Current Trends of Bioactive Peptides—New Sources and Therapeutic Effect
Abstract
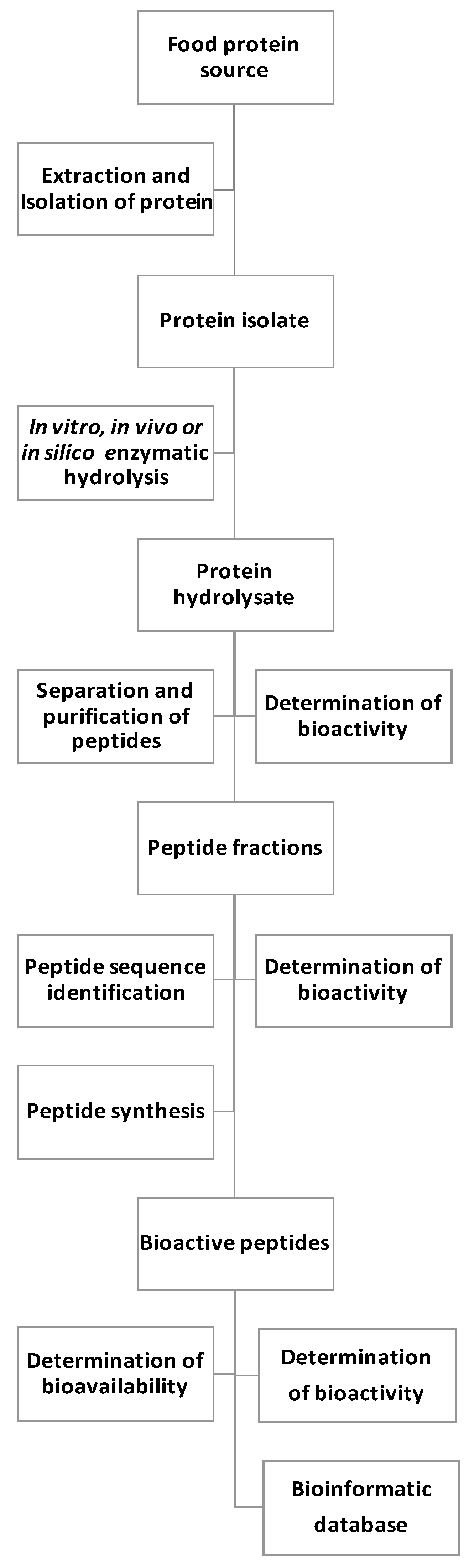
1. Introduction
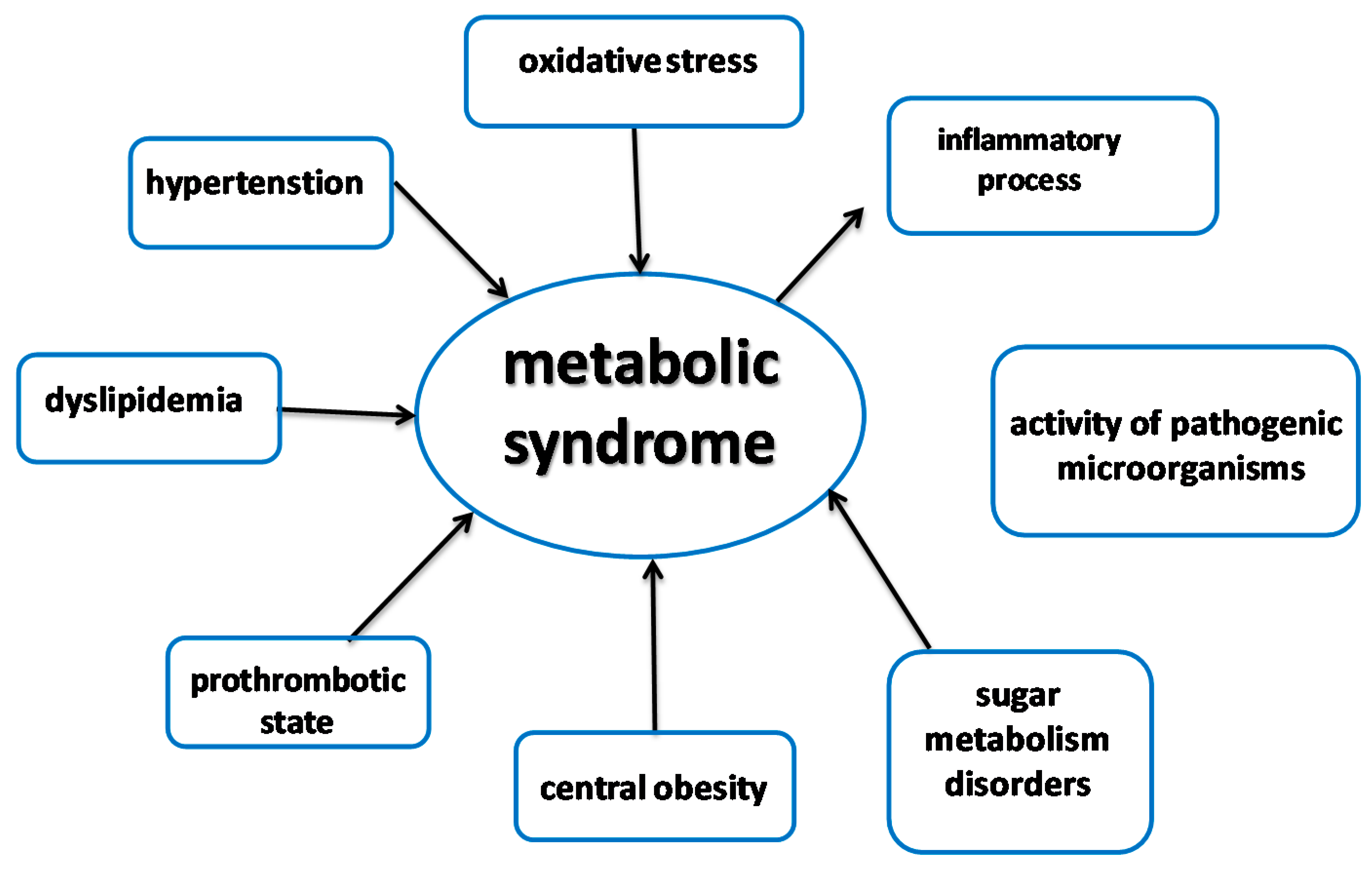
2. Peptides as Inhibitors of Enzymes Involved in Metabolic Syndrome
2.1. Metabolic Syndrome
2.2. ACE Inhibitory Peptides
2.3. Pancreatic Lipase Inhibitory Peptides
2.4. Peptide Inhibition of Diabetes Risk Factors
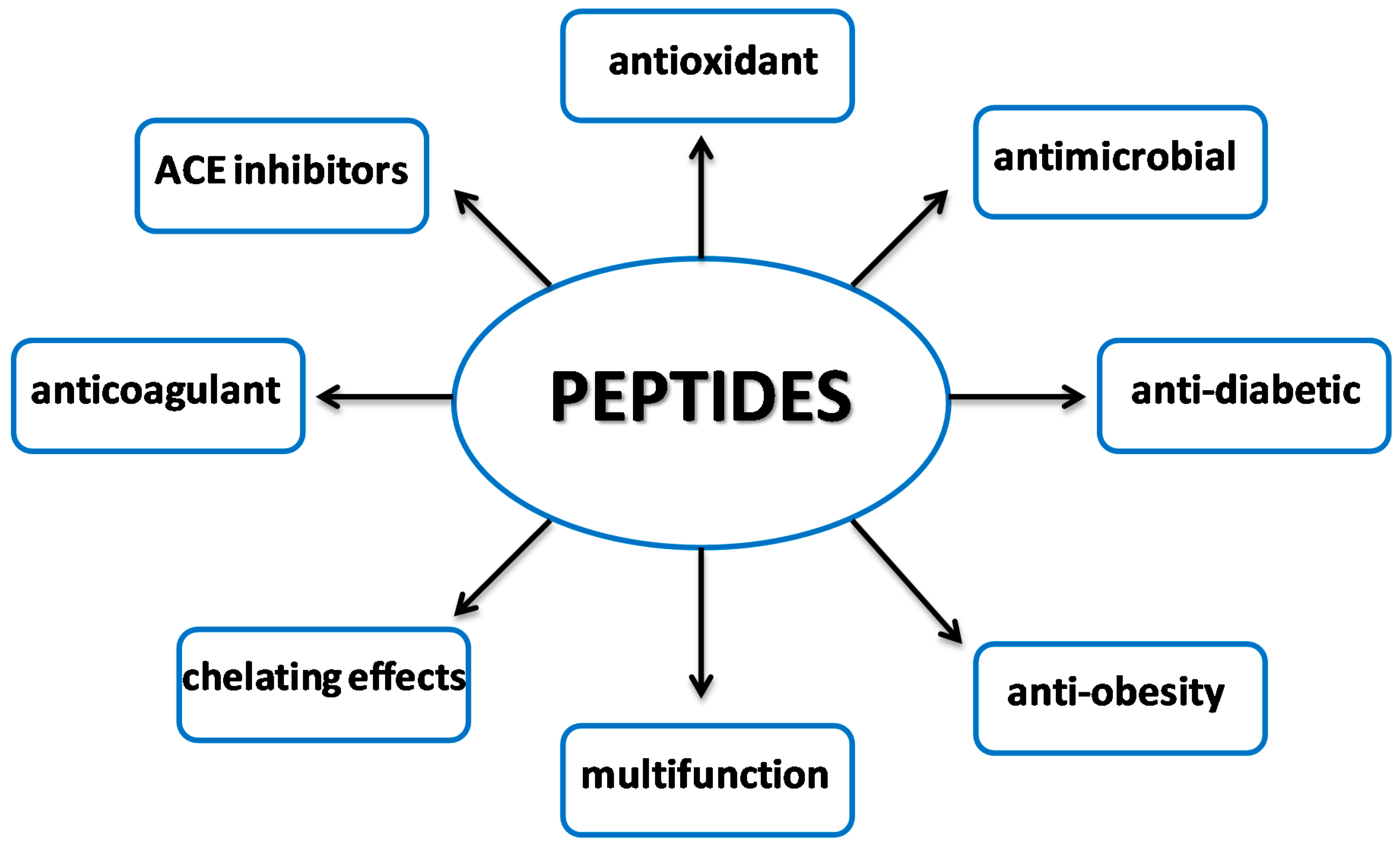
3. Antioxidant Peptides
3.1. Structure-Activity Relationship in Peptides
3.2. Stability and Bioavailability of Antioxidant Peptides
3.3. Investigations of Cellular Antioxidant Activity
3.4. Multifunctional Nature of Antioxidant Peptides
3.5. Bioinformatics Studies of Antioxidant Peptides
4. Peptides with Antimicrobial Properties
5. New Alternative Sources of Peptides
5.1. Peptides from Edible Insects
5.2. Peptides from Seafood By-Products
5.3. Peptides from Seeds and Plants
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dziki, D.; Gawlik-Dziki, U.; Biernacka, B. Cistus incanus L. as an Innovative Functional. Foods 2019, 8, 1–12. [Google Scholar]
- Szymanowska, U.; Baraniak, B. Antioxidant and potentially anti-inflammatory activity of anthocyanin fractions from pomace obtained from enzymatically treated raspberries. Antioxidants 2019, 8, 299. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Manabe, Y.; Sugawara, T.; Paul, N.A.; Zhao, J. Identification and biological activities of carotenoids from the freshwater alga Oedogonium intermedium. Food Chem. 2018, 242, 247–255. [Google Scholar] [CrossRef]
- Złotek, U.; Szymanowska, U.; Rybczyńska-Tkaczyk, K.; Jakubczyk, A. Effect of Jasmonic Acid, Yeast Extract Elicitation, and Drying Methods on the Main Bioactive Compounds and Consumer Quality of Lovage (Levisticum officinale Koch). Foods 2020, 3, 323. [Google Scholar] [CrossRef] [PubMed]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control 2015, 54, 111–119. [Google Scholar] [CrossRef]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Krystyna, J.; Lewicki, S.; Złotek, U. Different temperature treatments of millet grains affect the biological activity of protein hydrolyzates. Nutrients 2019, 11, 550. [Google Scholar] [CrossRef] [PubMed]
- Karaś, M. Influence of physiological and chemical factors on the absorption of bioactive peptides. Int. J. Food Sci. Technol. 2019, 54, 1486–1496. [Google Scholar] [CrossRef]
- Yada, R.Y. Plant proteases for bioactive peptides release: A review. Crit. Rev. Food Sci. Nutr. 2017, 8398, 2147–2163. [Google Scholar]
- Jakubczyk, A.; Karaś, M.; Złotek, U.; Szymanowska, U.; Baraniak, B. Peptides obtained from fermented faba bean seeds (Vicia faba) as potential inhibitors of an enzyme involved in the pathogenesis of metabolic syndrome. LWT-Food Sci. Technol. 2019, 105, 306–313. [Google Scholar] [CrossRef]
- Fideler, J.; Johanningsmeier, S.D.; Ekelöf, M.; Muddiman, D.C. Discovery and quanti fication of bioactive peptides in fermented cucumber by direct analysis IR-MALDESI mass spectrometry and LC-QQQ-MS. Food Chem. 2019, 271, 715–723. [Google Scholar] [CrossRef]
- Arulrajah, B.; Muhialdin, B.J.; Zarei, M.; Hasan, H.; Saari, N. Lacto-fermented Kenaf (Hibiscus cannabinus L.) seed protein as a source of bioactive peptides and their applications as natural preservatives. Food Control 2020, 110, 106969. [Google Scholar] [CrossRef]
- Mirzaei, M. In vitro and in silico studies of novel synthetic ACE-inhibitory peptides derived from Saccharomyces cerevisiae protein hydrolysate. Bioorg. Chem. 2019, 87, 647–654. [Google Scholar] [CrossRef]
- Souza, P.F.N.; Marques, L.S.M.; Oliveira, J.T.A.; Lima, P.G.; Dias, P.; Neto, N.A.S.; Lopes, F.E.S.; Sousa, J.S.; Silva, A.F.B.; Caneiro, R.F.; et al. Synthetic antimicrobial peptides: From choice of the best sequences to action mechanisms. Biochimie 2020. In press. [Google Scholar] [CrossRef]
- Złotek, U.; Jakubczyk, A.; Rybczyńska-Tkaczyk, K.; Ćwiek, P.; Baraniak, B.; Lewicki, S. Characteristics of new peptides GQLGEHGGAGMG, GEHGGAGMGGGQFQPV, EQGFLPGPEESGR, RLARAGLAQ, YGNPVGGVGH, and GNPVGGVGHGTTGT as inhibitors of enzymes involved in metabolic syndrome and antimicrobial potential. Molecules 2020, 25, 2492. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, L.; Quintieri, L.; Caputo, L.; Gallerani, R.; Mayo, B.; Leo, F. De Cloning and expression of synthetic genes encoding angiotensin-I converting enzyme (ACE)-inhibitory bioactive peptides in Bifidobacterium pseudocatenulatum. Fed. Eur. Microbiol. Scieties 2013, 340, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Qiao, R.; Chen, T.; Wu, J.; Du, S. Identification and molecular cloning of novel antimicrobial peptides from skin secretions of the Chinese bamboo leaf odorous frog (Odorrana versabilis ) and the North American pickerel frog (Rana palustris ). J. Tradit. Chin. Med. Sci. 2017, 4, 297–305. [Google Scholar] [CrossRef][Green Version]
