Extraction and Characterization of Antioxidant Peptides from Fruit Residues
Abstract
1. Introduction
2. Antioxidant Peptides in Fruit Residues
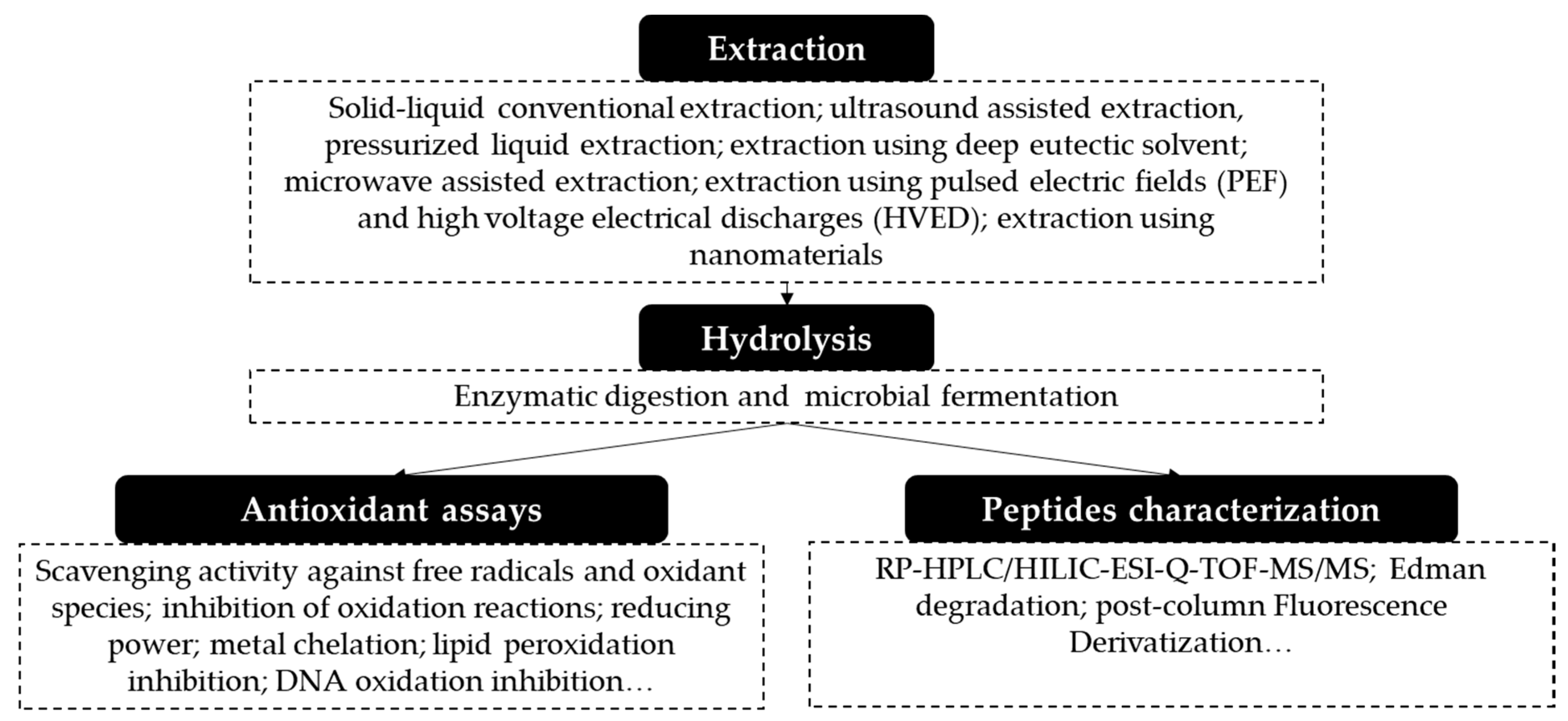
3. Obtaining Antioxidant Proteins and Peptides from Fruit Residues
4. Techniques Used in the Extraction and Purification of Proteins
4.1. Solid–Liquid Conventional Extraction
4.2. Ultrasound-Assisted Extraction of Proteins
4.3. Pressurized Liquid Extraction
4.4. Extraction Using Deep Eutectic Solvents
4.5. Microwave-Assisted Extraction
4.6. Extraction Using Pulsed Electric Field (PEF) and High Voltage Electrical Discharge (HVED)
4.7. Extraction and Purification Using Nanomaterials
5. Methods Used for the Release of Antioxidant Peptides
6. Evaluation of Antioxidant Activity of Peptides
7. Peptide Fractionation
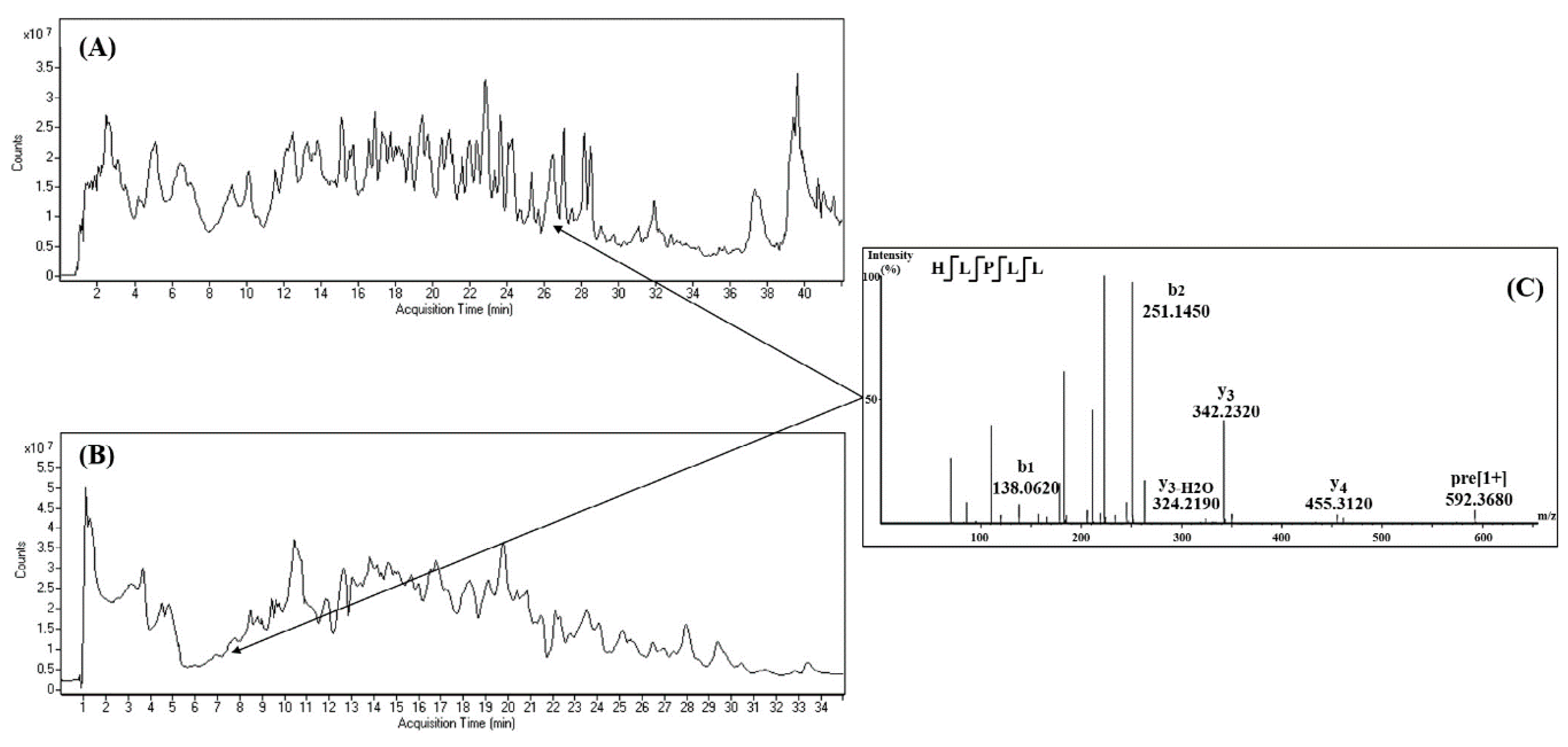
8. Peptide Identification
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019; 1: Comprensive Tables, 2–4; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Global Production of Fresh Fruit from 1990 to 2018 (in Million Metric Tons). Available online: https://www.statista.com/statistics/262266/global-production-of-fresh-fruit/ (accessed on 16 April 2020).
