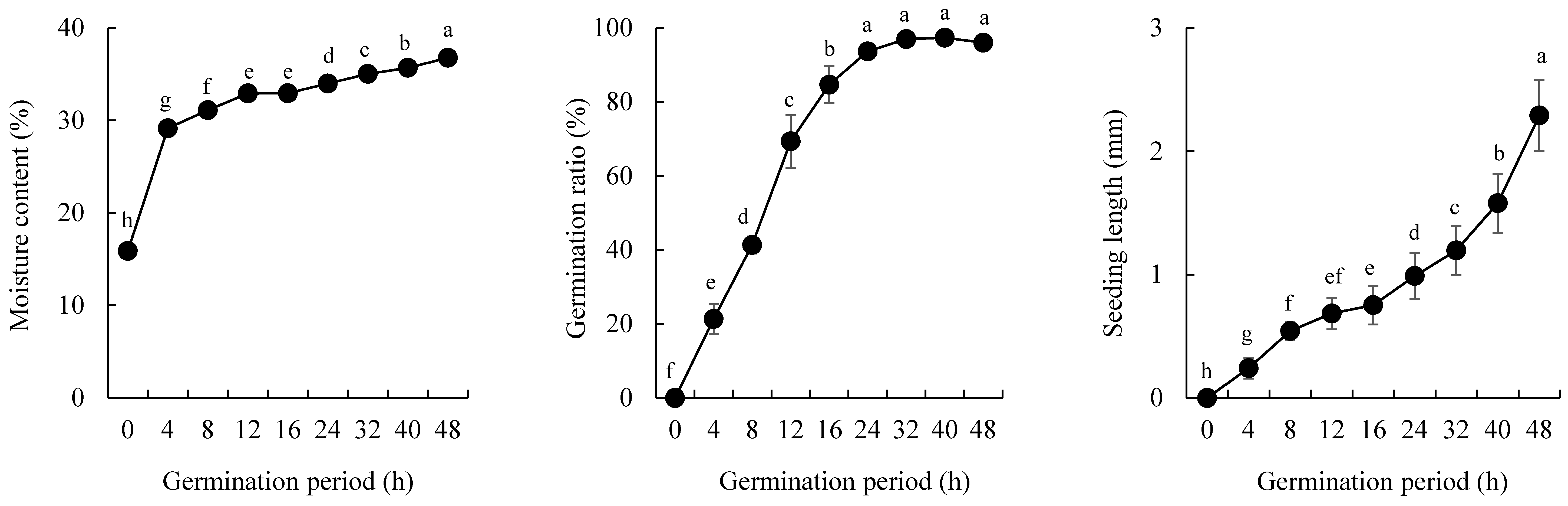
3.1. Germination Ratio, Seedling Length, and Moisture Content of GBR
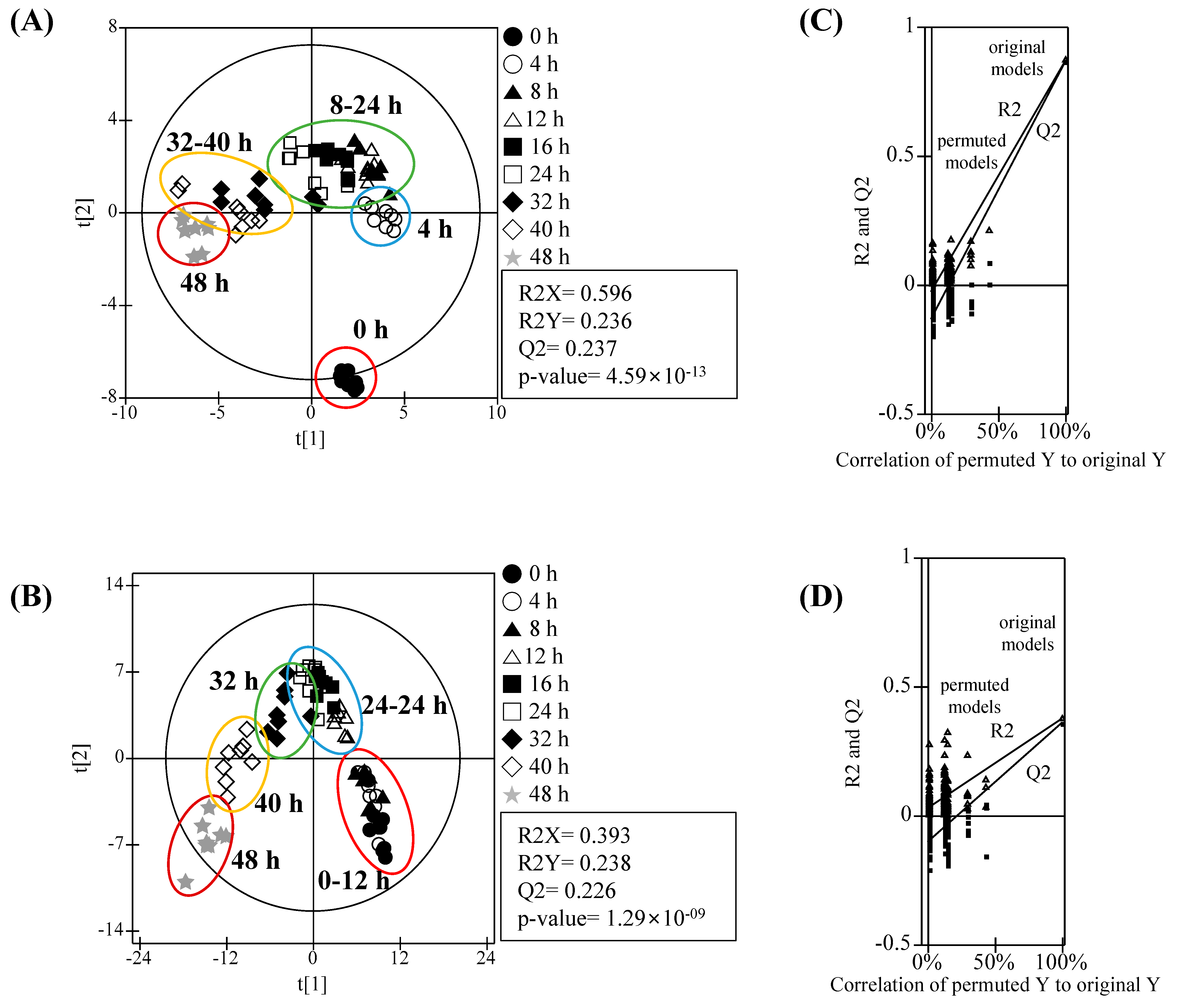
3.2. Metabolomic Analysis and PLS-DA Score Plots