- Okasha, H. Gene Reports Synthesis and molecular cloning of antimicrobial peptide chromogranin A N-46 gene using conventional PCR. Gene Rep. 2020, 18, 100571. [Google Scholar] [CrossRef]
- Vermeirssen, V.; Van Camp, J.; Decroos, K.; Van Wijmelbeke, L.; Verstraete, W. The Impact of Fermentation and In Vitro Digestion on the Formation of Angiotensin-I-Converting Enzyme Inhibitory Activity from Pea and Whey Protein. J. Dairy Sci. 2003, 86, 429–438. [Google Scholar] [CrossRef]
- Kim, S.-K.; Ngo, D.-H.; Vo, T.-S. Marine fish-derived bioactive peptides as potential antihypertensive agents. In Advances in Food and Nutrition Research; Jeya, H., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 65, pp. 249–260. [Google Scholar]
- Abdelhedi, O.; Jridi, M.; Jemil, I.; Mora, L.; Toldrá, F.; Aristoy, M.; Boualga, A.; Nasri, M.; Nasri, R. Combined biocatalytic conversion of smooth hound viscera: Protein hydrolysates elaboration and assessment of their antioxidant, anti-ACE and antibacterial activities. FRIN 2016, 86, 9–23. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M. Identification of antioxidant and anti-inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria). Int. J. Food Sci. Technol. 2018, 53, 2542–2551. [Google Scholar] [CrossRef]
- Rogozhin, E.A.; Slezina, M.P.; Slavokhotova, A.A.; Istomina, E.A.; Korostyleva, T.V.; Smirnov, A.N.; Grishin, E.V.; Egorov, T.A.; Odintsova, T.I. A novel antifungal peptide from leaves of the weed Stellaria media L. Biochimie 2015, 116, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M. Food Proteins and Bioactive Peptides: New and Novel Sources, Characterisation Strategies and Applications. Foods 2018, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xuan, G.; Fu, M.; He, G.; Mb, W.W. Effect of angiotensin I -converting enzyme inhibitory peptide from rice dregs protein on antihypertensive activity in spontaneously hypertensive rats. Asian Pac. J. Clin. Nutr. 2007, 16, 281–285. [Google Scholar]
- Min-Gu, K.; Sung Hun Yi, J.-S.L. Production and Characterization of a New α -Glucosidase Inhibitory Peptide from Aspergillus oryzae N159-1. Mycobiology 2013, 149–154. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Mathangasinghe, Y.; Jayawardena, R.; Hills, A.P.; Misra, A. Prevalence and trends of metabolic syndrome among adults in the asia-pacific region: A systematic review. BMC Public Health 2017, 17, 101. [Google Scholar] [CrossRef]
- Sigit, F.S.; Tahapary, D.L.; Trompet, S.; Sartono, E.; Van Dijk, K.W. The prevalence of metabolic syndrome and its association with body fat distribution in middle - aged individuals from Indonesia and the Netherlands: A cross—sectional analysis of two population - based studies. Diabetol. Metab. Syndr. 2020, 12, 1–11. [Google Scholar] [CrossRef]
- Durante, A.; Durante, A.; Peretto, G.; Laricchia, A.; Ancona, F.; Spartera, M.; Cianflone, D. Role of the Renin-Angiotensin-Aldosterone System in the Pathogenesis of Atherosclerosis. Curr. Pharm. Des. 2012, 18, 981–1004. [Google Scholar] [CrossRef]
- Wu, C.; Mohammadmoradi, S.; Chen, J.Z.; Sawada, H.; Daugherty, A.; Lu, H.S. Renin-Angiotensin System and Cardiovascular Functions. Arterosclerosis. Thromb. Vasc. Biol. 2018, 38, 108–116. [Google Scholar] [CrossRef]
- Li, Y.; Sadiq, F.A.; Fu, L.; Zhu, H.; Zhong, M.; Sohail, M. Identification of Angiotensin I-Converting Enzyme Inhibitory Peptides Derived from Enzymatic. Mar. Drug 2016, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Khueychai, S.; Jangpromma, N.; Choowongkomon, K. A novel ACE inhibitory peptide derived from alkaline hydrolysis of ostrich (Struthio camelus) egg white ovalbumin. Process Biochem. 2018, 73, 235–245. [Google Scholar] [CrossRef]
- Rao, S.; Ju, T.; Sun, J.; Su, Y.; Xu, R.; Yang, Y. Puri fi cation and characterization of angiotensin I-converting enzyme inhibitory peptides from enzymatic hydrolysate of hen egg white lysozyme. Food Res. Int. 2012, 46, 127–134. [Google Scholar] [CrossRef]
- Salampessy, J.; Reddy, N.; Phillips, M.; Kailasapathy, K. Isolation and characterization of nutraceutically potential ACE-Inhibitory peptides from leatherjacket (Meuchenia sp.) protein hydrolysates. LWT Food Sci. Technol. 2017, 80, 430–436. [Google Scholar] [CrossRef]
- Toopcham, T.; Mes, J.J.; Wichers, H.J.; Roytrakul, S.; Yongsawatdigul, J. Bioavailability of angiotensin I-converting enzyme (ACE) inhibitory peptides derived from Virgibacillus halodenitrificans SK1-3-7 proteinases hydrolyzed tilapia muscle proteins. Food Chem. 2017, 220, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Hur, S.J. Purification of novel angiotensin converting enzyme inhibitory peptides from beef myo fi brillar proteins and analysis of their effect in spontaneously hypertensive rat model. Biomed. Pharmacother. 2019, 116, 109046. [Google Scholar] [CrossRef]
- Sonklin, C.; Alashi, M.A.; Laohakunjit, N.; Kerdchoechuen, O.; Aluko, R.E. Identification of antihypertensive peptides from mung bean protein hydrolysate and their effects in spontaneously hypertensive rats. J. Funct. Foods 2020, 64, 103635. [Google Scholar] [CrossRef]