- Hernández-Corroto, E.; Marina, M.L.; García, M.C. Extraction and identification by high resolution mass spectrometry of bioactive substances in different extracts obtained from pomegranate peel. J. Chromatogr. A 2019, 1594, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Communication from the Commision to the Council and the European Parliament on the Interpretative Communication on Waste and By-Products; Commision of the European Communities: Brussels, Belguim, 2007.
- Padayachee, A.; Day, L.; Howell, K.; Gidley, M. Complexity and Health Functionality of Plant Cell Wall Fibres from Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2015, 57, 59–81. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.; Dhillon, S.; Brar, S. Perspective of apple processing wastes as low-cost substrates for bioproduction of high value products: A review. Renew. Sustain. Energy Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Wang, L.; Shen, F.; Yuan, H.; Zou, D.; Liu, Y.; Zhu, B.; Li, X. Anaerobic co-digestion of kitchen waste and fruit/vegetable waste: Lab-scale and pilot-scale studies. Waste Manag. 2014, 34, 2627–2633. [Google Scholar] [CrossRef]
- Burange, A.; Clark, J.H.; Luque, R. Trends in Food and Agricultural Waste Valorization. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 1–10. ISBN 978-1-119-95143-8. [Google Scholar]
- Deng, G.-F.; Shen, C.; Xu, X.-R.; Kuang, R.-D.; Guo, Y.-J.; Zeng, L.-S.; Gao, L.-L.; Lin, X.; Xie, J.-F.; Xia, E.-Q.; et al. Potential of fruit wastes as natural resources of bioactive compounds. Int. J. Mol. Sci. 2012, 13, 8308–8323. [Google Scholar] [CrossRef]
- Hajfathalian, M.; Ghelichi, S.; García-Moreno, P.J.; Moltke Sørensen, A.-D.; Jacobsen, C. Peptides: Production, bioactivity, functionality, and applications. Crit. Rev. Food Sci. Nutr. 2018, 58, 3097–3129. [Google Scholar] [CrossRef]
- Garcia, M.C.; Gonzalez-Garcia, E.; Vasquez-Villanueva, R.; Marina, M.L. Apricot and other seed stones: Amygdalin content and the potential to obtain antioxidant, angiotensin I converting enzyme inhibitor and hypocholesterolemic peptides. Food Funct. 2016, 7, 4693–4701. [Google Scholar] [CrossRef]
- García, M.C.; Puchalska, P.; Esteve, C.; Marina, M.L. Vegetable foods: A cheap source of proteins and peptides with antihypertensive, antioxidant, and other less occurrence bioactivities. Talanta 2013, 106, 328–349. [Google Scholar] [CrossRef]
- Gonzalez-Garcia, E.; Garcia, M.C.; Marina, M.L. Capillary liquid chromatography-ion trap-mass spectrometry methodology for the simultaneous quantification of four angiotensin-converting enzyme-inhibitory peptides in Prunus seed hydrolysates. J. Chromatogr. A 2018, 1540, 47–54. [Google Scholar] [CrossRef]
- González-García, E.; Marina, M.L.; García, M.C. Plum (Prunus Domestica L.) by-product as a new and cheap source of bioactive peptides: Extraction method and peptides characterization. J. Funct. Foods 2014, 11, 428–437. [Google Scholar] [CrossRef]
- Minkiewicz, P.; Iwaniak, A.; Darewicz, M. BIOPEP-UWM database of bioactive peptides: Current opportunities. Int. J. Mol. Sci. 2019, 20, 5978. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Corroto, E.; Marina, M.L.; Garcia, M.C. Multiple protective effect of peptides released from Olea europaea and Prunus persica seeds against oxidative damage and cancer cell proliferation. Food Res. Int. 2018, 106, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Esteve, C.; Marina, M.L.; Garcia, M.C. Novel strategy for the revalorization of olive (Olea europaea) residues based on the extraction of bioactive peptides. Food Chem. 2015, 167, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Aluko, R.E. 5-Amino acids, peptides, and proteins as antioxidants for food preservation. In Handbook of Antioxidants for Food Preservation; Shahidi, F., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 105–140. ISBN 978-1-78242-089-7. [Google Scholar]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Dash, P.; Ghosh, G. Proteolytic and antioxidant activity of protein fractions of seeds of Cucurbita moschata. Food Biosci. 2017, 18, 1–8. [Google Scholar] [CrossRef]
- Meshginfar, N.; Sadeghi Mahoonak, A.; Hosseinian, F.; Ghorbani, M.; Tsopmo, A. Production of antioxidant peptide fractions from a by-product of tomato processing: Mass spectrometry identification of peptides and stability to gastrointestinal digestion. J. Food Sci. Technol. 2018, 55, 3498–3507. [Google Scholar] [CrossRef]
- Karami, Z.; Akbari-Adergani, B. Bioactive food derived peptides: A review on correlation between structure of bioactive peptides and their functional properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef]
- Mäkinen, S.; Johannson, T.; Vegarud Gerd, E.; Pihlava, J.M.; Pihlanto, A. Angiotensin I-converting enzyme inhibitory and antioxidant properties of rapeseed hydrolysates. J. Funct. Foods 2012, 4, 575–583. [Google Scholar] [CrossRef]
- Barba, F.; Boussetta, N.; Vorobiev, E. Emerging Technologies for the Recovery of Isothiocyanates, Protein and Phenolic Compounds from Rapeseed and Rapeseed Press-Cake: Effect of High Voltage Electrical Discharges. Innov. Food Sci. Emerg. Technol. 2015, 31, 67–72. [Google Scholar] [CrossRef]
- Hernández-Corroto, E.; Plaza, M.; Marina, M.L.; García, M.C. Sustainable extraction of proteins and bioactive substances from pomegranate peel (Punica granatum L.) using pressurized liquids and deep eutectic solvents. Innov. Food Sci. Emerg. Technol. 2020, 60, 102314. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Extraction assisted by pulsed electric energy as a potential tool for green and sustainable recovery of nutritionally valuable compounds from mango peels. Food Chem. 2016, 192, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xue, J.; Zhao, H.; Zhao, X.; Xue, H.; Sun, Y.; Xue, W. Isolation and Structural Characterization of Antioxidant Peptides from Degreased Apricot Seed Kernels. J. AOAC Int. 2019, 101, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Vasquez-Villanueva, R.; Marina, M.L.; Garcia, M.C. Identification by hydrophilic interaction and reversed-phase liquid chromatography-tandem mass spectrometry of peptides with antioxidant capacity in food residues. J. Chromatogr. A 2016, 1428, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Garcia, E.; Marina, M.L.; Garcia, M.C.; Righetti, P.G.; Fasoli, E. Identification of plum and peach seed proteins by nLC-MS/MS via combinatorial peptide ligand libraries. J. Proteom. 2016, 148, 105–112. [Google Scholar] [CrossRef]