3.3. Identification of Major Metabolites
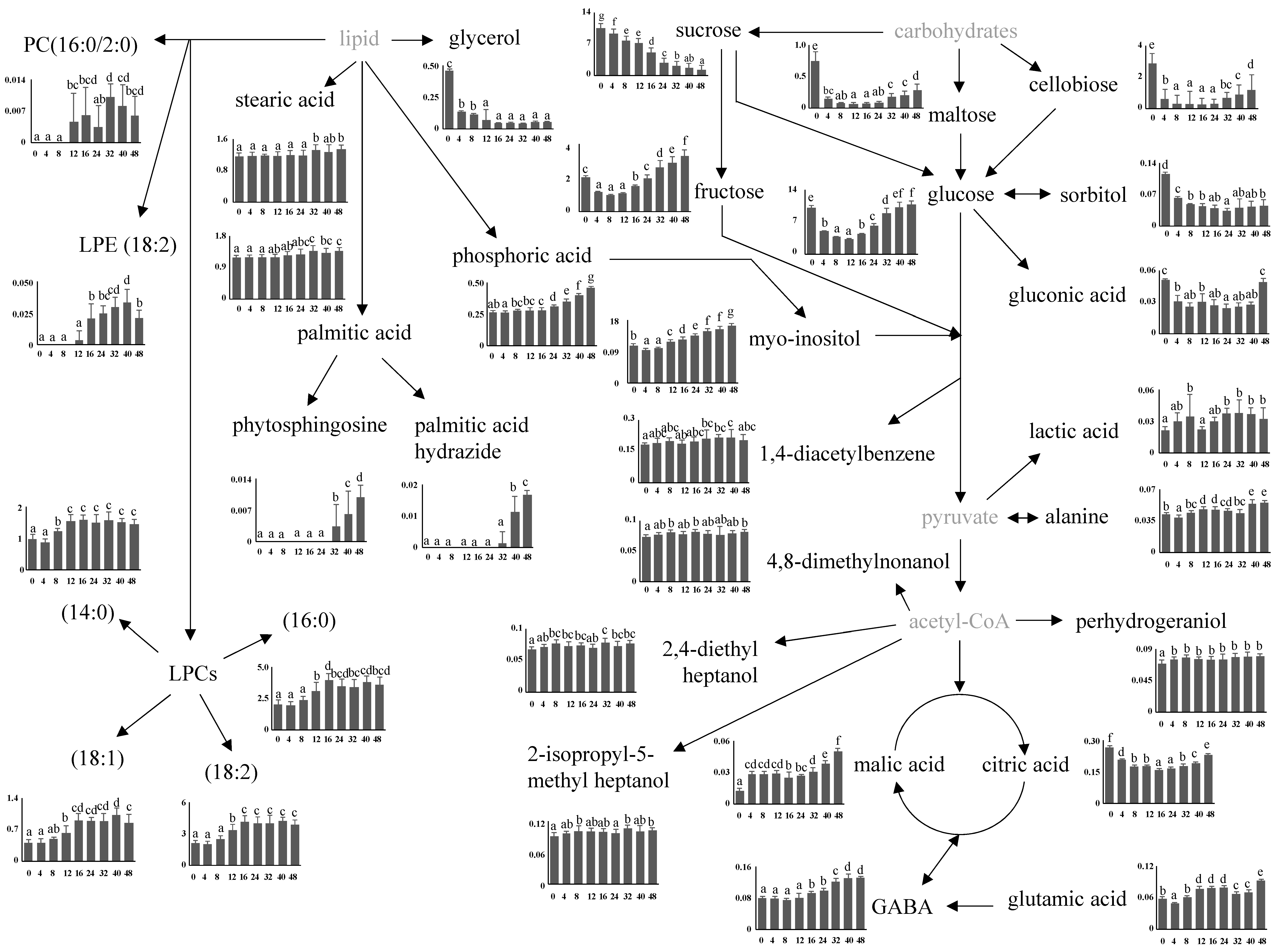
3.4. Relative Abundance of Identified Metabolites and Proposed Metabolomic Pathway
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Quarterly Global Report. In Crop Prospects and Food Situation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2007; Available online: http://www.fao.org/3/ai480e/ai480e00.htm (accessed on 1 May 2020).