- Zhang, P.; Chang, C.; Liu, H.; Li, B.; Yan, Q.; Jiang, Z. Identification of novel angiotensin I-converting enzyme (ACE ) inhibitory peptides from wheat gluten hydrolysate by the protease of Pseudomonas aeruginosa. J. Funct. Foods 2020, 65, 103751. [Google Scholar] [CrossRef]
- Stanton, C. Bioactive Peptides from Muscle Sources: Meat and Fish. Nutrients 2011, 3, 765–791. [Google Scholar]
- Forghani, B.; Zarei, M.; Ebrahimpour, A.; Philip, R.; Bakar, J.; Abdul Hamid, A.; Saari, N. Purification and characterization of angiotensin converting enzyme-inhibitory peptides derived from Stichopus horrens: Stability study against the ACE and inhibition kinetics. J. Funct. Foods 2016, 20, 276–290. [Google Scholar] [CrossRef]
- Tu, M.; Wang, C.; Chen, C.; Zhang, R.; Liu, H.; Lu, W. Identi fication of a novel ACE-inhibitory peptide from casein and evaluation of the inhibitory mechanisms. Food Chem. 2018, 256, 98–104. [Google Scholar] [CrossRef]
- Wang, R.; Lu, X.; Sun, Q.; Gao, J.; Ma, L.; Huang, J. Novel ACE Inhibitory Peptides Derived from Simulated Gastrointestinal Digestion in Vitro of Sesame (Sesamum indicum L.) Protein and Molecular Docking Study. Int. J. Mol. Sci. 2020, 21, 1059. [Google Scholar] [CrossRef]
- Sousa, R.; Portmann, R.; Dubois, S.; Recio, I.; Egger, L. Protein digestion of di ff erent protein sources using the INFOGEST static digestion model. Food Res. Int. 2020, 130, 108996. [Google Scholar] [CrossRef] [PubMed]
- Giromini, C.; Cheli, F.; Rebucci, R.; Baldi, A. Invited review: Dairy proteins and bioactive peptides: Modeling digestion and the intestinal barrier. J. Dairy Sci. 2019, 102, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Karaś, M.; Baraniak, B.; Jakubczyk, A. Evaluation of ACE, α-glucosidase, and lipase inhibitory activities of peptides obtained by in vitro digestion of selected species of edible insects. Eur. Food Res. Technol. 2020, 246, 1361–1369. [Google Scholar] [CrossRef]
- Bryan, D.D.S.L.; Abbott, D.A.; Classen, H.L. Digestion kinetics of protein sources determined using an in vitro chicken model. Anim. Feed Sci. Technol. 2019, 248, 106–113. [Google Scholar] [CrossRef]
- Ko, S.; Kang, N.; Kim, E.; Cheol, M.; Lee, S.; Kang, S.; Lee, J.; Jeon, B.; Kim, S.; Park, S.; et al. A novel angiotensin I-converting enzyme (ACE) inhibitory peptide from a marine Chlorella ellipsoidea and its antihypertensive effect in spontaneously hypertensive rats. Process Biochem. 2012, 47, 2005–2011. [Google Scholar] [CrossRef]
- Marques, C.; Manuela, M.; Odila, J.; Guardão, L. In vitro ACE-inhibitory peptide KGYGGVSLPEW facilitates noradrenaline release from sympathetic nerve terminals: Relationship with the lack of antihypertensive effect on spontaneous hypertensive rats. Peptides 2017, 71, 72–76. [Google Scholar] [CrossRef]
- Hou, X.; Guan, X.; Cao, Y.; Weng, Z.; Hu, Q.; Liu, H.; Jia, S. Inhibition of pancreatic lipase by the constituents in St. John ’ s Wort: In vitro and in silico investigations. Int. J. Biol. Macromol. 2020, 145, 620–633. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef]
- Man, B.; Cheung, Y.; Cheung, T.T.; Samaranayake, N.R. Safety of antiobesity drugs. Ther. Adv. Drug Saf. 2013, 1–11. [Google Scholar] [CrossRef]
- Hüttl, C.; Hettrich, C.; Miller, R.; Paulke, B.-R.; Henklein, P.; Rawel, H.M.; Bier, F.F. Self-assembled peptide amphiphiles function as multivalent binder with increased hemagglutinin affinity. BMC Biotechnol. 2013, 13, 51. [Google Scholar] [CrossRef]
- Stefanucci, A.; Dimmito, M.P.; Zengin, G.; Luisi, G.; Mirzaie, S.; Novellino, E.; Mollica, A. Discovery of novel amide tripeptides as pancreatic lipase inhibitors by virtual screening. R. Soc. Chem. 2019, 1, 1–3. [Google Scholar] [CrossRef]
- Lunder, M.; Bratkovi, T.; Kreft, S.; Štrukelj, B. Peptide inhibitor of pancreatic lipase selected by phage display using different elution strategies. J. Lipid Res. 2017, 21, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Villaluenga, C.; Rupasinghe, S.G.; Schuler, M.A.; De, E.G. Peptides from purified soybean b -conglycinin inhibit fatty acid synthase by interaction with the thioesterase catalytic domain. FEBS J. 2010, 277, 1481–1493. [Google Scholar] [CrossRef] [PubMed]
- Ramadhan, A.H.; Nawas, T.; Zhang, X.; Pembe, M.; Xia, W.; Xu, Y. Purification and identification of a novel antidiabetic peptide from Chinese giant salamander (Andrias davidianus) protein hydrolysate against α -amylase and α -glucosidase. Int. J. Food Prop. 2018, 2912, 1–13. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, R.; Chen, X.; Zeng, Z.; Ma, H.; Chen, S. Dipeptidyl Peptidase IV-Inhibitory Peptides Derived from Silver Carp (Hypophthalmichthys molitrix Val.) Proteins. J. Agric. Food Chem. 2016, 13, 1–9. [Google Scholar] [CrossRef]
- Kumar, T.V.; Lakshmanasenthil, S.; Geetharamani, D.; Marudhupandi, T.; Suja, G.; Suganya, P. Fucoidan—A -d-glucosidase inhibitor from Sargassum wightii with relevance to type 2 diabetes mellitus therapy. Int. J. Biol. Macromol. 2015, 72, 1044–1047. [Google Scholar] [CrossRef]
