Effects of Ultra-Sonication and Agitation on Bioactive Compounds and Structure of Amaranth Extract
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Chemical and Reagents
2.3. Extraction of Amaranth Powder
Ultra-Sonication and Agitation Extraction
2.4. Hunter Color Values
2.5. Determination of Amaranthus Pigments
2.6. Determination of Total Phenol Content
2.7. Determination of the Flavonoid Content
2.8. Determination of Phenolic Compounds Using High-Performanceliquid Chromatography (HPLC)
2.9. Antioxidant Capacity Using DPPH+ Assay
2.10. Antioxidant Capacity Using ABTS+ Assay
2.11. Fourier-Transformed Infrared (FTIR) Spectrometry
2.12. Statistical Analysis
3. Results and Discussion
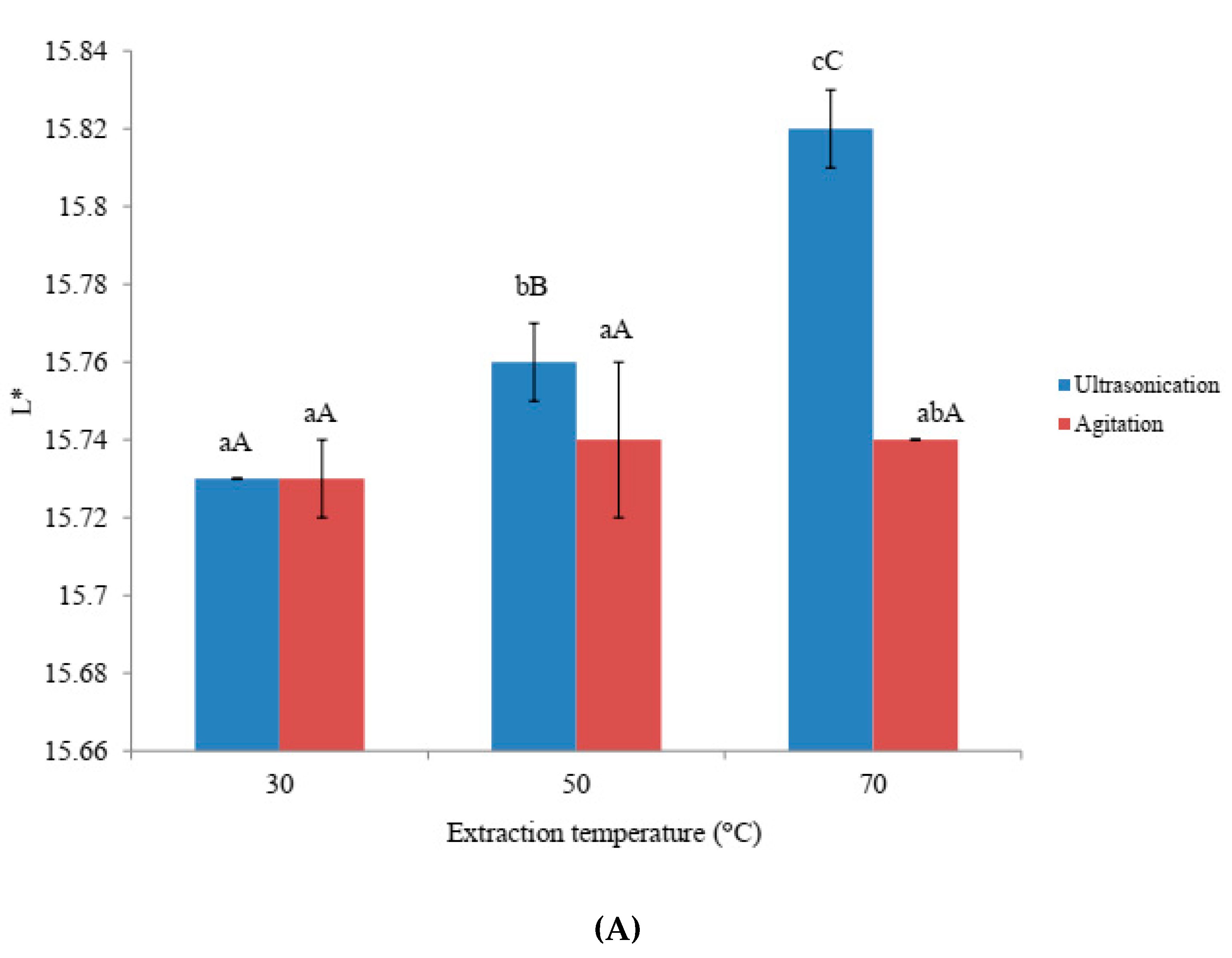
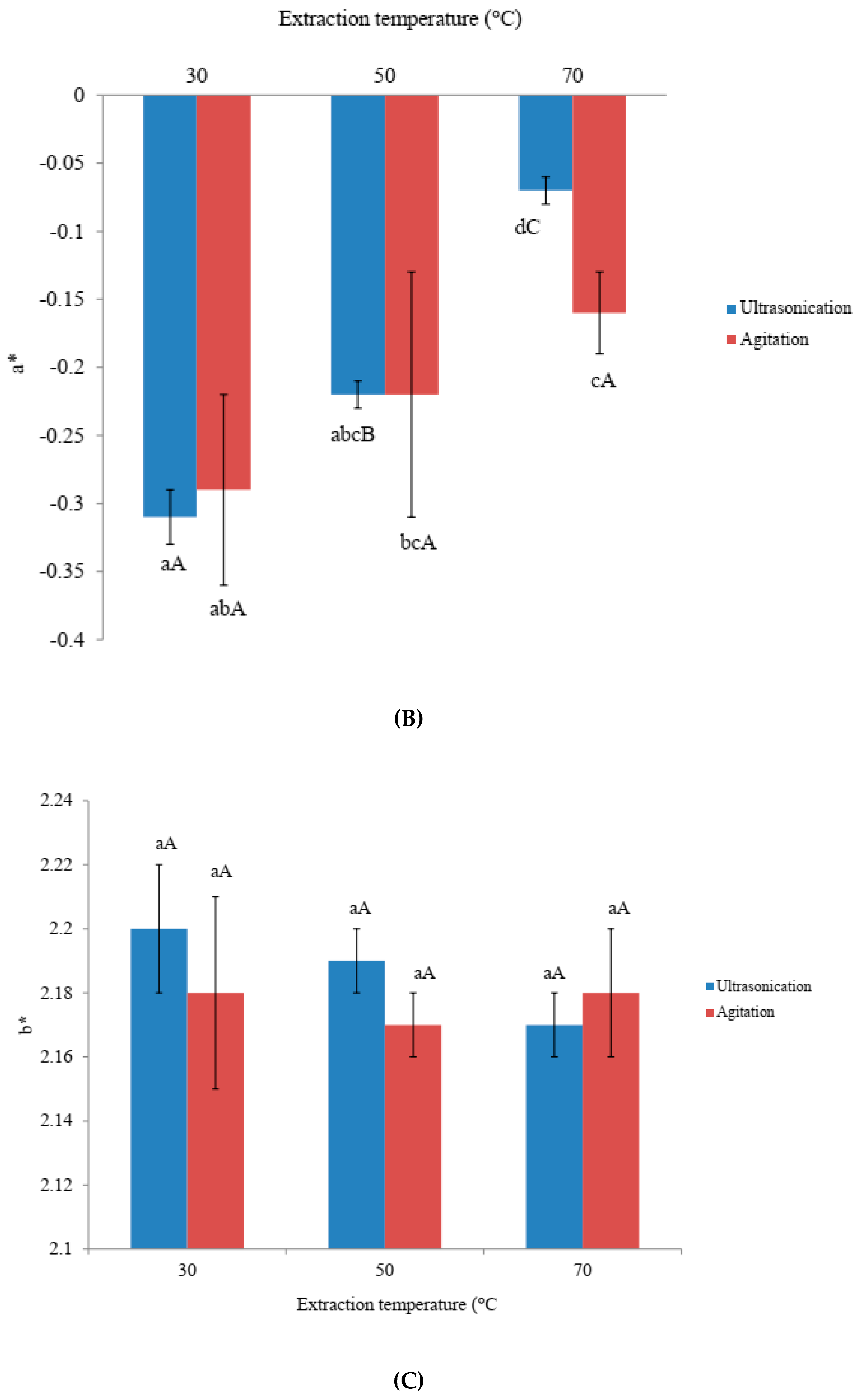
3.1. Effects of Ultra-Sonication and Agitation on Color Values of Amaranthus Extracts
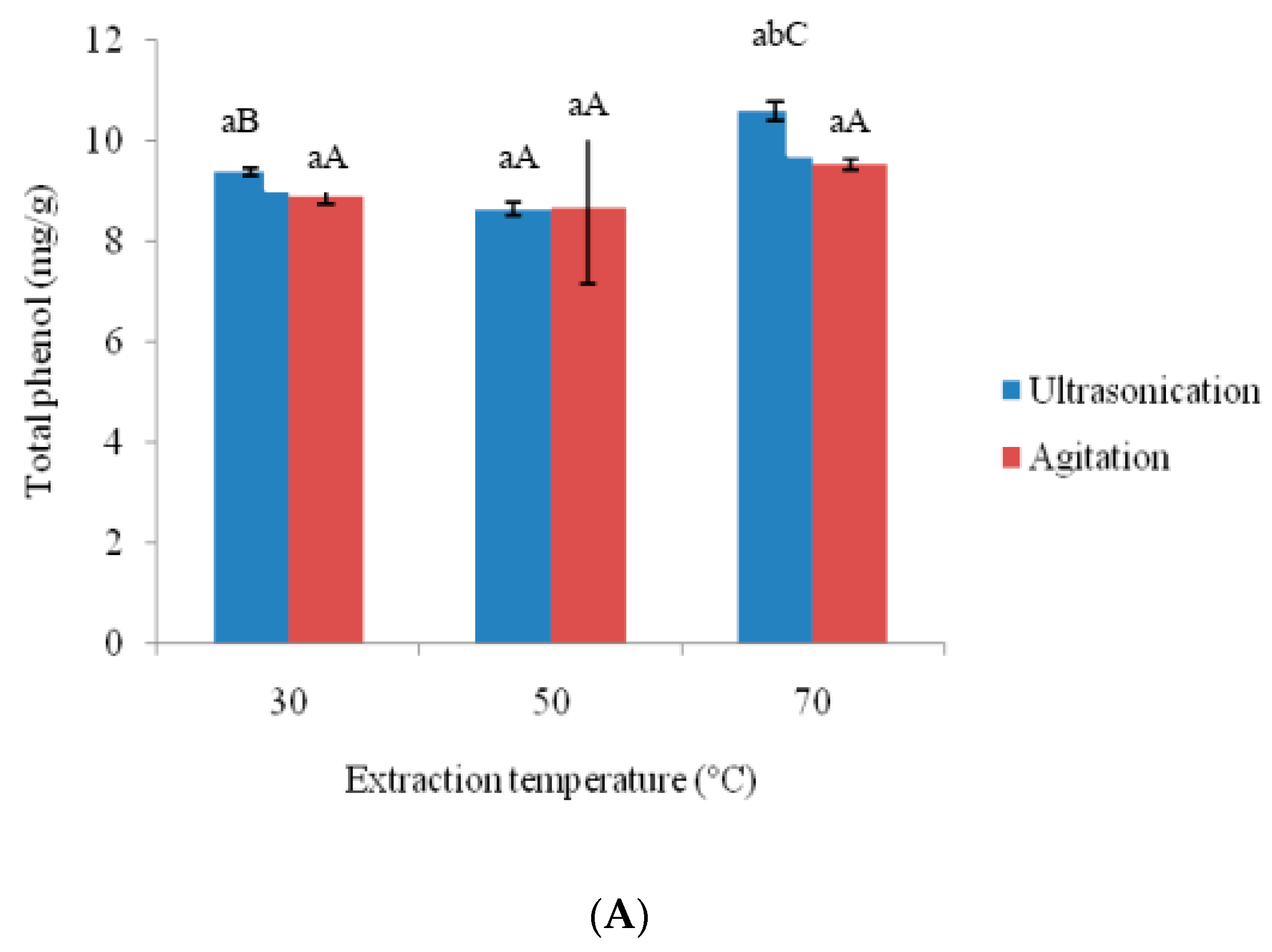
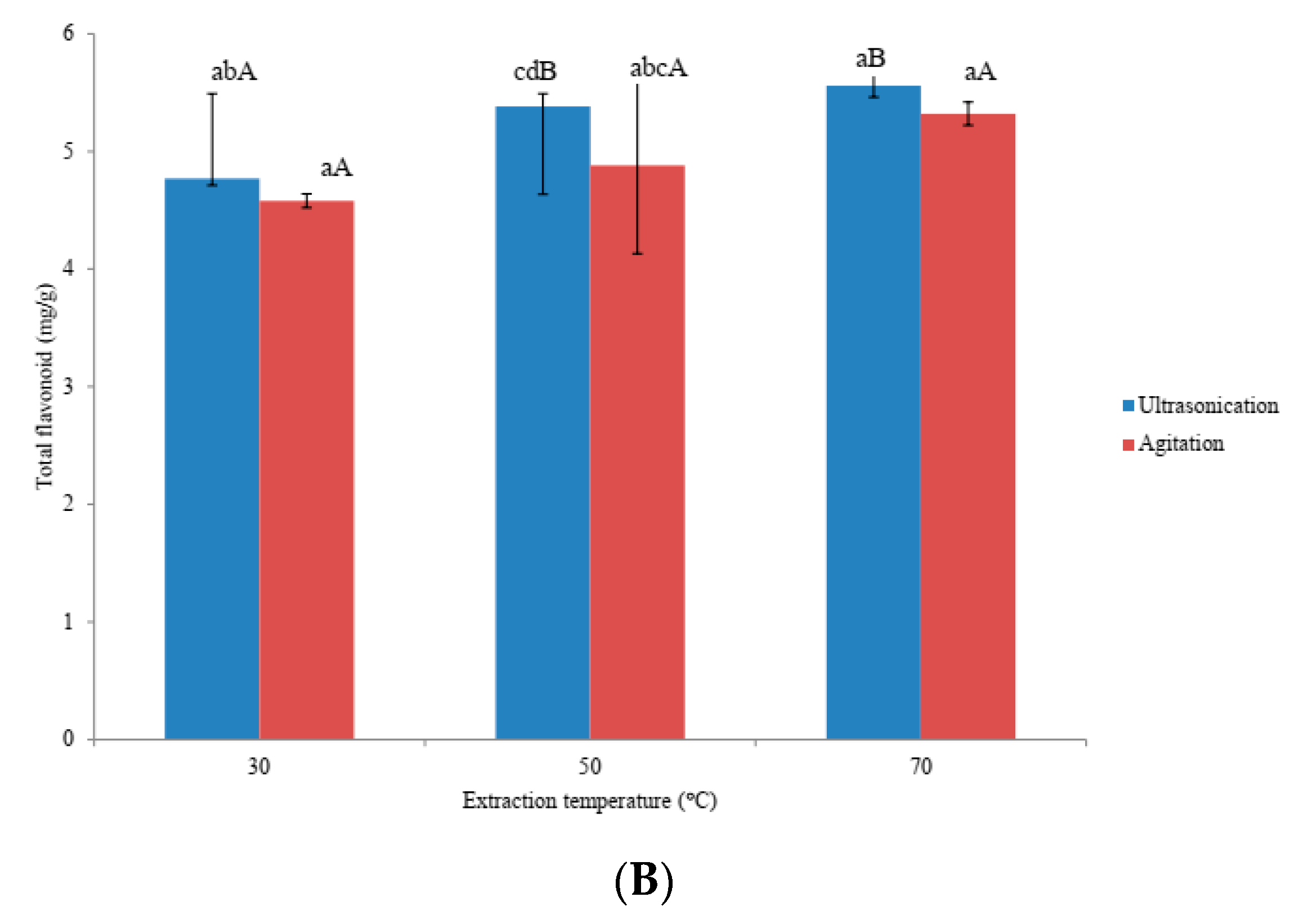
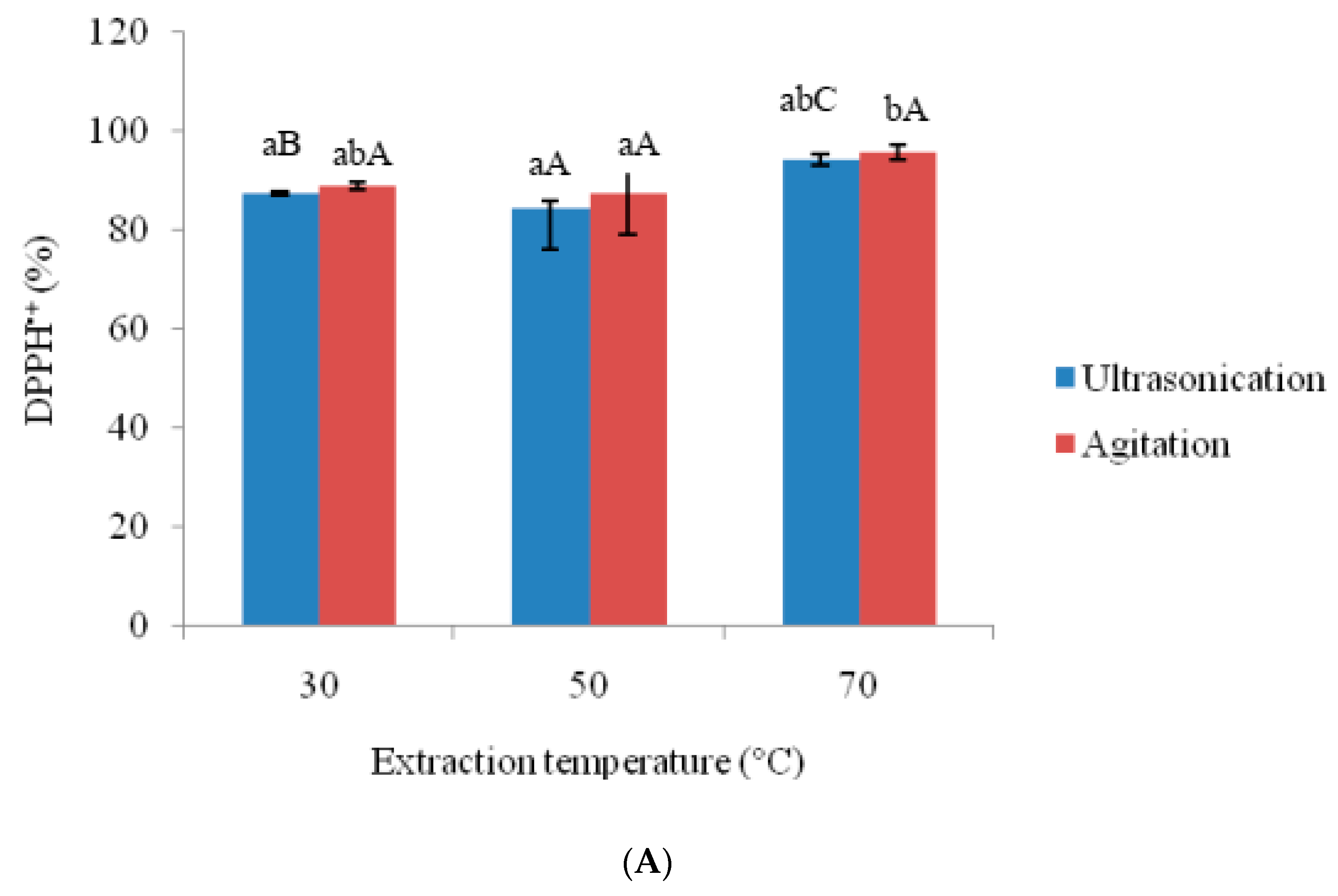
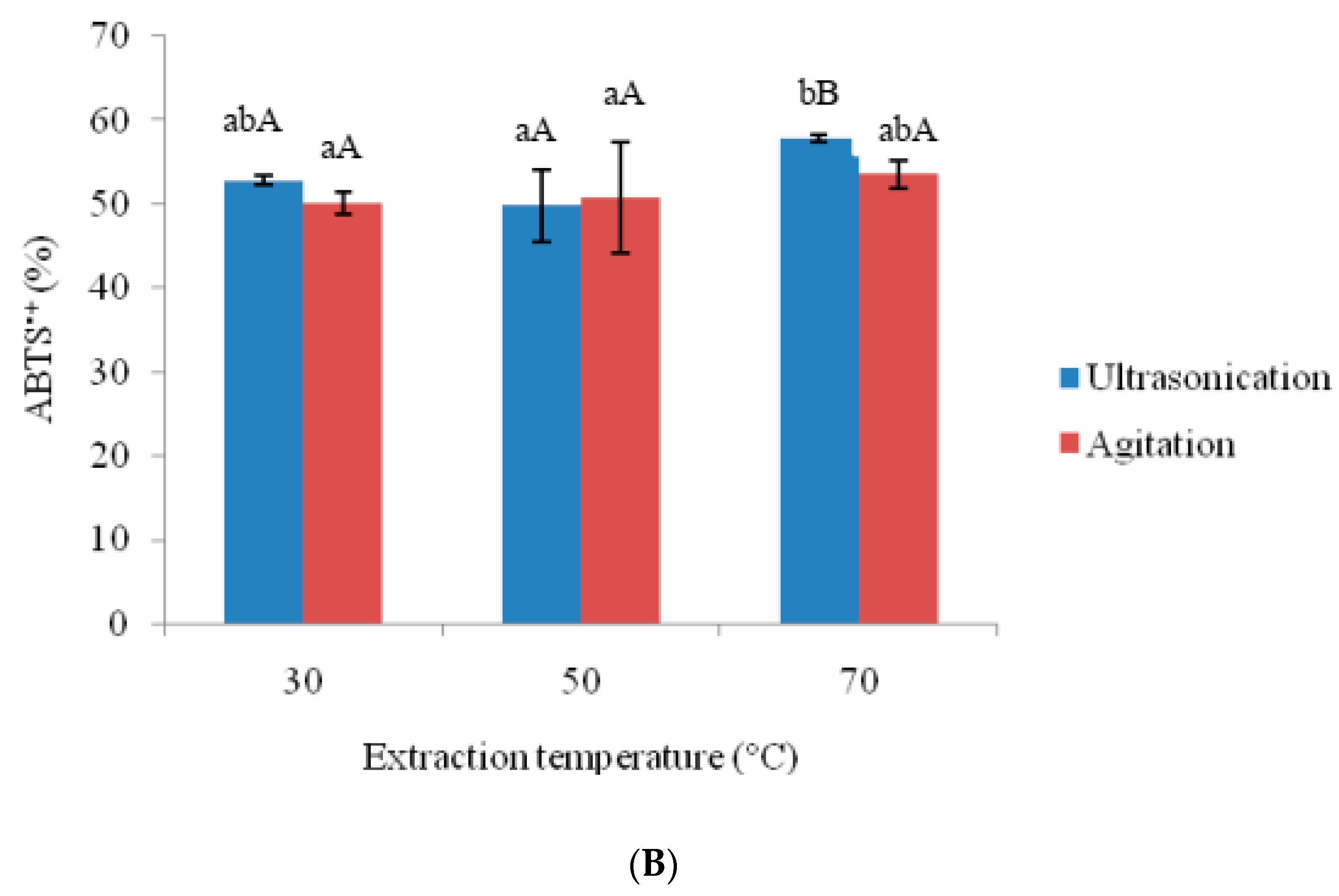
3.2. Effects of Ultra-Sonication and Agitation on Anti-Oxidative Properties
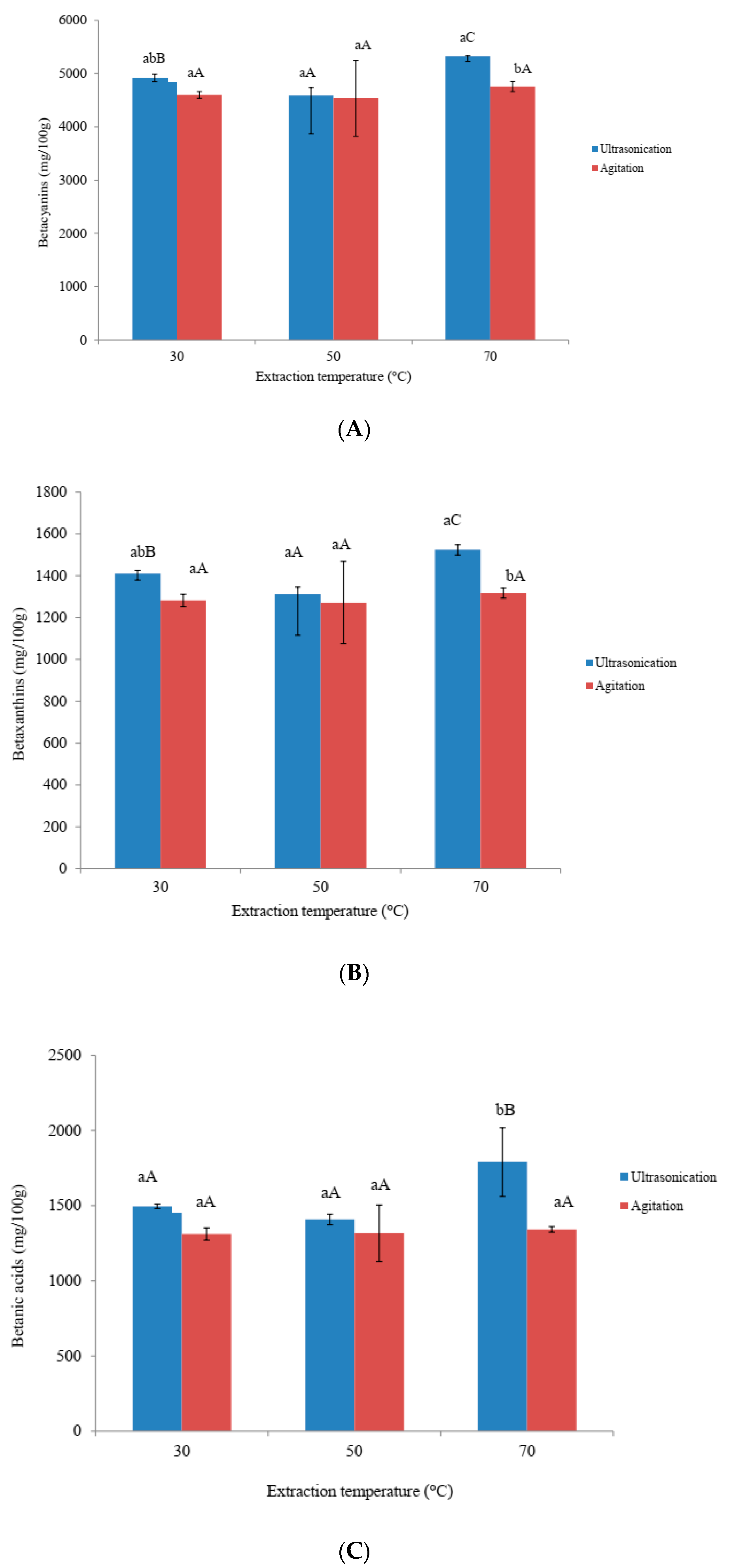
3.3. Effects of Ultra-Sonication and Agitation on Betacyanins, Betaxanthins and Betalamic Acid
3.4. Effects of Ultra-Soniation and Agitation on Individual Phenolic Compounds
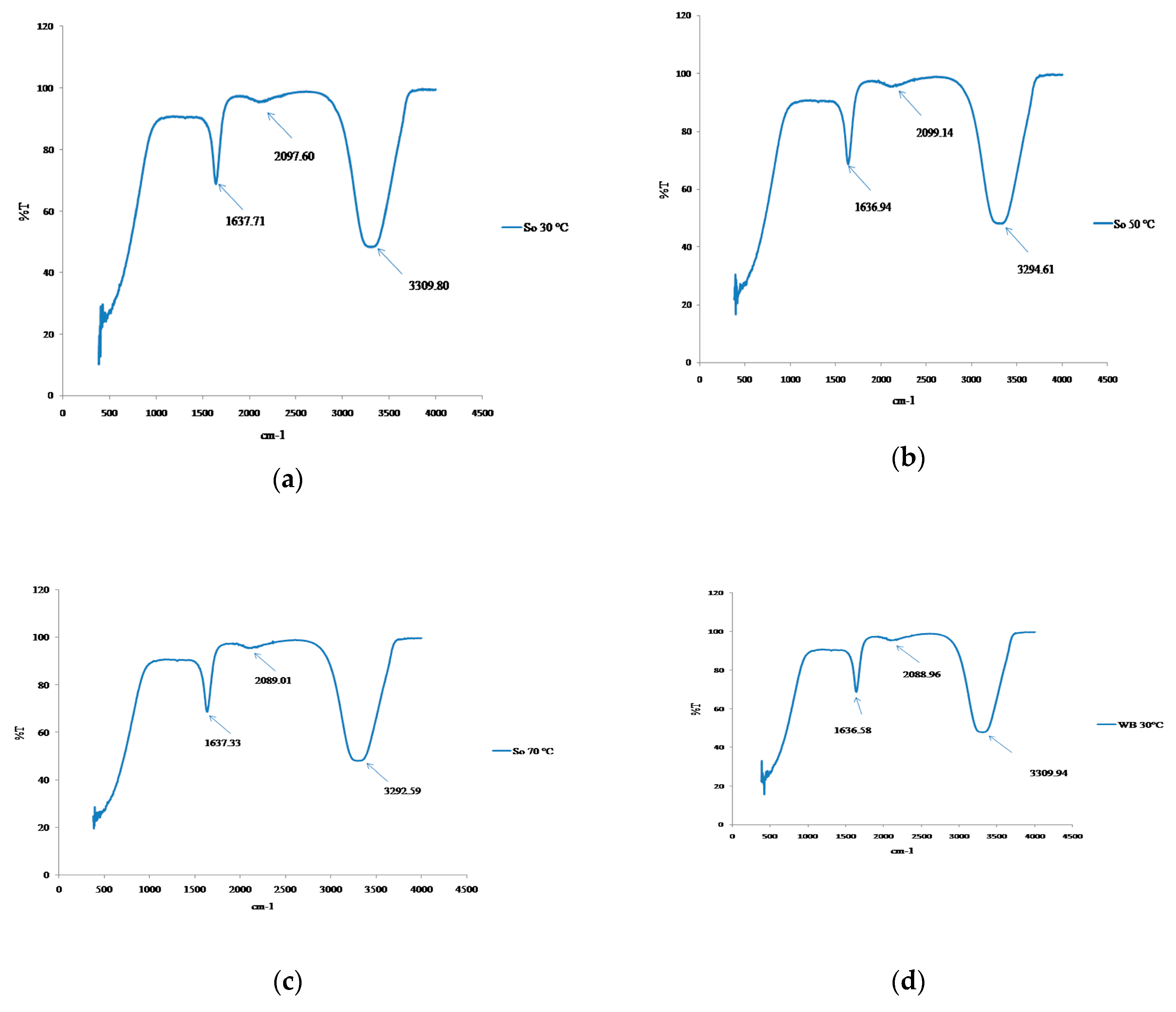
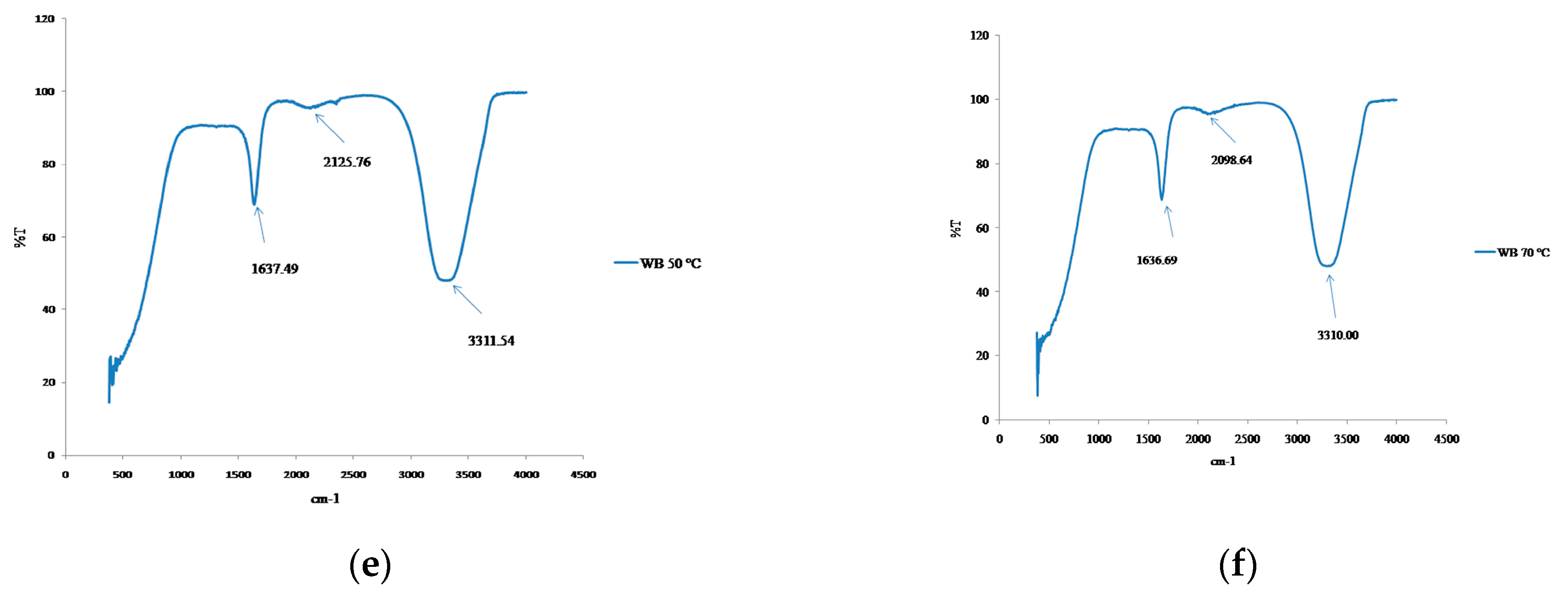
3.5. Structural Changes
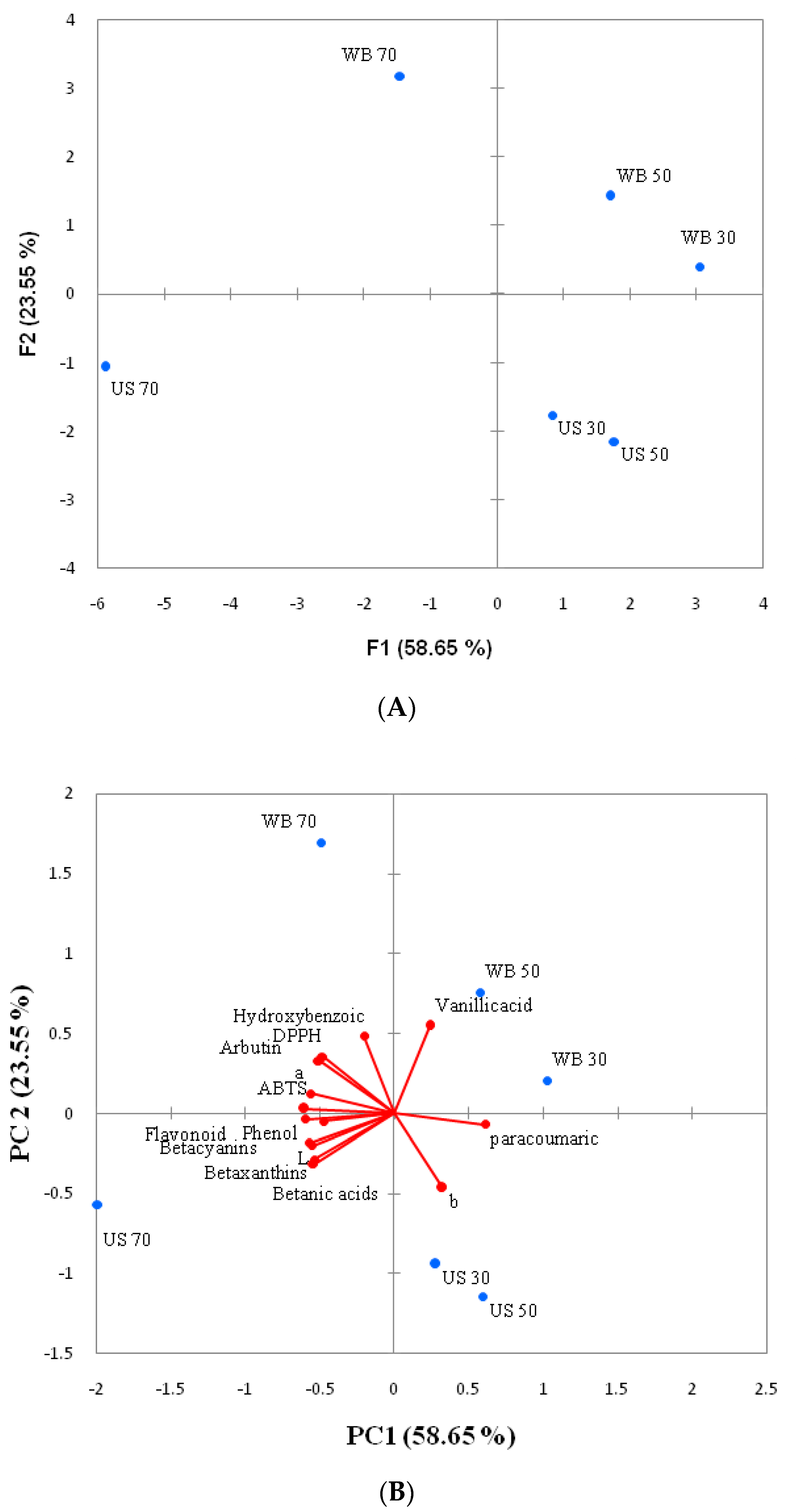