- Choudhary, R.R.; Verma, H.N.; Sharma, N.K. Antioxidant and Dehalogenase Activity of Lagenaria sciceraria Seed Protein Fraction. J. Plant. Sci. Res. 2013, 29, 145–158. [Google Scholar]
- Dash, P.; Panda, P.; Ghosh, G. Free Radical Scavenging Activities and Nutritional Value of Lagenaria siceraria: A Nutriment Creeper. Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 1743–1752. [Google Scholar] [CrossRef]
- Dash, P.; Rath, G.; Ghosh, G. In Vivo Antioxidant Potential of Protein Hydrolysates of some Cucurbitaceae Seed. JDDT [Internet] 2020, 10, 128–132. [Google Scholar]
- García, M.C.; Endermann, J.; González-García, E.; Marina, M.L. HPLC-Q-TOF-MS Identification of Antioxidant and Antihypertensive Peptides Recovered from Cherry (Prunus cerasus L.) Subproducts. J. Agric. Food Chem. 2015, 63, 1514–1520. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Barba, F.J.; Parniakov, O.; Galanakis, C.M.; Lebovka, N.; Grimi, N.; Vorobiev, E. High Voltage Electrical Discharges, Pulsed Electric Field, and Ultrasound Assisted Extraction of Protein and Phenolic Compounds from Olive Kernel. Food Bioprocess. Technol. 2015, 8, 885–894. [Google Scholar] [CrossRef]
- González-García, E.; Puchalska, P.; Marina, M.L.; García, M.C. Fractionation and identification of antioxidant and angiotensin-converting enzyme-inhibitory peptides obtained from plum (Prunus domestica L.) stones. J. Funct. Foods 2015, 19, 376–384. [Google Scholar] [CrossRef]
- Mechmeche, M.; Kachouri, F.; Ksontini, H.; Hamdi, M. Production of bioactive peptides from tomato seed isolate by Lactobacillus plantarum fermentation and enhancement of antioxidant activity. Food Biotechnol. 2017, 31, 94–113. [Google Scholar] [CrossRef]
- Mechmeche, M.; Ksontini, H.; Hamdi, M.; Kachouri, F. Production of Bioactive Peptides in Tomato Seed Protein Isolate Fermented by Water Kefir Culture: Optimization of the Fermentation Conditions. Int. J. Pept. Res. Ther. 2019, 25, 137–150. [Google Scholar] [CrossRef]
- Moayedi, A.; Hashemi, M.; Safari, M. Valorization of tomato waste proteins through production of antioxidant and antibacterial hydrolysates by proteolytic Bacillus subtilis: Optimization of fermentation conditions. J. Food Sci. Technol. 2016, 53, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Moayedi, A.; Mora, L.; Aristoy, M.C.; Safari, M.; Hashemi, M.; Toldrá, F. Peptidomic analysis of antioxidant and ACE-inhibitory peptides obtained from tomato waste proteins fermented using Bacillus subtilis. Food Chem. 2018, 250, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Moayedi, A.; Mora, L.; Aristoy, M.-C.; Hashemi, M.; Safari, M.; Toldrá, F. ACE-Inhibitory and Antioxidant Activities of Peptide Fragments Obtained from Tomato Processing By-Products Fermented Using Bacillus subtilis: Effect of Amino Acid Composition and Peptides Molecular Mass Distribution. Appl. Biochem. Biotechnol. 2017, 181, 48–64. [Google Scholar] [CrossRef]
- Mechmeche, M.; Kachouri, F.; Ksontini, H.; Setti, K.; Hamdi, M. Bioprocess development and preservation of functional food from tomato seed isolate fermented by kefir culture mixture. J. Food Sci. Technol. 2018, 55, 3911–3921. [Google Scholar] [CrossRef]
- Mechmeche, M.; Kachouri, F.; Chouabi, M.; Ksontini, H.; Setti, K.; Hamdi, M. Optimization of Extraction Parameters of Protein Isolate from Tomato Seed Using Response Surface Methodology. Food Anal. Methods 2017, 10, 809–819. [Google Scholar] [CrossRef]
- Meshginfar, N.; Mahoonak, A.S.; Hosseinian, F.; Tsopmo, A. Physicochemical, antioxidant, calcium binding, and angiotensin converting enzyme inhibitory properties of hydrolyzed tomato seed proteins. J. Food Biochem. 2019, 43, e12721. [Google Scholar] [CrossRef]
- Sharma, S.; Verma, H.N.; Sharma, N.K. Cationic Bioactive Peptide from the Seeds of Benincasa hispida. Int. J. Pept. 2014, 2014, 156060. [Google Scholar] [CrossRef]
- Kanbargi, K.D.; Sonawane, S.K.; Arya, S.S. Functional and antioxidant activity of Ziziphus jujube seed protein hydrolysates. J. Food Meas. Charact. 2016, 10, 226–235. [Google Scholar] [CrossRef]
- Kanbargi, K.D.; Sonawane, S.K.; Arya, S.S. Encapsulation characteristics of protein hydrolysate extracted from Ziziphus jujube seed. Int. J. Food Prop. 2017, 20, 3215–3224. [Google Scholar] [CrossRef]
- Siddeeg, A.; Xu, Y.; Jiang, Q.; Xia, W. In vitro antioxidant activity of protein fractions extracted from seinat (Cucumis melo var. tibish) seeds. CYTA J. Food 2015, 13, 1–10. [Google Scholar] [CrossRef][Green Version]
- Arise, R.; Yekeen, A.; Ekun, O.; Olatomiwa, O. Protein Hydrolysates from Citrullus lanatus Seed: Antiradical and Hydrogen Peroxide-scavenging properties and kinetics of Angiotensin-I converting enzyme inhibition. Ceylon J. Sci. 2016, 45, 39. [Google Scholar] [CrossRef]
- Dash, P.; Ghosh, G. Fractionation, amino acid profiles, antimicrobial and free radical scavenging activities of Citrullus lanatus seed protein. Nat. Prod. Res. 2017, 31, 2945–2947. [Google Scholar] [CrossRef] [PubMed]
- Arise, R.; Yekeen, A.; Ekun, O. In vitro antioxidant and α-amylase inhibitory properties of watermelon seed protein hydrolysates. Environ. Exp. Biol. 2016, 14, 163–172. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Feng, Y.; Duan, Y.; Ma, H.; Zhang, H. Purification and identification of novel antioxidant peptides from watermelon seed protein hydrolysates and their cytoprotective effects on H2O2-induced oxidative stress. Food Chem. 2020, 327, 127059. [Google Scholar] [CrossRef] [PubMed]