- Kim, K.; Rioux, L.; Turgeon, S.L. Phytochemistry Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef]
- Lakshmanasenthil, S.; Vinothkumar, T.; Geetharamani, D.; Marudhupandi, T. Fucoidan—A novel α -amylase inhibitor from Turbinaria ornata with relevance to type 2 diabetes mellitus therapy. Biocatal. Agric. Biotechnol. 2014, 1–5. [Google Scholar] [CrossRef]
- Cho, M.; Han, J.H.; You, S. Inhibitory effects of fucan sulfates on enzymatic hydrolysis of starch. LWT Food Sci. Technol. 2011, 44, 1164–1171. [Google Scholar] [CrossRef]
- Wang, J.; Wu, T.; Fang, L.; Liu, C.; Liu, X.; Li, H.; Shi, J.; Li, M.; Min, W. Anti-diabetic ef ect by walnut (Juglans mandshurica Maxim.)-derived peptide LPLLR through inhibiting αglucosidase and α-amylase, and alleviating insulin resistance of hepatic HepG2 cells. J. Funct. Foods 2020, 69, 103944. [Google Scholar] [CrossRef]
- Saufi, B.; Nurul, S.; Afifah, H.; Gan, C. Antioxidative and Amylase Inhibitor Peptides from Basil Seeds. Int. J. Pept. Res. Ther. 2015, 1–8. [Google Scholar]
- Wang, R.; Zhao, H.; Pan, X.; Orfila, C.; Lu, W.; Ma, Y. Preparation of bioactive peptides with antidiabetic, antihypertensive, and antioxidant activities and identification of α-glucosidase inhibitory peptides from soy protein. Food Sci. Nutr. 2019, 7, 1848–1856. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim Mohamed, B.M.; Neitz, A.W. Gaspar Structural properties of bioactive peptides with α-glucosidase inhibitory activity. Chem. Biol. Drug Des. 2018, 91, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Asoodeh, A.; Homayouni-tabrizi, M.; Shabestarian, H. ScienceDirect Biochemical characterization of a novel antioxidant and angiotensin I-converting enzyme inhibitory peptide from Struthio camelus egg white protein hydrolysis. J. Food Drug Anal. 2015, 24, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Gong, Y.; Li, Z.; Yu, D.; Chi, C.; Ma, J. Isolation and characterisation of five novel antioxidant peptides from ethanol-soluble proteins hydrolysate of spotless smoothhound (Mustelus griseus) muscle. J. Funct. Foods 2014, 6, 176–185. [Google Scholar] [CrossRef]
- Moronta, J.; Smaldini, P.L.; Docena, G.H.; Añón, M.C. Peptides of amaranth were targeted as containing sequences with potential anti-inflammatory properties. J. Funct. Foods 2016, 21, 463–473. [Google Scholar] [CrossRef]
- Song, J.J.; Wang, Q.; Du, M.; Ji, X.M.; Mao, X.Y. Identification of dipeptidyl peptidase-IV inhibitory peptides from mare whey protein hydrolysates. J. Dairy Sci. 2017, 100, 6885–6894. [Google Scholar] [CrossRef] [PubMed]
- Shwaiki, L.N.; Arendt, E.K.; Lynch, K.M.; Thery, T.L.C. International Journal of Food Microbiology Inhibitory effect of four novel synthetic peptides on food spoilage yeasts. Int. J. Food Microbiol. 2019, 300, 43–52. [Google Scholar] [CrossRef]
- Villadóniga, C.; María, A.; Cantera, B. New ACE-inhibitory peptides derived from α -lactalbumin produced by hydrolysis with Bromelia antiacantha peptidases. Biocatal. Agric. Biotechnol. 2019, 20, 101258. [Google Scholar] [CrossRef]
- Balti, R.; Nedjar-arroume, N.; Bougatef, A.; Guillochon, D.; Nasri, M. Three novel angiotensin I-converting enzyme (ACE) inhibitory peptides from cuttlefish (Sepia officinalis ) using digestive proteases. Food Res. Int. 2010, 43, 1136–1143. [Google Scholar] [CrossRef]
- Zhang, P.; Roytrakul, S.; Sutheerawattananonda, M. Production and purification of glucosamine and angiotensin-I converting enzyme (ACE) inhibitory peptides from mushroom hydrolysates. J. Funct. Foods 2017, 36, 72–83. [Google Scholar] [CrossRef]
- Cao, X.; Lyu, Y.; Ning, J.; Tang, X.; Shen, X. Synthetic peptide, Ala-Arg-Glu-Gly-Glu-Met, abolishes pro- proliferative and anti-apoptotic effects of high glucose in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2017, 485, 215–220. [Google Scholar] [CrossRef]
- Joy, O.; Liu, L.; Zhang, S.; Lu, J.; Pang, X.; Lv, J. α-Glucosidase and ACE dual inhibitory protein hydrolysates and peptide fractions of sprouted quinoa yoghurt beverages inoculated with Lactobacillus casei. Food Chem. 2019, 299, 124985. [Google Scholar]
- Montoya-Rodríguez, A.; González, E.; Mejía, D. Pure peptides from amaranth (Amaranthus hypochondriacus) proteins inhibit LOX-1 receptor and cellular markers associated with atherosclerosis development in vitro. Food Res. Int. 2015, 77, 204–214. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 1–13. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Wong, F.C.; Xiao, J.; Wang, S.; Ee, K.Y.; Chai, T.T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- Selamassakul, O.; Laohakunjit, N. Bioactive peptides from brown rice protein hydrolyzed by bromelain: Relationship between biofunctional activities and flavor characteristics. J. Food Sci. 2020, 1, 1–11. [Google Scholar] [CrossRef]