3.6. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khandaker, L.; Ali, M.B.; Oba, S. Total polyphenol and antioxidant activity of red amaranth (Amaranthus tricolor L.) as affected by different sunlight level. J. Jpn. Soc. Hortic. Sci. 2008, 77, 395–401. [Google Scholar] [CrossRef]
- Biswas, M.; Dey, S.; Sen, R. Betalains from Amaranthus tricolor L. J. Pharmacogn. Phytochem. 2013, 1, 87–95. [Google Scholar]
- Li, H.; Deng, Z.; Liu, R.; Zhu, H.; Draves, J.; Marcone, M.; Sun, Y.; Tsao, R. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, flower and stalk extracts of three Amaranthus species. J. Food Compos. Anal. 2015, 37, 75–81. [Google Scholar] [CrossRef]
- Al-Mamun, M.A.; Husna, J.; Khatun, M.; Hasan, R.; Kamruzzaman, M.; Hoque, K.M.F.; Reza, M.A.; Ferdousi, Z. Assessment of antioxidant, anticancer and antimicrobial activity of two vegetable species of Amaranthus in Bangladesh. BMC Complement. Altern. Med. 2016, 16, 1–11. [Google Scholar]
- Khanam, U.K.S.; Oba, S. Bioactive substances in leaves of two amaranth species, Amaranthus tricolor and A. hypochondriacus. Can. J. Plant Sci. 2013, 93, 47–58. [Google Scholar]
- Sandoval-Sicairos, E.S.; Domínguez-Rodríguez, M.; Montoya-Rodríguez, A.; Milán-Noris, A.K.; Reyes-Moreno, C.; Milán-Carrillo, J. Phytochemical Compounds and Antioxidant Activity Modified by Germination and Hydrolysis in Mexican Amaranth. Plant Foods Hum. Nutr. 2020, 75, 192–199. [Google Scholar] [CrossRef]
- Sindhuja, A.; Sudha, M.L.; Rahim, A. Effect of incorporation of amaranth flour on the quality of cookies. Eur. Food Res. Technol. 2005, 221, 597–601. [Google Scholar]