- Saleh, A.S.M.; Wang, P.; Wang, N.; Yang, L.; Xiao, Z. Brown rice versus white rice: Nutritional quality, potential health benefits, development of food products, and preservation technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1070–1096. [Google Scholar] [CrossRef]
- Zhou, Z.K.; Robards, K.; Helliwell, S.; Blanchard, C. Composition and functional properties of rice. Int. J. Food Sci. Technol. 2002, 37, 849–868. [Google Scholar] [CrossRef]
- Feng, W.; Yang, N.; Touré, A.; Jin, Z.; Xu, X. Germinated brown rice and its role in human health. Crit. Rev. Food Sci. Nutr. 2013, 53, 451–463. [Google Scholar] [CrossRef]
- Komatsuzaki, N.; Tsukahara, K.; Toyoshima, H.; Suzuki, T.; Shimizu, N.; Kimura, T. Effect of soaking and gaseous treatment on GABA content in germinated brown rice. J. Food Eng. 2007, 78, 556–560. [Google Scholar] [CrossRef]
- Patil, S.B.; Khan, M.K. Germinated brown rice as a value added rice product: A review. J. Food Sci. Technol. 2011, 48, 661–667. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, H.; Guo, X.; Qian, H. The impact of germination on the characteristics of brown rice flour and starch. J. Sci. Food Agric. 2012, 92, 380–387. [Google Scholar] [CrossRef]
- Dong, K.; Zhen, S.; Cheng, Z.; Cao, H.; Ge, P.; Yan, Y. Proteomic analysis reveals key proteins and phosphoproteins upon seed germination of wheat (Triticum aestivum L.). Front. Plant Sci. 2015, 6, 1017. [Google Scholar] [CrossRef]
- He, D.; Yang, P. Proteomics of rice seed germination. Front. Plant Sci. 2013, 4, 246. [Google Scholar] [CrossRef]
- Mak, Y.; Willows, R.D.; Roberts, T.H.; Wrigley, C.W.; Sharp, P.J.; Copeland, L. Germination of wheat: A functional proteomics analysis of the embryo. Cereal Chem. 2009, 86, 281–289. [Google Scholar] [CrossRef]
- Pramai, P.; Hamid, N.A.A.; Mediani, A.; Maulidiani, M.; Abas, F.; Jiamyangyuen, S. Metabolite profiling, antioxidant, and a-glucosidase inhibitory activities of germinated rice: Nuclear-magnetic-resonance-based metabolomics study. J. Food Drug Anal. 2018, 26, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Dong, Y.; Guo, N.; Li, L.; Ren, H. Metabolomic analysis of the polyphenols in germinating mung beans (Vigna radiata) seeds and sprouts. J. Sci. Food Agric. 2014, 94, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Kusano, M.; Fukushima, A.; Kobayashi, M.; Hayashi, N.; Jonsson, P.; Moritz, T.; Ebana, K.; Saito, K. Application of a metabolomic method combining one-dimensional and two-dimensional gas chromatography-time-of-flight/mass spectrometry to metabolic phenotyping of natural variants in rice. J. Chromatogr. B 2007, 855, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, H.; Wang, X.; He, J.; Gong, X.; Yi, F. Germination period of rice beans analyzed by ultra-performance liquid chromatography quadrupole-time of flight mass spectrometry (UPLC-Q-TOF MS): Effects of methyl jasmonate and metabolites profile. Int. J. Food Prop. 2019, 22, 1662–1672. [Google Scholar] [CrossRef]
- Han, C.; Zhen, S.; Zhu, G.; Bian, Y.; Yan, Y. Comparative metabolome analysis of wheat embryo and endosperm reveals the dynamic changes of metabolites during seed germination. Plant Physiol. Biochem. 2017, 115, 320–327. [Google Scholar] [CrossRef]