- Gallego, M.; Mauri, L.; Aristoy, M.C.; Toldrá, F.; Mora, L. Antioxidant peptides pro fi le in dry-cured ham as a ff ected by gastrointestinal digestion. J. Funct. Foods 2020, 69, 103956. [Google Scholar] [CrossRef]
- Toldrá, F.; Gallego, M.; Reig, M.; Aristoy, M. Bioactive peptides generated in the processing of dry-cured ham. Food Chem. 2020, 321, 126689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, H.; Zhang, G.; Kong, F.; Hu, Y.; Xiong, S.; Zhao, S. Identification and characterization of novel antioxidant peptides from crucian carp (Carassius auratus) cooking juice released in simulated gastrointestinal digestion by UPLC-MS/MS and in silico analysis. J. Chromatogr. B. 2019, 1136, 121893. [Google Scholar] [CrossRef] [PubMed]
- Jakubczyk, A.; Karaś, M.; Złotek, U.; Szymanowska, U. Identification of potential inhibitory peptides of enzymes involved in the metabolic syndrome obtained by simulated gastrointestinal digestion of fermented bean (Phaseolus vulgaris L.) seeds. Food Res. Int. 2017, 100, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Cai, S.; Gao, M.; Chu, X.; Pan, X.; Gong, K.; Xiao, C.; Chen, Y.; Zhao, Y.; Wang, B.; et al. Purification of antioxidant peptides of Moringa oleifera seeds and their protective effects on H2O2 oxidative damaged Chang liver cells. J. Funct. Foods 2019, 64, 103698. [Google Scholar] [CrossRef]
- Phongthai, S.; Rawdkuen, S. Fractionation and characterization of antioxidant peptides from rice bran protein hydrolysates stimulated by. Cereal Chem. 2019, 97, 316–325. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein - derived antioxidant peptides. J. Food Bioc. 2019, 43, e12761. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, L.; Sun, Q.; Song, G.; Huang, J. Extraction, identification and structure-activity relationship of antioxidant peptides from sesame (Sesamum indicum L.) protein hydrolysate. Food Res. Int. 2018, 116, 707–7016. [Google Scholar] [CrossRef] [PubMed]
- Najafian, L.; Babji, A.S. Purification and Identification of Antioxidant Peptides from Fermented Fish Sauce (Budu) Purification and Identification of Antioxidant Peptides from. J. Aquat. Food Prod. Technol. 2018, 8850, 1–12. [Google Scholar]
- Yang, J.; Huang, J.; Dong, X.; Zhang, Y.; Zhou, X.; Huang, M.; Zhou, G. Purification and identification of antioxidant peptides from duck plasma proteins. Food Chem. 2020, 126534. [Google Scholar] [CrossRef]
- Wu, D.; Sun, N.; Ding, J.; Zhu, B.; Lin, S. Evaluation and structure-activity relationship analysis of antioxidant shrimp peptides. Food Funct. 2019, 10, 5605–5615. [Google Scholar] [CrossRef]
- García-Mora, P.; Martín-Martínez, M.; Bonache, M.A.; González-Múniz, R.; Peñas, E.; Frias, J.; Martínez-, C. Identification, functional gastrointestinal stability and molecular docking studies of lentil peptides with dual antioxidant and angiotensin I converting enzyme inhibitory activities. Food Chem. 2016, 221, 464–472. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, H.; Wang, Y.; Yu, Y.; Liu, J.; Liu, B.; Zhang, T. Identification of antioxidant peptides derived from egg-white protein and its protective effects on H2O2-induced cell damage. Int. J. Food Sci. Technol. 2019, 54, 2219–2227. [Google Scholar] [CrossRef]
- Sheng, J.; Yang, X.; Chen, J.; Peng, T.; Yin, X.; Liu, W.; Liang, M.; Wan, J.; Yang, X. Antioxidative E ff ects and Mechanism Study of Bioactive Peptides from Defatted Walnut (Juglans regia L.) Meal Hydrolysate. J. Agric. Food Chem. 2019, 67, 3305–3312. [Google Scholar] [CrossRef] [PubMed]
- Matsui, R.; Honda, R.; Kanome, M.; Hagiwara, A.; Matsuda, Y.; Togitani, T. Designing antioxidant peptides based on the antioxidant properties of the amino acid side-chains. Food Chem. 2018, 245, 750–755. [Google Scholar] [CrossRef]
- Agrawal, H.; Joshi, R.; Gupta, M. Purification, identification and characterization of two novel antioxidant peptides from finger millet (Eleusine coracana) protein hydrolysate. Food Res. Int. 2018, 120, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Shi, C.; Zhou, C.; Sun, X.; Ang, Y.; Dong, X.; Huang, M.; Zhou, G. Purification and characterization of novel antioxidant peptides from duck breast protein hydrolysates. LWT Food Sci. Technol. 2020, 109215. [Google Scholar] [CrossRef]
- Sun, X.; Acquah, C.; Aluko, R.E.; Udenigwe, C.C. Considering food matrix and gastrointestinal e ff ects in enhancing bioactive peptide absorption and bioavailability. J. Funct. Foods 2020, 64, 103680. [Google Scholar] [CrossRef]
- Song, W.; Kong, X.; Hua, Y.; Li, X.; Zhang, C. Antioxidant and antibacterial activity and in vitro digestion stability of cottonseed protein hydrolysates. LWT Food Sci. Technol. 2020, 118, 108724. [Google Scholar] [CrossRef]
- Xu, F.; Zhang, J.; Wang, Z.; Yao, Y.; Atungulu, G.G.; Ju, X.; Wang, L. Absorption and Metabolism of Peptide WDHHAPQLR Derived from Rapeseed Protein and Inhibition of HUVECs. J. Agric. Food Chem. 2018, 66, 5178–5189. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, J.; Yin, Z.; Li, H. Evaluation of antioxidant peptides generated from Jiuzao (residue after Baijiu distillation) protein hydrolysates and their effect of enhancing healthy value of Chinese Baijiu. Soc. Chem. Ind. 2019, 100, 59–73. [Google Scholar] [CrossRef]