- Priatni, S.; Pradita, A. Stability study of betacyanin extract from red dragon fruit (Hylocereuspolyrhizus) peels. Procedia Chem. 2015, 16, 438–444. [Google Scholar]
- Kumar, S.S.; Manoj, P.; Shetty, N.P.; Prakash, M.; Girindhar, P. Characterization of major betalain pigments-gomphrenin, betanin and isobetanin from basellaruba L. fruit and evaluation of efficacy as a natural colourant in product (ice cream) development. J Food Sci. Technol. 2015, 52, 4994–5002. [Google Scholar] [CrossRef]
- De Azeredo, H.M.C.; Pereira, A.C.; de Souza, A.C.R.; Gouveia, S.T.; Mendes, K.C.B. Study on efficiency of betacyanin extraction from red beetroots. Int. J. Food Sci. Technol. 2009, 44, 2464–2469. [Google Scholar]
- Medina Meza, I.G.; Boioli, P.; Barbosa Cánovas, G.V. Assessment of the Effects of Ultrasonics and Pulsed Electric Fields on Nutritional and Rheological Properties of Raspberry and Blueberry Purees. Food Bioprocess Technol. 2016, 9, 520–531. [Google Scholar] [CrossRef]
- Sue-Siang, T.; Birch, E.J. Effect of ultrasonic treatment on the polyphenol content and antioxidant capacity of extract from defatted hemp, flax and canola seed cakes. Ultrason. Sonochem. 2014, 21, 346–353. [Google Scholar]
- Altemimi, A.; Watson, D.G.; Choudhary, R.; Dasari, M.R.; Lightfoot, D.A. Ultrasound Assisted Extraction of Phenolic Compounds from Peaches and Pumpkins. PLoS ONE 2016, 11, e0148758. [Google