- Sonawane, S.K.; Arya, S.S. Citrullus lanatus protein hydrolysate optimization for antioxidant potential. J. Food Meas. Charact. 2017, 11, 1834–1843. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Duan, Y.; Ma, H. Effects of divergent ultrasound pretreatment on the structure of watermelon seed protein and the antioxidant activity of its hydrolysates. Food Chem. 2019, 299, 125165. [Google Scholar] [CrossRef]
- Parniakov, O.; Roselló-Soto, E.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. New approaches for the effective valorization of papaya seeds: Extraction of proteins, phenolic compounds, carbohydrates, and isothiocyanates assisted by pulsed electric energy. Food Res. Int. 2015, 77, 711–717. [Google Scholar] [CrossRef]
- Guo, P.; Qi, Y.; Zhu, C.; Wang, Q. Purification and identification of antioxidant peptides from Chinese cherry (Prunus pseudocerasus Lindl.) seeds. J. Funct. Foods 2015, 19, 394–403. [Google Scholar] [CrossRef]
- Osukoya, O.; Nwoye-Ossy, M.; Olayide, I.; Ojo, O.; Adewale, O.; Kuku, A. Antioxidant activities of peptide hydrolysates obtained from the seeds of Treculia africana Decne (African breadfruit). Prep. Biochem. Biotechnol. 2020, 50, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, C.Z.; Opoku, A.R.; Terblanche, S.E. Antioxidative effects of pumpkin seed (Cucurbita pepo) protein isolate in CCl4-induced liver injury in low-protein fed rats. Phytother. Res. 2006, 20, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Vaštag, Ž.; Popović, L.; Popović, S.; Krimer, V.; Peričin, D. Production of enzymatic hydrolysates with antioxidant and angiotensin-I converting enzyme inhibitory activity from pumpkin oil cake protein isolate. Food Chem. 2011, 124, 1316–1321. [Google Scholar] [CrossRef]
- Nourmohammadi, E.; SadeghiMahoonak, A.; Alami, M.; Ghorbani, M. Amino acid composition and antioxidative properties of hydrolysed pumpkin (Cucurbita pepo L.) oil cake protein. Int. J. Food Prop. 2017, 20, 3244–3255. [Google Scholar] [CrossRef]
- Fan, S.; Hu, Y.; Li, C.; Liu, Y. Optimization of preparation of antioxidative peptides from pumpkin seeds using response surface method. PLoS ONE 2014, 9, e92335. [Google Scholar] [CrossRef]
- Nkosi, C.Z.; Opoku, A.R.; Terblanche, S.E. In Vitro antioxidative activity of pumpkin seed (Cucurbita pepo) protein isolate and its In Vivo effect on alanine transaminase and aspartate transaminase in acetaminophen-induced liver injury in low protein fed rats. Phytother. Res. 2006, 20, 780–783. [Google Scholar] [CrossRef] [PubMed]
- Popović, L.; Peričin, D.; Vaštag, Ž.; Popović, S.; Krimer, V.; Torbica, A. Antioxidative and Functional Properties of Pumpkin Oil Cake Globulin Hydrolysates. J. Am. Oil Chem. Soc. 2013, 90, 1157–1165. [Google Scholar] [CrossRef]
- Venuste, M.; Zhang, X.; Shoemaker, C.F.; Karangwa, E.; Abbas, S.; Kamdem, P.E. Influence of enzymatic hydrolysis and enzyme type on the nutritional and antioxidant properties of pumpkin meal hydrolysates. Food Funct. 2013, 4, 811–820. [Google Scholar] [CrossRef]
- Liu, R.-L.; Yu, P.; Ge, X.-L.; Bai, X.-F.; Li, X.-Q.; Fu, Q. Establishment of an Aqueous PEG 200-Based Deep Eutectic Solvent Extraction and Enrichment Method for Pumpkin (Cucurbita moschata) Seed Protein. Food Anal. Methods 2017, 10, 1669–1680. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities of date seed protein hydrolysates prepared using Alcalase, Flavourzyme and Thermolysin. J. Funct. Foods 2015, 18, 1125–1137. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; Shahidi, F. Antioxidant potential of date (Phoenix dactylifera L.) seed protein hydrolysates and carnosine in food and biological systems. J. Agric. Food Chem. 2015, 63, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Romelle, F.D.; Rani, A.; Manohar, R.S. Chemical composition of some selected fruit peels. Eur. J. Food Sci. Technol. 2016, 4, 12–21. [Google Scholar]
- Lima, B.N.B.; Lima, F.F.; Tavares, M.I.B.; Costa, A.M.M.; Pierucci, A.P.T.R. Determination of the centesimal composition and characterization of flours from fruit seeds. Food Chem. 2014, 151, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Raihana, A.R.N.; Marikkar, J.M.N.; Amin, I.; Shuhaimi, M. A Review on Food Values of Selected Tropical Fruits’ Seeds. Int. J. Food Prop. 2015, 18, 2380–2392. [Google Scholar] [CrossRef]
- Lam, S.K.; Ng, T.B. Passiflin, a novel dimeric antifungal protein from seeds of the passion fruit. Phytomed. Int. J. Phytother. Phytopharm. 2009, 16, 172–180. [Google Scholar] [CrossRef]
- Osborne, T.B. The Vegetable Proteins, 2nd ed.; Longmans Green and Co: London, UK, 1924. [Google Scholar]
- Ozuna, C.; Paniagua-Martínez, I.; Castaño-Tostado, E.; Ozimek, L.; Amaya-Llano, S.L. Innovative applications of high-intensity ultrasound in the development of functional food ingredients: Production of protein hydrolysates and bioactive peptides. Food Res. Int. 2015, 77, 685–696. [Google Scholar] [CrossRef]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. TRAC Trends Anal. Chem. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- González-García, E.; Maly, M.; de la Mata, F.J.; Gómez, R.; Marina, M.L.; García, M.C. Proof of concept of a “greener” protein purification/enrichment method based on carboxylate-terminated carbosilane dendrimer-protein interactions. Anal. Bioanal. Chem. 2016, 408, 7679–7687. [Google Scholar] [CrossRef]
- González-García, E.; Sánchez-Nieves, J.; de la Mata, F.J.; Marina, M.L.; García, M.C. Feasibility of cationic carbosilane dendrimers for sustainable protein sample preparation. Colloids Surf. B Biointerfaces 2020, 186, 110746. [Google Scholar] [CrossRef]
- González-García, E.; Gutiérrez Ulloa, C.E.; de la Mata, F.J.; Marina, M.L.; García, M.C. Sulfonate-terminated carbosilane dendron-coated nanotubes: A greener point of view in protein sample preparation. Anal. Bioanal. Chem. 2017, 409, 5337–5348. [Google Scholar] [CrossRef]