- Tonolo, F.; Fiorese, F.; Moretto, L.; Folda, A.; Scalcon, V.; Grinzato, A.; Ferro, S.; Arrigoni, G.; Bindoli, A.; Feller, E.; et al. Identification of New Peptides from Fermented Milk Showing Antioxidant Properties: Mechanism of Action. Antioxidants 2020, 9, 117. [Google Scholar] [CrossRef]
- He, R.; Wang, Y.; Yang, Y.; Wang, Z.; Ju, X.; Yuan, J. Rapeseed protein-derived ACE inhibitory peptides LY, RALP and GHS show antioxidant and anti-inflammatory effects on spontaneously hypertensive rats. J. Funct. Foods 2019, 55, 211–219. [Google Scholar] [CrossRef]
- Ballatore, M.B.; Bettiol, R.; Braber, N.L.V.; Aminahuel, C.A.; Rossi, Y.E.; Petroselli, G.; Erra, R.; Cavaglieri, L.R.; Montenegro, M.A. Antioxidant and cytoprotective effect of peptides produced by hydrolysis of whey protein concentrate with trypsin. Food Chem. 2020, 319, 126472. [Google Scholar] [CrossRef] [PubMed]
- Minkiewicz, P.; Iwaniak, A. BIOPEP-UWM Database of Bioactive Peptides: Current Opportunities. Int. J. Mole 2019, 20, 5978. [Google Scholar] [CrossRef] [PubMed]
- Salas, C.E.; Badillo-Corona, J.; Ramírez-Sotelo, G.; Oliver-Salvador, M.D.C. Biologically Active and Antimicrobial Peptides from Plants. BioMed Res. Int. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Amadou, I.; Le, G.-W.; Amza, T.; Sun, J.; Shi, Y.-H. Purification and characterization of foxtail millet-derived peptides with antioxidant and antimicrobial activities. Food Res. Int. 2013, 51, 422–428. [Google Scholar] [CrossRef]
- Lei, J.; Sun, L.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931. [Google Scholar] [PubMed]
- Bahar, A.A.; Ren, D. Antimicrobial Peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef]
- Pelegrini, P.B.; Del Sarto, R.P.; Silva, O.N.; Franco, O.L.; Grossi-De-Sá, M.F. Antibacterial Peptides from Plants: What They Are and How They Probably Work. Biochem. Res. Int. 2011, 2011, 1–9. [Google Scholar] [CrossRef]
- Lum, K.Y.; Tay, S.T.; Le, C.F.; Lee, V.S.; Sabri, N.H.; Velayuthan, R.D.; Hassan, H.; Sekaran, S.D. Activity of Novel Synthetic Peptides against Candida albicans. Sci. Rep. 2015, 5, 1–12. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef]
- Barreto-Santamaría, A.; Hernando, C.; Gabriela, A.-P.; Chonny Herrera, D.S.; Pérez, W.H.; Patarroyo, M.E. A New Synthetic Peptide Having Two Target of Antibacterial Action in. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef]
- Oliver-Salvador, M.D.E.L.C.; Ariza-Ortega, T.D.J.; Zenón-briones, Y.; Luis, J. Angiotensin-I-converting enzyme inhibitory, antimicrobial, and antioxidant effect of bioactive peptides obtained from different varieties of common beans (Phaseolus vulgaris L.) with in vivo antihypertensive activity in spontaneously hypertensive rats. Eur. Food Res. Technol. 2014, 239, 785–794. [Google Scholar]
- Cusimano, M.G.; Spinello, A.; Barone, G.; Schillaci, D.; Cascioferro, S.; Magistrato, A.; Parrino, B.; Arizza, V.; Vitale, M. A Synthetic Derivative of Antimicrobial Peptide Holothuroidin 2 from Mediterranean Sea Cucumber (Holothuria tubulosa ) in the Control of Listeria monocytogenes. Mar. Drugs 2019, 17, 159. [Google Scholar] [CrossRef]
- Tong, Y.; Tang, J. Candida albicans infection and intestinal immunity. Microbiol. Res. 2017, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Satana, D.; Genc, G.E.; Erturan, Z. The antifungal susceptibilities of oral Candida spp isolates from HIV-infected patients. Afr. J. Microbiol. Res. 2010, 4, 466–470. [Google Scholar]
- Boas, L.; Campos, M.; Lorrayne, R.; Berlanda, A. Antiviral peptides as promising therapeutic drugs. Cell. Mol. Life Sci. 2019, 17, 1–18. [Google Scholar]
- Ngai, P.H.K.; Ng, T.B. Phaseococcin, an antifungal protein with antiproliferative and anti-HIV-1 reverse transcriptase activities from small scarlet runner beans. Biochem. Cell Biol. 2005, 220, 212–220. [Google Scholar] [CrossRef]
- Wong, J.H.; Ng, T.B. Sesquin, a potent defensin-like antimicrobial peptide from ground beans with inhibitory activities toward tumor cells and HIV-1 reverse transcriptase. Peptides 2005, 26, 1120–1126. [Google Scholar] [CrossRef]
- Karaś, M.; Jakubczyk, A.; Szymanowska, U.; Złotek, U.; Zielińska, E. Digestion and bioavailability of bioactive phytochemicals. Int. J. Food Sci. Technol. 2017, 52, 291–305. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Unlocking the biological potential of proteins from edible insects through enzymatic hydrolysis: A review. Innov. Food Sci. Emerg. Technol. 2017, 43, 239–252. [Google Scholar] [CrossRef]