Scholar] [CrossRef] [PubMed]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Skenderidis, P.; Petrotos, K.; Giavasis, I.; Hadjichristodoulou, C.; Tsakalof, A. Optimization of ultrasound assisted extraction of of goji berry (Lycium barbarum) fruits and evaluation of extracts’ bioactivity. J. Food Process. Eng. 2017, 40, e12522. [Google Scholar] [CrossRef]
- Sifaoui, I.; Chammem, N.; Abderrabba, M.; Mejri, M. Optimization of Phenolic compounds Extractionfrom Olive Leaves using Experimental Design Methodology. J. Mater. Environ. Sci. 2016, 7, 1119–1127. [Google Scholar]
- Okafor, S.N.; Obonga, W.; Ezeokonkwo, M.A.; Nurudeen, J.; Orovwigho, U.; Ahiabuike, J. Assessment of the Health implications of Synthetic and Natural Food Colourants A Critical Review. UK J. Pharm. Biosci. 2016, 4, 1–11. [Google Scholar] [CrossRef]
- Das, M.; Saeid, A.; Hossain, M.F.; Jiang, G.H.; Eun, J.B.; Ahmed, M. Influence of extraction parameters and stability of betacyaninsextracted from red amaranth during storage. J. Food Sci. Technol. 2019, 56, 643–653. [Google Scholar]
- Lee, B.R.; Muneera, S.; Jung, W.J.; Aviced, J.C.; Ourryd, A.; Kima, T.H. Mycorrhizal colonization alleviates drought-induced oxidative damage and lignification in the leaves of drought-stressed perennial ryegrass (Loliumperenne). Physiol. Plant. 2012, 145, 440–449. [Google Scholar] [CrossRef]
- Hajimahmoodi, M.; Hanifeh, M.; Oveisi, M.R.; Sadeghi, N.; Jannat, B. Determination of total antioxidant capacity of green teas by the ferric reducing/antioxidant power assay. Iran. J. Environ. Health Sci. Eng. 2008, 3, 167–172. [Google Scholar]