- Vásquez-Villanueva, R.; Pena González, C.; Sánchez-Nieves, J.; Mata, F.; Marina, M.; García, M. Gold nanoparticles coated with carbosilane dendrons in protein sample preparation. Microchim. Acta 2019, 186, 508. [Google Scholar] [CrossRef] [PubMed]
- Grosso, C.; Valentão, P.; Ferreres, F.; Andrade, P.B. Alternative and efficient extraction methods for marine-derived compounds. Mar. Drugs 2015, 13, 3182–3230. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Simó, C.; Ibáñez, E.; Cifuentes, A. Capillary electrophoresis-mass spectrometry of Spirulina platensis proteins obtained by pressurized liquid extraction. Electrophor. 2005, 26, 4215–4224. [Google Scholar] [CrossRef][Green Version]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Lores, H.; Romero, V.; Costas, I.; Bendicho, C.; Lavilla, I. Natural deep eutectic solvents in combination with ultrasonic energy as a green approach for solubilisation of proteins: Application to gluten determination by immunoassay. Talanta 2017, 162, 453–459. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Wahlström, R.; Rommi, K.; Willberg-Keyriläinen, P.; Ercili-Cura, D.; Holopainen-Mantila, U.; Hiltunen, J.; Mäkinen, O.; Nygren, H.; Mikkelson, A.; Kuutti, L. High Yield Protein Extraction from Brewer’s Spent Grain with Novel Carboxylate Salt-Urea Aqueous Deep Eutectic Solvents. ChemistrySelect 2017, 2, 9355–9363. [Google Scholar] [CrossRef]
- Huang, J.; Guo, X.; Xu, T.; Fan, L.; Zhou, X.; Wu, S. Ionic deep eutectic solvents for the extraction and separation of natural products. J. Chromatogr. A 2019, 1598, 1–19. [Google Scholar] [CrossRef]
- Bleakley, S.; Hayes, M. Algal Proteins: Extraction, Application, and Challenges Concerning Production. Foods 2017, 6, 33. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme assisted extraction of biomolecules as an approach to novel extraction technology: A review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E. Extraction of valuable biocompounds assisted by high voltage electrical discharges: A review. Comptes Rendus Chimie 2014, 17, 197–203. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E.; Reess, T.; De Ferron, A.; Pecastaing, L.; Ruscassié, R.; Lanoisellé, J.-L. Scale-up of high voltage electrical discharges for polyphenols extraction from grape pomace: Effect of the dynamic shock waves. Innov. Food Sci. Emerg. Technol. 2012, 16, 129–136. [Google Scholar] [CrossRef]
- González-García, E.; Marina, M.L.; García, M.C. Nanomaterials in Protein Sample Preparation. Sep. Purif. Rev. 2019, 49, 1–36. [Google Scholar] [CrossRef]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Vásquez-Villanueva, R.; Muñoz-Moreno, L.; José Carmena, M.; Luisa Marina, M.; Concepción García, M. In vitro antitumor and hypotensive activity of peptides from olive seeds. J. Funct. Foods 2018, 42, 177–184. [Google Scholar] [CrossRef]
- Puchalska, C.; Esteve, M.L.; Marina, M.C. García Peptides. In Handbook of Food Analysis; CRC Press: Boca Raton, FL, USA, 2015; pp. 329–355. ISBN 978-1-4665-5654-6. [Google Scholar]
| Residue | Extracting Media | Extraction Conditions | Protein Purification Conditions | Refs. |
|---|---|---|---|---|
| SOLID–LIQUID CONVENTIONAL EXTRACTION | ||||
| Tomato seeds | NaCl (1.5%, pH 11.5) | DGS: extracting medium at ratio 1:10 (w/v); stirring (room temperature and 1 h) | Centrifugation and precipitation at pH 4.0 | [21,43] |
| Tomato seeds | NaOH aq. (pH 7.5–1.5) | 1 g of DGS and 82 mL water; stirring (50 °C and 50 h) | Centrifugation and filtration | [37,41] |
| Tomato seeds | NaOH aq. (pH 7.5) | DGS: extracting medium at ratio 1:82.81 (30 °C and 50 h) | Centrifugation and precipitation at pH 3.9 | [42] |
| Watermelon seeds | Alkali (0.8%) | DGS: extracting medium at ratio 1:30 (40 °C and 30 min) | Precipitation at pH 4.5 | [52] |
| Watermelon seeds | NaOH aq. (pH 12.0) | DGS: extracting medium at ratio 1:10 (w/v); stirring (1 h); 2 additional extractions after centrifugation | Precipitation at pH 4.0 | [48,50] |
| Jujube seeds | (I) Water; (II) 50 mM Tris-HCl (pH 7.5); (III) 0.6 M NaCl (0.1% HCl); and (IV) acetic acid (5%) | (I and II) 1.5 g ground seeds and 20 mL solvent; stirring (4 °C and 2 h); (III) Idem at ground seeds:solvent 1:3 (w/v); and (IV) 5 g and 20 mL solvent. Shaking overnight (80 rpm) | (I, II, and III) Precipitation with (NH₄)₂SO₄ and dialysis (24 h, 4 °C); (IV) filtration and precipitation with acetone | [45] |
| Chinese cherry seeds | NaOH aq. (pH 10.0) | DGS: extracting medium at 1:20 (w/v); stirring (40 °C and 40 min) | Filtration and precipitation at pH 3.84 | [55] |
| African breadfruit seeds | NaOH aq. (pH 9.0) | DGS: extracting medium at ratio 1:10 (w/v); stirring (30 min at room temperature) | Precipitation at pH 4.5 | [56] |
| Pumpkin (Cucurbita pepo) seeds | NaOH aq. (pH 10.0) | DGS | Precipitation at pH 5.0 | [57,61] |
| Pumpkin (Cucurbita pepo) oil cake | NaOH aq. (pH 10.0) | DGS | Filtration and precipitation at pH 5.0 | [58,62] |
| Pumpkin (Cucurbita pepo) oil cake | NaOH aq. (pH 10.0) | Defatted oil cake: extracting media at ratio 1:10 (w/v) | Precipitation at pH 5.0 | [59] |
| Pumpkin (Cucurbita pepo) oil cake | NaOH aq. (pH 11.0) | DGS: extracting medium at 1:30 (w/v); stirring (50 °C and 1.5 h) | Precipitation at pH 5.3 | [60] |
| Pumpkin (Cucurbita moschata), watermelon, and bottle gourd seeds | Tris HCl (pH 8.0) | 200 mg of DGS and 50 mL buffer (1 h) | Centrifugation and precipitation with acetone | [32] |
| Bottle gourd seeds | 50 mM phosphate buffer (10 mM EDTA, 100 mM KCl, 1 mM DTT, and 1% SDS) | Sample: extracting medium at ratio 1:3 (w/v) (3 times) | Filtration and precipitation with chilled ethanol | [30] |
| Wax gourd seed | 20 mM phosphate buffer (pH 6.5, 5.0 mM EDTA, and 10 mM DTT) (buffer I) and phosphate (2.0 mM EDTA, 1 mM DTT, urea 4 M, and 2% Triton X 100) (buffer II) | 500 g of DGS and buffer I, 3 h; centrifugation and 2nd extraction under same conditions; centrifugation and 3rd extraction with buffer II and centrifugation | Dialysis, centrifugation, and filtration | [44] |