- Vercruysse, L.; Smagghe, G.; Herregods, G.; Van Camp, J. ACE inhibitory activity in enzymatic hydrolysates of insect protein. J. Agric. Food Chem. 2005, 53, 5207–5211. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Karaś, M.; Jakubczyk, A.; Zieliński, D.; Baraniak, B. Edible Insects as Source of Proteins; Mérillon, J.M., Ramawat, K., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Tao, M.; Wang, C.; Liao, D.; Liu, H.; Zhao, Z.; Zhao, Z. Purification, modification and inhibition mechanism of angiotensin I-converting enzyme inhibitory peptide from silkworm pupa (Bombyx mori) protein hydrolysate. Process Biochem. 2017, 54, 172–179. [Google Scholar] [CrossRef]
- Wang, W.; Wang, N.; Liu, C.; Jin, J. Effect of Silkworm Pupae Peptide on the Fermentation and Quality of Yogurt. J. Food Process. Preserv. 2017, 41, e12893. [Google Scholar] [CrossRef]
- Jia, J.; Wu, Q.; Yan, H.; Gui, Z. Purification and molecular docking study of a novel angiotensin-I converting enzyme (ACE) inhibitory peptide from alcalase hydrolysate of ultrasonic-pretreated silkworm pupa (Bombyx mori) protein. Process Biochem. 2015, 50, 876–883. [Google Scholar] [CrossRef]
- Wu, Q.; Jia, J.; Yan, H.; Du, J.; Gui, Z. A novel angiotensin-I converting enzyme (ACE) inhibitory peptide from gastrointestinal protease hydrolysate of silkworm pupa (Bombyx mori) protein: Biochemical characterization and molecular docking study. Peptides 2015, 68, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hall, F.; Liceaga, A. Effect of microwave-assisted enzymatic hydrolysis of cricket (Gryllodes sigillatus) protein on ACE and DPP-IV inhibition and tropomyosin-IgG binding. J. Funct. Foods 2020, 64, 103634. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, N.; Wang, W.; Wang, J.; Zhu, Z.; Li, X. Molecular mechanisms of novel peptides from silkworm pupae that inhibit α-glucosidase. Peptides 2016, 76, 45–50. [Google Scholar] [CrossRef]
- Dutta, P.; Sahu, R.K.; Dey, T.; Lahkar, M.D.; Manna, P.; Kalita, J. Beneficial role of insect-derived bioactive components against inflammation and its associated complications (colitis and arthritis) and cancer. Chem. Biol. Interact. 2019, 313, 108824. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M. Antioxidant and anti-inflammatory activities of hydrolysates and peptide fractions obtained by enzymatic hydrolysis of selected heat-treated edible insects. Nutrients 2017, 9, 1–14. [Google Scholar] [CrossRef]
- Faruck, M.O.; Yusof, F.; Chowdhury, S. An overview of antifungal peptides derived from insect. Peptides 2016, 80, 80–88. [Google Scholar] [CrossRef]
- de Castro, R.J.S.; Ohara, A.; Aguilar, J.G.d.S.; Domingues, M.A.F. Nutritional, functional and biological properties of insect proteins: Processes for obtaining, consumption and future challenges. Trends Food Sci. Technol. 2018, 76, 82–89. [Google Scholar] [CrossRef]
- Jantzen da Silva Lucas, A.; Menegon de Oliveira, L.; da Rocha, M.; Prentice, C. Edible insects: An alternative of nutritional, functional and bioactive compounds. Food Chem. 2020, 311. [Google Scholar] [CrossRef]
- Rahnamaeian, M.; Cytryńska, M.; Zdybicka-Barabas, A.; Dobslaff, K.; Wiesner, J.; Twyman, R.M.; Zuchner, T.; Sadd, B.M.; Regoes, R.R.; Schmid-Hempel, P.; et al. Insect antimicrobial peptides show potentiating functional interactions against Gram-negative bacteria. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150293. [Google Scholar] [CrossRef]
- Da Rocha, M.; Alemán, A.; Baccan, G.C.; López-Caballero, M.E.; Gómez-Guillén, C.; Montero, P.; Prentice, C. Anti-Inflammatory, Antioxidant, and Antimicrobial Effects of Underutilized Fish Protein Hydrolysate. J. Aquat. Food Prod. Technol. 2018, 27, 592–608. [Google Scholar] [CrossRef]
- Liu, Y.; Wan, S.; Liu, J.; Zou, Y.; Liao, S. Antioxidant Activity and Stability Study of Peptides from Enzymatically Hydrolyzed Male Silkmoth. J. Food Process. Preserv. 2017, 41, e13081. [Google Scholar] [CrossRef]
- Hall, F.; Johnson, P.E.; Liceaga, A. Effect of enzymatic hydrolysis on bioactive properties and allergenicity of cricket (Gryllodes sigillatus) protein. Food Chem. 2018, 262, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Karaś, M.; Jakubczyk, A. Antioxidant activity of predigested protein obtained from a range of farmed edible insects. Int. J. Food Sci. Technol. 2017, 52, 306–312. [Google Scholar] [CrossRef]
- Sasidharan, A.; Venugopal, V. Proteins and Co-products from Seafood Processing Discards: Their Recovery, Functional Properties and Applications. Waste Biomass. Valor. 2019. [Google Scholar] [CrossRef]
- Maestri, E.; Pavlicevic, M.; Montorsi, M.; Marmiroli, N. Meta-Analysis for Correlating Structure of Bioactive Peptides in Foods of Animal Origin with Regard to Effect and Stability. Compr. Rev. Food Sci. Food Saf. 2018, 18, 3–30. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Guha, S.; Majumder, K. Food-derived bioactive peptides in human health: Challenges and opportunities. Nutrients 2018, 10, 1–17. [Google Scholar] [CrossRef]
- Nikoo, M.; Benjakul, S. Potential application of seafood-derived peptides as bifunctional ingredients, antioxidant-cryoprotectant: A review. J. Funct. Foods 2015, 19, 753–764. [Google Scholar] [CrossRef]