- Yim, S.; Nam, S. Physiochemical, nutritional and functional characterization of 10 different pear cultivars (Pyrus spp.). J. Appl. Bot. Food Qual. 2016, 89, 73–81. [Google Scholar]
- Dong, W.; Hu, R.; Chu, Z.; Zhao, J.; Tan, L. Effect of different drying techniques on bioactive components, fatty acid composition, and volatile profile of robusta coffee beans. Food Chem. 2017, 234, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Călinoiu, L.F.; Vodnar, D.C. Thermal Processing for the Release of PhenolicCompounds from Wheat and Oat Bran. Biomolecules 2020, 10, 2–14. [Google Scholar]
- Ashokkumar, M.; Sunartio, D.; Kentish, S.; Mawson, R.; Simons, L.; Vilkhu, K.; Versteeg, C. Modification of food ingredients by ultrasound to improve functionality: A preliminary study on a model system. Innov. Food Sci. Emerg. Technol. 2008, 9, 155–160. [Google Scholar] [CrossRef]
- Abid, M.; Jabbar, S.; Wu, T.; Hashim, M.M.; Hu, B.; Lei, S.; Zeng, X. Effect of ultrasound on different quality parameters of apple juice. Ultrason. Sonochem. 2013, 20, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Goh, K.M. Sonication treatment convalesce the overall quality of hand-pressed strawberry juice. Food Chem. 2017, 215, 470–476. [Google Scholar] [CrossRef]
- Muhamad, N.; Muhmed, S.A.; Yusoff, M.M.; Gimbun, J. Influence of Solvent Polarity and Conditions on Extraction of Antioxidant, Flavonoids and Phenolic Content from Averrhoabilimbi. J. Food Sci. Eng. 2014, 4, 255–260. [Google Scholar]