- Thomason, K.; Babar, M.A.; Erickson, J.E.; Mulvaney, M.; Beecher, C.; MacDonald, G. Comparative physiological and metabolomics analysis of wheat (Triticum aestivum L.) following post-anthesis heat stress. PLoS ONE 2018, 13, e0197919. [Google Scholar] [CrossRef]
- Zhen, S.; Dong, K.; Deng, X.; Zhou, J.; Xu, X.; Han, C.; Zhang, W.; Xu, Y.; Wang, Z.; Yan, Y. Dynamic metabolome profiling reveals significant metabolic changes during grain development of bread wheat (Triticum aestivum L.). J. Sci. Food Agric. 2016, 96, 3731–3740. [Google Scholar] [CrossRef]
- Gu, E.J.; Kim, D.W.; Jang, G.J.; Song, S.H.; Lee, J.I.; Lee, S.B.; Kim, B.M.; Cho, Y.; Lee, H.J.; Kim, H.J.; et al. Mass-based metabolomic analysis of soybean sprouts during germination. Food Chem. 2017, 217, 311–319. [Google Scholar] [CrossRef]
- Shu, X.L.; Frank, T.; Shu, Q.Y.; Engel, K.H. Metabolite profiling of germinating rice seeds. J. Agric. Food Chem. 2008, 56, 11612–11620. [Google Scholar] [CrossRef]
- Counce, P.A.; Keisling, T.C.; Mitchell, A.J. A uniform, objective, and adaptive system for expressing rice development. Crop Sci. 2000, 40, 436–443. [Google Scholar] [CrossRef]
- Fortier-McGill, B.E.; Majumdar, R.D.; Lam, L.; Soong, R.; Liaghati-Mobarhan, Y.; Sutrisno, A.; de Visser, R.; Simpson, M.J.; Wheeler, H.L.; Campbell, M.; et al. Comprehensive multiphase (CMP) NMR monitoring of the structural changes and molecular flux within a growing seed. J. Agric. Food Chem. 2017, 65, 6779–6788. [Google Scholar] [CrossRef]
- Sreenivasulu, N.; Usadel, B.; Winter, A.; Radchuk, V.; Scholz, U.; Stein, N.; Weschke, W.; Strickert, M.; Close, T.J.; Stitt, M.; et al. Barley grain maturation and germination: Metabolic pathway and regulatory network commonalities and differences highlighted by new MapMan/PageMan profiling tools. Plant Physiol. 2008, 146, 1738–1758. [Google Scholar] [CrossRef]
- Frank, T.; Scholz, B.; Peter, S.; Engel, K.H. Metabolite profiling of barley: Influence of the malting process. Food Chem. 2011, 124, 948–957. [Google Scholar] [CrossRef]
- Tavener, R.J.A.; Laidman, D.L. The induction of triglyceride metabolism in the germinating wheat grain. Phytochemistry 1972, 11, 981–987. [Google Scholar] [CrossRef]
- Yang, P.; Li, X.; Wang, X.; Chen, H.; Chen, F.; Shen, S. Proteomic analysis of rice (Oryza sativa) seeds during germination. Proteomics 2007, 7, 3358–3368. [Google Scholar] [CrossRef] [PubMed]
- Saikusa, T.; Horino, T.; Mori, Y. Accumulation of γ-aminobutyric acid (Gaba) in the rice germ during water soaking. Biosci. Biotechnol. Biochem. 1994, 58, 2291–2292. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Suzuki, K.; Yasui, Y.; Kasumi, T. Bio-functional components in the processed pre-germinated brown rice by a twin-screw extruder. J. Food Compos. Anal. 2005, 18, 303–316. [Google Scholar] [CrossRef]
- Pracharoenwattana, I.; Cornah, J.E.; Smith, S.M. Arabidopsis peroxisomal citrate synthase is required for fatty acid respiration and seed germination. Plant Cell 2005, 17, 2037–2048. [Google Scholar] [CrossRef]
- Rosental, L.; Nonogak, H.; Fait, A. Activation and regulation of primary metabolism during seed germination. Seed Sci. Res. 2014, 24, 1–15. [Google Scholar] [CrossRef]