| Jujube seeds | Tris-HCl (pH 7.5) | 1.5 g of ground seeds and 50 mM buffer (2 h) | Centrifugation, precipitation with (NH₄)₂SO₄ (4 °C), centrifugation, and dialysis | [46] |
| Milled rapeseed | 50 mM Tris-HCl (pH 8.5, 750 mM NaCl, 5 mM EDTA, and 0.3% Na₂O₅S₂) | 0.1 g/L (room temperature, 1 h) | Centrifugation | [23] |
| Pumpkin (Cucurbita moschata) seeds | Osborne method: (1) water; (2) Tris-HCl (5% NaCl); (3) isopropanol (55%); and (4) acetic acid (0.2 N) | 150 mg of DGS and 10 mL extracting medium (60 min). Next extractions at extracting medium:sample ratio 7:1 | (I, II, and III) Centrifugation and precipitation with acetone | [20] |
| Muskmelon seeds | Osborne method: (1) water; (2) NaCl (5%); (3) NaOH (0.1 M); and (4) ethanol (70%) | 100 g of DGS and 500 mL extracting medium (60 min) | (I) Centrifugation; (II) centrifugation and dialysis; (III) precipitation at pH 4.0; and (IV) evaporation at 40 °C | [47] |
| Bottle gourd seeds | Osborne method: (1) water; (2) Tris-HCl (100 mM, pH 8.1, 0.5 M NaCl); (3) isopropanol (55%); and (4) acetic acid (0.2 N) | 8 g of DGS and 60 mL extracting medium (60 min). Next extractions at extracting medium:sample ratio 7:1 | (I, II, III, and IV) Centrifugation and precipitation with acetone | [31] |
| Watermelon seeds | Osborne method | - | - | [49] |
| ULTRASOUND-ASSISTED EXTRACTION | ||||
| Pomegranate peel | 100 mM Tris-HCl (pH 7.5, 0.5%, SDS, and 0.25% DTT) | 150 mg milled peel and 5 mL buffer using HIFU (30%, 1 min) | Evaporation and precipitation with cold acetone | [3] |
| Peach, plum, apricot, cherry, and olive seeds | 100 mM Tris-HCl (pH 7.5, 0.5% SDS, and 0.5% DTT) | 30 mg DGS and 5 mL buffer using HIFU (30%, 1 min) | Precipitation with cold acetone | [11,13] |
| Plum seeds | 100 mM Tris-HCl (pH 7.5, 1% SDS, and 0.25% DTT) | 30 mg DGS and 5 mL buffer using HIFU (30%, 1 min) | Precipitation with cold acetone and filtration | [14,35] |
| Olive and peach seeds | 100 mM Tris-HCl (pH 7.5, 0.5% SDS, and 0.5% DTT) | 30 mg DGS and 5 mL buffer using HIFU (30%, 5 min) | Precipitation with cold acetone | [16] |
| Olive seeds | 125 mM Tris-HCl (pH 7.5, 1% SDS, and 0.1% DTT) | 30 mg milled seeds and 5 mL buffer using HIFU (30%, 5 min) | Precipitation with cold acetone | [17] |
| Plum and peach seeds | 50 mM Tris-HCl (pH 7.4) and 15 mM NaCl (buffer I)50 mM Tris-HCl (pH 7.4) and 15 mM NaCl and 1% SDS and 25 mM DTT (buffer II) | 200 mg DGS and 10 mL of buffer I or II (10 min) and shaking (overnight) | Evaporation and precipitation with cold acetone | [29] |
| Cherry seeds | 100 mM Tris-HCl (pH 7.5, 1% SDS, and 0.5% DTT) | 30 mg DGS and 5 mL buffer using HIFU (30%, 5 min) | Precipitation with cold acetone | [33] |
| PRESSURIZED LIQUID EXTRACTION | ||||
| Pomegranate peel | Ethanol (70% (v/v)) | 2 g ground dried peels and 8 g sand (1500 psi; 120 °C; static extraction time, 3 min; extraction time, 12 min; static cycles, 1) | Evaporation and precipitation with cold ethanol | [25] |
| EXTRACTION USING DEEP EUTECTIC SOLVENTS | ||||
| Pomegranate peel | Choline chloride:AA:H₂O in 1:1:10 molar ratio | 150 mg dried peels and 5 mL extracting medium (HIFU, 11 min, and 30%) | Evaporation and precipitation with cold ethanol | [25] |
| ULTRASOUND–MICROWAVE SYNERGISTIC EXTRACTION | ||||
| Pumpkin (Cucurbita moschata) seeds | PEG 200-choline chloride at 3:1 molar ratio | Microwave-assisted extraction (6 min, 120 W); ultrasound-assisted extraction (30 min, 240 W); water bath extraction (43 °C and 60 min); ultrasound–microwave synergistic extraction (28%, 28 g /L, 140 W, 43 °C and 4 min) | Isoelectric point precipitation; ethanol precipitation; centrifugation; centrifugation, isoelectric point precipitation, and ethanol precipitation | [64] |
| EXTRACTION USING PULSED ELECTRIC FIELD (PEF) AND HIGH VOLTAGE ELECTRICAL DISCHARGE (HVED) | ||||
| Olive seeds | Water | Sample: extracting medium at ratio 10 (w/w); pretreatment with HVED, PEF, and ultrasound; extraction (2 min and 150 rpm) | - | [34] |
| Rapeseed press-cake | Water | Sample: extracting medium at ratio 20 (v/w); HVED (240 kJ/kg) | - | [24] |
| Mango peels | I and II) Water; III) water (pH 11.0); IV) water and water (pH 11.0) | 300 g of sample at ratio 1/10 (w/v); (I) PEF (13.3 kV/cm, 0.5 Hz); (II) HVED (40 kV/cm, 0.5 Hz); (III) aqueous extraction (20–60 °C and pH = 2.5, 6.0, 11.0); (IV) PEF; and (I) and aqueous extraction (50 °C, pH 6.0, and 3 h) | - | [26] |
| Papaya peels | (I and II) Water; (III) water (pH 11.0); (IV) water and water (pH 11.0) | 300 g sample at ratio 1:10 (w/v); (I) PEF (13.3 kV/cm, 0,5 Hz); (II) HVED (40 kV/cm, 0.5 Hz); (III) aqueous extraction (20–60 °C, pH = 2.5, 6.0, 11.0); (IV) PEF; and (I) aqueous extraction (50 °C, pH 7.0, and 3 h) | - | [54] |
| METHODS USING NANOMATERIALS | ||||
| Plum seeds | 100 mM Tris–HCl buffer (pH 7.5, 1% SDS, and 0.25% DTT) | 30 mg DGS and 5 mL buffer using HIFU (30%, 1 min) | 3G carboxylate-terminated dendrimers at pH 1.8 (30 min) | [74] |
| Plum seeds | 100 mM Tris–HCl buffer (pH 7.5, 1% SDS, and 0.25% DTT) | 30 mg DGS and 5 mL buffer using HIFU (30%, 1 min) | 2G dimethylamino-terminated dendrimers at pH 7.5 (30 min) | [75] |
| Plum seeds | - | 3G single wall carbon nanotubes functionalized with sulphonate-terminated carbosilane dendrimers at pH 7.5 with shaking (1 h) | Ultrafiltration | [76] |
| Peach seeds | - | 2G gold nanoparticles coated with carbosilane dendrimers with carboxylate groups at pH 2.5 with shaking (2 h) | Ultrafiltration | [77] |
| Fruit Residue | Enzyme/Microorganisms | Buffer (pH) | Temperature (°C) | Time (h) | Refs. |
|---|---|---|---|---|---|
| RELEASE OF PEPTIDES BY MICROBIAL FERMENTATION | |||||
| Tomato seed | Lactobacillus plantarum | — | 37 | 24 | [36] |
| Tomato seed | Water kefir microbial mixture | — | 37 | 24 | [37,41] |
| Tomato seed | Bacillus subtilis | — | 40 | 20 | [38] |
| Tomato seed | Bacillus subtilis | — | 37 | 24 | [39,40] |