- Okoh, S.O.; Asekun, O.T.; Familoni, O.B.; Afolayan, A.J. Antioxidant and Free Radical Scavenging Capacity of Seed and Shell Essential Oils Extracted from Abrus precatorius (L.). Antioxidants 2014, 3, 278–287. [Google Scholar] [CrossRef]
- Gokhale, S.V.; Lele, S.S. Betalain content and antioxidant activity of beta vulgaris: Effect of hot air convective drying and storage. J. Food Process. Preserv. 2014, 38, 585–590. [Google Scholar] [CrossRef]
- Paśko, P.; Sajewicz, M.; Gorinstein, S.; Achwieja, Z.Z. Analysis of Selected Phenolic Acids and Flavonoids in Amaranthuscruentus and Chenopodium quinoa Seeds and Sprouts by HPLC. Acta Chromatogr. 2008, 20, 661–672. [Google Scholar]
- Mphahlele, R.R.; Fawole, O.A.; Makunga, N.P.; Opara, U.L. Effect of drying on the bioactive compounds, antioxidant, antibacterial and antityrosinase activities of pomegranate peel. BMC Complement. Altern. Med. 2016, 16, 143. [Google Scholar] [CrossRef] [PubMed]
- Venskutonis, P.R.; Kraujalis, P. Nutritional components of amaranth seeds and vegetables: A Review on composition, properties, and uses. Compr. Rev. Food Sci. Food Saf. 2013, 12, 381–412. [Google Scholar] [CrossRef]
- Narayan, M.R. Review: Dye sensitized solar cells based on natural photosensitizers. Renew. Sustain. Energy Rev. 2012, 16, 208–215. [Google Scholar] [CrossRef]