- Tian, S.; Nakamura, K.; Kayahara, H. Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J. Agric. Food Chem. 2004, 52, 4808–4813. [Google Scholar] [CrossRef]
| RT a (min) | Compound | RI b | VIP c | p-Value d |
|---|---|---|---|---|
| 6.73 | 4,8-dimethylnonanol | 1073 | 0.46 | 1.77 × 10−1 |
| 12.24 | 1,4-diacetylbenzene | 1426 | 0.5 | 5.82 × 10−2 |
| 6.27 | lactic acid | 1045 | 0.52 | 5.98 × 10−3 |
| 10.26 | 2-isopropyl-5-methyl-1-heptanol | 1292 | 0.52 | 8.45 × 10−3 |
| 21.29 | stearic acid | 2226 | 0.56 | 1.10 × 10−2 |
| 14.01 | p-hydroxydiisopropylbenzene | 1560 | 0.61 | 1.24 × 10−2 |
| 10.39 | 2,4-diethyl-1-heptanol | 1300 | 0.62 | 1.15 × 10−2 |
| 6.66 | perhydrogeraniol | 1068 | 0.67 | 2.71 × 10−3 |
| 19.38 | palmitic acid | 2029 | 0.69 | 3.56 × 10−4 |
| 7.01 | alanine | 1089 | 0.88 | 1.12 × 10−16 |
| 14.54 | glutamic acid | 1601 | 0.90 | 8.99 × 10−33 |
| 12.82 | malic acid | 1469 | 1.05 | 8.68 × 10−30 |
| 17.42/17.54 | fructose | 1845/1856 | 1.13 | 2.23 × 10−27 |
| 13.42 | γ-aminobutyric acid (GABA) | 1514 | 1.14 | 6.88 × 10−31 |
| 9.70 | phosphoric acid | 1256 | 1.15 | 2.89 × 10−38 |
| 19.74 | myo-inositol | 2065 | 1.16 | 8.40 × 10−35 |
| 18.81 | gluconic acid | 1973 | 1.17 | 1.76 × 10−22 |
| 24.53 | sucrose | 2601 | 1.23 | 1.18 × 10−44 |
| 17.70/17.92 | glucose | 1870/1890 | 1.29 | 1.11 × 10−40 |
| 18.13 | sorbitol | 1909 | 1.39 | 1.43 × 10−24 |
| 16.86 | citric acid | 1795 | 1.40 | 9.30 × 10−42 |
| 9.73 | glycerol | 1258 | 1.43 | 1.28 × 10−38 |
| 25.54 | maltose | 2731 | 1.44 | 1.89 × 10−29 |
| 25.33 | cellobiose | 2703 | 1.45 | 2.46 × 10−31 |
| RT a (min) | Compound | Exact Mass | MS Fragments | VIP b | p-Value c |
|---|---|---|---|---|---|
| (M + H) | |||||
| 1.65 | tryptophan | 205.0821 | 188 | 0.47 | 7.32 × 10−1 |
| 5.37 | PC (16:0/2:0) | 538.3476 | 184, 440 | 0.99 | 2.15 × 10−6 |
| 5.79 | phytosphingosine | 318.2986 | 282 | 1.24 | 1.43 × 10−12 |
| 6.94 | LPC (16:0) | 496.3404 | 184, 104 | 1.35 | 2.15 × 10−13 |
| 6.56 | LPE (18:2) | 478.2904 | 337, 460 | 1.37 | 4.64 × 10−19 |
| 7.12 | LPC (18:1) | 522.3558 | 184, 104 | 1.43 | 5.43 × 10−17 |
| 6.13 | LPC (14:0) | 468.3089 | 184, 104 | 1.44 | 8.35 × 10−14 |
| 5.02 | palmitic acid hydrazide | 271.2731 | 254 | 1.47 | 3.92 × 10−28 |
| 6.60 | LPC (18:2) | 520.3405 | 184, 104 | 1.50 | 1.22 × 10−17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, O.-W.; Ahn, J.-H.; Kim, B.-M.; Oh, J.; Kim, H.-J. Metabolomic Analysis of Germinated Brown Rice at Different Germination Stages. Foods 2020, 9, 1130. https://doi.org/10.3390/foods9081130
Kim H, Kim O-W, Ahn J-H, Kim B-M, Oh J, Kim H-J. Metabolomic Analysis of Germinated Brown Rice at Different Germination Stages. Foods. 2020; 9(8):1130. https://doi.org/10.3390/foods9081130
Chicago/Turabian StyleKim, Hoon, Oui-Woung Kim, Jae-Hwan Ahn, Bo-Min Kim, Juhong Oh, and Hyun-Jin Kim. 2020. "Metabolomic Analysis of Germinated Brown Rice at Different Germination Stages" Foods 9, no. 8: 1130. https://doi.org/10.3390/foods9081130
APA StyleKim, H., Kim, O.-W., Ahn, J.-H., Kim, B.-M., Oh, J., & Kim, H.-J. (2020). Metabolomic Analysis of Germinated Brown Rice at Different Germination Stages. Foods, 9(8), 1130. https://doi.org/10.3390/foods9081130