| RELEASE OF PEPTIDES BY ENZYMATIC DIGESTION | |||||
| Pumpkin oil cake | Alcalase | Tris-HCl (0.1 M and pH 8.0) | 50 | 1 | [58] |
| Flavourzyme | 1 | ||||
| Alcalase and flavourzyme | 2 | ||||
| Pumpkin oil cake | Alcalase | Phosphate (pH 8.0) | 50 | 0–2.5 | [62] |
| Flavourzyme | Phosphate (pH 7.0) | 50 | |||
| Pepsin | Phosphate (pH 3.0) | 37 | |||
| Pumpkin oil cake | Alcalase | Tris-HCl (pH 9.0) | 50 | 3.5 | [59] |
| Trypsin | Tris-HCl (pH 8.0) | 35 | 5 | ||
| Pumpkin seed | Acid protease | pH 2.5 | 50 | 5 | [60] |
| Pumpkin meal | Alcalase | pH 8.0 | 55 | 5 | [63] |
| Flavourzyme | pH 7.0 | 50 | |||
| Protamex | pH 6.5 | 50 | |||
| Neutrase | pH 7.0 | 50 | |||
| Peach, plum, apricot, and olive seeds | Pepsin and pancreatin | pH 2.0 and pH 8.0 | 37 | 3 | [11] |
| Apricot seeds | Alcalase | Borate (5 mM and pH 8.5) | 50 | 4 | [11] |
| Thermolysin | Phosphate (5 mM and pH 8.0) | 4 | |||
| Flavourzyme | Phosphate (5 mM and pH 7.5) | 8 | |||
| Plum seeds | Alcalase | Borate (5 mM and pH 8.5) | 50 | 3 | [14,35] |
| Thermolysis | Phosphate (5 mM and pH 8.0) | 50 | 4 | ||
| Flavourzyme | Phosphate (5 mM and pH 7.0) | 50 | 7 | ||
| Protease P | Phosphate (5 mM and pH 7.5) | 40 | 24 | ||
| Apricot seeds | Alkaline and flavor proteases | - | - | - | [27] |
| Peach seeds | Alcalase | Phosphate (5 mM and pH 8.0) | 50 | 4 | [28] |
| Thermolysin | Phosphate (5 mM and pH 8.0) | 50 | 4 | ||
| Flavourzyme | Ammonium bicarbonate (5 mM and pH 6.5) | 50 | 3 | ||
| Protease P | Phosphate (5 mM and pH 7.5) | 40 | 7 | ||
| Cherry seeds | Alcalase | Borate (pH 8.5) | 50 | 7 | [33] |
| Thermolysin | Phosphate (pH 8.0) | ||||
| Flavourzyme | Bicarbonate (pH 6.0) | ||||
| Chinese cherry seeds | Alcalase and Neutrase | Water (pH 7.5) | 50 | 2 | [55] |
| Olive and peaches seeds | Alcalase | Borate (5 mM and pH 8.5) | 50 | 4 | [16] |
| Olive seeds | Alcalase | Phosphate (5 mM and pH 8.0) | 50 | 2 | [17] |
| Thermolysin | Phosphate (5 mM and pH 8.0) | ||||
| Flavourzyme | Ammonium bicarbonate (5 mM and pH 6.0) | ||||
| Trypsin | Tris-HCl (5 mM and pH 9.0) | ||||
| Neutrase | Phosphate (5 mM and pH 7.0) | ||||
| Tomato seeds | Alcalase | Phosphate (pH 8.0) | 50 | 0.5–3 | [43] |
| Tomato seeds | Alcalase | Phosphate (pH 8.0) | 50 | 2.3 | [21] |
| Milled rapeseed | Pepsin | Phosphate (0.1 M and pH 2.0) | 40 | 3 | [23] |
| Trypsin | Phosphate (0.1 M and pH 7.0) | 40 | 3 | ||
| Alcalase | Phosphate (0.1 M and pH 7.0) | 50 | 3 | ||
| Subtilisin | Phosphate (0.1 M and pH 8.0) | 60 | 3 | ||
| Thermolysin | Phosphate (0.1 M and pH 8.0) | 60 | 24 | ||
| Jujube seeds | Papain | Tris-HCl (50 mM and pH 6.5–7.5) | 65 | 1.5 | [45,46] |
| Alcalase | Tris-HCl (50 mM and pH 6.5–8.5) | 60 | |||
| Protease P | Tris-HCl (50 mM and pH 7.5) | 37 | |||
| Muskmelon seeds | Pepsin and Trypsin | pH 2.0 and pH 7.0 | 37 | 6 | [47] |
| Pumpkin (Cucurbita moschata), watermelon, and bottle gourd seeds | Trypsin | Tris-HCl (50 mM and pH 7.5) | - | 4 | [32] |
| Watermelon seeds | Alcalase | Phosphate (5 mM and pH 8.0) | 60 | 5 | [48,50] |
| Trypsin | Phosphate (5 mM and pH 8.0) | 37 | |||
| Pepsin | Glycine (5 mM and pH 2.2) | 37 | |||
| Watermelon seeds | Alcalase | pH 8.5 | 55 | 3 | [51] |
| Watermelon seeds | Papain | — | — | — | [52] |
| Pepsin | pH 2.4 | 37 | 3 | ||
| Protease | — | — | — | ||
| Pancreatin | — | — | — | ||
| Trypsin | — | — | — | ||
| Chymotrypsin | — | — | — | ||
| Watermelon seeds | Alcalase | NaOH aq. (pH 9.0) | 50 | 0.8 | [53] |
| African breadfruit seeds | Trypsin, pepsin, and pancreatin | Water | - | [56] | |
| Date palm seeds | Alcalase | pH 8.0 | 50 | 1 | [65,66] |
| Flavourzyme | pH 7.0 | 2 | |||
| Thermolysin | pH 8.0 | 3 | |||
| Pomegranate peel | Alcalase | Borate (5 mM and pH 9.0) | 50 | 2 | [3] |
| Thermolysin | Phosphate (5 mM and pH 7.5) | 70 | 1 | ||
| Pomegranate peel | Alcalase Thermolysin | Borate (5–10 mM and pH 9.0) Phosphate (5 mM and pH 7.5) or borate (100 mM and pH 7.5) | 50 70 | 2 1 | [25] |
| Assay | Methodology | Refs. |
|---|---|---|
| EVALUATION OF THE CAPACITY TO SCAVENGE FREE RADICALS AND OXIDANT SPECIES | ||
| Scavenging effect on hydrogen peroxide (H₂O₂) radicals | Measurement of the reduction in the absorbance of a H₂O₂ solution at 230 nm after incubation with potential antioxidants. | [20,31,48] |
| Scavenging effect on ABTS (2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) radicals | ABTS radicals that absorb at 734 nm are produced by the reaction of ABTS with potassium persulfate. The method evaluates the reduction in the absorbance of ABTS radicals due to the presence of potential antioxidants. | [3,11,14,16,17,20,24,25,28,31,33,34,35,36,37,41,42,45,46,49,51,52,54,55,58,62,65] |
| Scavenging effect on nitric oxide (NO) radicals | Nitric oxide radicals are formed from nitroprusside and the incubation of formed nitric oxide radicals with a Griess reagent (1% sulphanilamide, 2% H3PO4, and 0.1% naphthylethylene diamine dihydrochloride) results in nitrite ions. Nitrite ions can be measured by the formation of a compound that absorbs at 546 nm. The scavenging of nitric oxide radicals by potential antioxidants reduces nitrite ion formation and their absorbance at 546 nm. | [20,31,49] |
| Scavenging effect on DPPH (1,1-diphenyl-2-picrylhydrazyl) radicals | Measurement of the decrease in the absorption of DPPH radicals at 515–517 nm when potential antioxidants are added. | [14,17,20,21,26,27,30,31,34,36,37,38,39,40,41,42,43,44,45,46,47,48,49,51,52,53,54,55,56,59,60,61,63,65] |
| EVALUATION OF THE CAPACITY TO INHIBIT OXIDATION REACTIONS | ||
| Inhibition of formation of superoxide (O²⁻) radicals | The assay measures the rate of pyrogallol autooxidation in presence and absence of potential antioxidants at 320–420 nm. | [50,55] |
| Inhibition of formation of hydroxyl (OH•) radicals | Hydroxyl radicals are generated by the oxidation of Fe2+ to Fe3+ in the presence of H₂O₂. The presence of Fe2+ is monitored by the formation of a complex with 1,10-phenanthroline that absorbs at 536 nm. The presence of potential antioxidants inhibits the oxidation of Fe2+ and results in an absorbance increase. | [3,11,14,16,17,25,27,28,33,35,47,55,65] |