- Skenderidis, P.; Mitsagga, C.; Giavasis, I.; Petrotos, K.; Lampakis, D.; Leontopoulos, S.; Hadjichristodoulou, C.; Tsakalof, A. The in vitro antimicrobial activity assessment of ultrasound assisted Lycium barbarum fruit extracts and pomegranate fruit peels. J. Food Meas. Charact. 2019, 13, 2017–2031. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, H.; Huang, R.; Corke, H. Characterization and Quantification of Betacyanin Pigments from Diverse Amaranthus Species. J. Agric. Food Chem. 1998, 46, 2063–2070. [Google Scholar] [CrossRef]
| Parameters | Extraction Method | |||||
|---|---|---|---|---|---|---|
| US 30 | US 50 | US 70 | AG 30 | AG 50 | AG 70 | |
| Arbutin | A 26485.60 ± 2544.45 a | A 26691.20 ± 1821.51 a | B 48458.40 ± 3920.77 c | A 19419.4 ± 1549.13 a | B 35148.4 ± 2588.01 b | C 53958.8 ± 5624.61 c |
| Hydroxybenzoic acid | AB 1817.20 ± 101.26 ab | A 1519.40 ± 135.48 a | B 1898.80 ± 110.31 ab | A 1654.2 ± 189.22 a | A 2183.88 ± 76.54 b | A 2153.2 ± 236.46 b |
| Vanillic acid | A 35.08 ± 4.92 a | A 31.72 ± 2.55 a | A 35.84 ± 1.33 a | A 158.4 ± 1.7 b | A 147.8 ± 91.36 b | A 146.6 ± 74.39 b |
| p-coumaric acid | A 57.84 ± 1.7 bc | A 57.48 ± 15.67 bc | A 28.80 ± 0.09 a | A 69.96 ± 7.86 c | A 61.4 ± 14.65 bc | A 39.36 ± 2.26 ab |
| Ferulic acid | A 79.84 ± 5.88 ab | A 77.68 ± 10.3 ab | A 76.00 ± 2.38 a | AB 85.16 ± 3.11 ab | A 73.8 ± 5.6 a | B 93.76 ± 7.01 b |
| Total phenolic compounds | A 28475.56 ± 2644.97 a | A 28377.45 ± 1980.36 a | C 50371.20 ± 3924.38 c | A 23657.24 ± 1481.36 a | B 36819.16 ± 1650.274 b | C 53311.12 ± 1569.55 c |
| Loading | PC 1 | PC2 |
|---|---|---|
| L* | −0.871 | −0.315 |
| a* | −0.884 | 0.195 |
| b* | 0.516 | −0.737 |
| Phenol | −0.945 | −0.056 |
| Flavonoid | −0.747 | −0.080 |
| DPPH+ | −0.756 | 0.562 |
| ABTS+ | −0.949 | 0.044 |
| Betacyanins | −0.903 | −0.289 |
| Betaxanthins | −0.847 | −0.453 |
| Betanic acids | −0.851 | −0.498 |
| Arbutin | −0.795 | 0.521 |
| Hydroxybenzoic | −0.317 | 0.762 |
| Vanillicacid | 0.379 | 0.863 |
| Paracoumaric | 0.966 | −0.109 |
| Ferulic acid | −0.005 | 0.616 |
| Scores | ||
| US 30 | 0.831 | −1.771 |
| US 50 | 1.767 | −2.152 |
| US 70 | −5.891 | −1.068 |
| WB 30 | 3.042 | 0.390 |
| WB50 | 1.704 | 1.420 |
| WB70 | −1.454 | 3.180 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, M.; Ramachandraiah, K.; Jiang, G.-H.; Eun, J.B. Effects of Ultra-Sonication and Agitation on Bioactive Compounds and Structure of Amaranth Extract. Foods 2020, 9, 1116. https://doi.org/10.3390/foods9081116
Ahmed M, Ramachandraiah K, Jiang G-H, Eun JB. Effects of Ultra-Sonication and Agitation on Bioactive Compounds and Structure of Amaranth Extract. Foods. 2020; 9(8):1116. https://doi.org/10.3390/foods9081116
Chicago/Turabian StyleAhmed, Maruf, Karna Ramachandraiah, Gui-Hun Jiang, and Jong Bang Eun. 2020. "Effects of Ultra-Sonication and Agitation on Bioactive Compounds and Structure of Amaranth Extract" Foods 9, no. 8: 1116. https://doi.org/10.3390/foods9081116
APA StyleAhmed, M., Ramachandraiah, K., Jiang, G.-H., & Eun, J. B. (2020). Effects of Ultra-Sonication and Agitation on Bioactive Compounds and Structure of Amaranth Extract. Foods, 9(8), 1116. https://doi.org/10.3390/foods9081116