| Oxygen radical antioxidant capacity (ORAC) | The method is based on the oxidation of fluorescein by reactive oxygen species (ROS) resulting from the radical initiator 2,2’-azobis(2-methylpropionamidine) dihydrochloride. The inhibition of fluorescein oxidation by the presence of potential antioxidants is measured from the increase in fluorescence intensity. | [51,65] |
| EVALUATION OF THE REDUCING POWER | ||
| Ferric reducing antioxidant power (FRAP) | Measures the ability of potential antioxidants to reduce Fe³⁺ from the ferricyanide complex to Fe²⁺-complex. Formation of Fe²⁺-complex is measured at 700 nm. | [14,16,20,27,28,30,31,33,35,38,39,40,45,46,47,49,50,52,56,58,62,63,65] |
| Ammonium phosphomolybdenum | The method evaluates the capacity of potential antioxidants to reduce Mo⁶⁺ to Mo⁵⁺. Presence of Mo⁵⁺ is monitored by the subsequent formation of a green phosphor/Mo⁵⁺ complex that absorbs at 695–65 nm. | [20,21,30,31,59] |
| EVALUATION OF THE METAL QUELATION ACTIVITY | ||
| Ferrous ion chelation activity (FICA) | Ferrozine reacts with Fe²⁺ to form a complex that absorbs at 562 nm. In the presence of chelating agents, the complex is disrupted, resulting in a decrease in absorption at 562 nm. | [45,46,52,53,59,61,63,65] |
| Cuprous ion chelation activity (CICA) | Reaction of pyrocatechol and Cu²⁺ results in a substance that absorbs at 632 nm. The presence of a metal chelator disrupts this molecule and reduces the absorbance. | [47] |
| EVALUATION OF THE CAPACITY TO INHIBIT LIPIDS AND LIPOPROTEINS OXIDATION | ||
| Ferric thiocyanate | Primary products resulting from the oxidation of linoleic acid are incubated with EtOH, ammonium thiocyanate, and FeCl₂, leading to the formation of Fe(SCN)²⁺ that absorbs at 500 nm. Presence of potential antioxidants results in the inhibition of linoleic acid oxidation and the reduction of absorption. | [14,16,17,28,33,35,44,49,56,63] |
| Thiobarbituric acid reactive substances (TBARS) | The presence of secondary oxidation products formed during oxidation of linoleic acid is measured by the reaction of one of them, the malondialdehyde, with SDS, acetic acid, and TBA at 532 nm. The presence of potential antioxidants reduces this absorbance. | [20,23,31,32,44,53,57,66] |
| β-carotene linoleate | It measures the ability of potential antioxidants to decrease the oxidative bleaching of β-carotene in an oil-in-water emulsion. The reaction is monitored by measuring the absorbance at 470 nm immediately after the addition of a potential antioxidant. | [66] |
| Inhibition of Cu²⁺-induced low-density lipoprotein (LDL) peroxidation | This assay measures the peroxidation induced by cupric sulfate in LDL. Presence of potential antioxidants results in the inhibition of the oxidation and the reduction of the absorbance of conjugated dienes at 344 nm. | [66] |
| EVALUATION OF THE CAPACITY TO INHIBIT DNA OXIDATION | ||
| Supercoiled-to-Nicked-Circular-Conversion (SNCC) | Oxidation of supercoiled DNA into nicked circular DNA in the presence of Cu²⁺ and H₂O₂ is monitored by measuring the fluorescent intensity of ethidium-stained nicked circular DNA. The presence of a potential antioxidant inhibits this reaction, and the signal corresponding to the oxidized form of DNA decreases. | [30] |
| Inhibition of peroxyl and hydroxyl radical-induced supercoiled strands scission | Strand scission of supercoiled DNA is measured in the presence of peroxyl and hydroxyl radicals. After incubation, DNA is separated by gel electrophoresis, and the intensity of supercoiled DNA bands in the presence and absence of potential antioxidant are compared. | [66] |
| EVALUATION OF THE CAPACITY TO INHIBIT OXIDATIVE DAMAGE INDUCED IN CELLS | ||
| 2’, 7’-dichloro-dihydro-fluorescein diacetate (DCFH-DA) fluorescent probe | Oxidative stress in cells is induced by the addition of a strong oxidant (H₂O₂ or other peroxide). DCFH-DA fluorescence probe, added to cell culture, reacts with ROS to produce fluorescent DCF that is measured at an λexcitation of 488 nm and an λemission of 585 and 530 nm. The presence of a potential antioxidant inhibits ROS generation and DCF signal decreases. | [16,51] |
| Intracellular concentration of Ca²⁺ determination | Intracellular Ca²⁺ is measured with fluorescent dye Fura-2 AM. Fura-2AM is cleaved by intracellular esterase, and the resulting Fura-2 can bind to Ca²⁺ and cause strong fluorescence under a 330–350 nm excitation light. Fluorescence intensity decreases in H₂O₂-damaged cells treated with potential antioxidants. | [51] |
| Acridine orange/ethidium bromide (AO/EB) fluorescent staining | Cell membrane damage is measured by evaluating the staining of DNA with EB or AO using an inverted fluorescence microscope. The presence of potential antioxidants will reduce the number of red cells resulting from the staining with EB and will increase the number of green cells resulting from the staining with AO. | [51] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivares-Galván, S.; Marina, M.L.; García, M.C. Extraction and Characterization of Antioxidant Peptides from Fruit Residues. Foods 2020, 9, 1018. https://doi.org/10.3390/foods9081018
Olivares-Galván S, Marina ML, García MC. Extraction and Characterization of Antioxidant Peptides from Fruit Residues. Foods. 2020; 9(8):1018. https://doi.org/10.3390/foods9081018
Chicago/Turabian StyleOlivares-Galván, Saúl, María Luisa Marina, and María Concepción García. 2020. "Extraction and Characterization of Antioxidant Peptides from Fruit Residues" Foods 9, no. 8: 1018. https://doi.org/10.3390/foods9081018
APA StyleOlivares-Galván, S., Marina, M. L., & García, M. C. (2020). Extraction and Characterization of Antioxidant Peptides from Fruit Residues. Foods, 9(8), 1018. https://doi.org/10.3390/foods9081018