- Lee, J.K.; Patel, S.K.S.; Sung, B.H.; Kalia, V.C. Biomolecules from municipal and food industry wastes: An overview. Bioresour. Technol. 2020, 298, 122346. [Google Scholar] [CrossRef]
- Fang, Z.; Xu, L.; Lin, Y.; Cai, X.; Wang, S. The preservative potential of Octopus scraps peptides−Zinc chelate against Staphylococcus aureus: Its fabrication, antibacterial activity and action mode. Food Control 2019, 98, 24–33. [Google Scholar] [CrossRef]
- Shaviklo, A.R.; Rezapanah, S.; Motamedzadegan, A.; Damavandi-Kamali, N.; Mozafari, H. Optimum conditions for protein extraction from tuna processing by-products using isoelectric solubilization and precipitation processes. Iran. J. Fish. Sci. 2017, 16, 774–792. [Google Scholar]
- Nikoo, M.; Xu, X.; Gavlighi, H.A. Seafood Waste-Derived Peptides: Their Antioxidant Activity and Potential as Alternative Preservatives in Fish Products. In Protein Byproducts: Transformation from Environmental Burden Into Value-Added Products; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 315–332. ISBN 9780128026113. [Google Scholar]
- Bruno, S.F.; Ekorong, F.J.A.A.; Karkal, S.S.; Cathrine, M.S.B.; Kudre, T.G. Green and innovative techniques for recovery of valuable compounds from seafood by-products and discards: A review. Trends Food Sci. Technol. 2019, 85, 10–22. [Google Scholar] [CrossRef]
- Mangano, V.; Gervasi, T.; Rotondo, A.; De Pasquale, P.; Dugo, G.; Macrì, F.; Salvo, A. Protein hydrolysates from anchovy waste: Purification and chemical characterization. Nat. Prod. Res. 2019, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-R.; Zhao, Y.-Q.; Qiu, Y.-T.; Chi, C.-F.; Wang, B. Preparation and Characterization of Gelatin and Antioxidant Peptides from Gelatin Hydrolysate of Skipjack Tuna (Katsuwonus pelamis) Bone Stimulated by in vitro Gastrointestinal Digestion. Mar. Drugs 2019, 17, 78. [Google Scholar] [CrossRef] [PubMed]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Wang, Y.M.; Zhang, B.; Deng, S.G. Isolation and characterization of three antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) heads. J. Funct. Foods 2015, 12, 1–10. [Google Scholar] [CrossRef]
- Chi, C.F.; Wang, B.; Hu, F.Y.; Wang, Y.M.; Zhang, B.; Deng, S.G.; Wu, C.W. Purification and identification of three novel antioxidant peptides from protein hydrolysate of bluefin leatherjacket (Navodon septentrionalis) skin. Food Res. Int. 2015, 73, 124–129. [Google Scholar] [CrossRef]
- Lassoued, I.; Mora, L.; Nasri, R.; Jridi, M.; Toldrá, F.; Aristoy, M.C.; Barkia, A.; Nasri, M. Characterization and comparative assessment of antioxidant and ACE inhibitory activities of thornback ray gelatin hydrolysates. J. Funct. Foods 2015, 13, 225–238. [Google Scholar] [CrossRef]
- Umayaparvathi, S.; Meenakshi, S.; Vimalraj, V.; Arumugam, M.; Sivagami, G.; Balasubramanian, T. Antioxidant activity and anticancer effect of bioactive peptide from enzymatic hydrolysate of oyster (Saccostrea cucullata). Biomed. Prev. Nutr. 2014, 4, 343–353. [Google Scholar] [CrossRef]
- Hajji, S.; Younes, I.; Rinaudo, M.; Jellouli, K.; Nasri, M. Characterization and In Vitro Evaluation of Cytotoxicity, Antimicrobial and Antioxidant Activities of Chitosans Extracted from Three Different Marine Sources. Appl. Biochem. Biotechnol. 2015, 177, 18–35. [Google Scholar] [CrossRef]
- Cai, L.; Wu, X.; Lv, Y.; Xu, Y.; Mi, G.; Li, J. The neuroprotective and antioxidant activities of protein hydrolysates from grass carp (Ctenopharyngodon idella) skin. J. Food Sci. Technol. 2015, 52, 3750–3755. [Google Scholar] [CrossRef][Green Version]
- Harnedy, P.A.; Parthsarathy, V.; McLaughlin, C.M.; O’Keeffe, M.B.; Allsopp, P.J.; McSorley, E.M.; O’Harte, F.P.M.; FitzGerald, R.J. Atlantic salmon (Salmo salar) co-product-derived protein hydrolysates: A source of antidiabetic peptides. Food Res. Int. 2018, 106, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Neves, A.C.; Harnedy, P.A.; O’Keeffe, M.B.; FitzGerald, R.J. Bioactive peptides from Atlantic salmon (Salmo salar) with angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory, and antioxidant activities. Food Chem. 2017, 218, 396–405. [Google Scholar] [CrossRef]
- Kang, N.; Ko, S.-C.; Kim, H.-S.; Yang, H.-W.; Ahn, G.; Lee, S.-C.; Lee, T.-G.; Lee, J.-S.; Jeon, Y.-J. Structural Evidence for Antihypertensive Effect of an Antioxidant Peptide Purified from the Edible Marine Animal Styela clava. J. Med. Food 2020, 23, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Vo, T.S.; Ryu, B.M.; Kim, S.K. Angiotensin- I- converting enzyme (ACE) inhibitory peptides from Pacific cod skin gelatin using ultrafiltration membranes. Process Biochem. 2016, 51, 1622–1628. [Google Scholar] [CrossRef]
- Jin, R.; Teng, X.; Shang, J.; Wang, D.; Liu, N. Identification of novel DPP–IV inhibitory peptides from Atlantic salmon (Salmo salar) skin. Food Res. Int. 2020, 133, 109161. [Google Scholar] [CrossRef]
- Hou, H.; Fan, Y.; Wang, S.; Si, L.; Li, B. Immunomodulatory activity of Alaska pollock hydrolysates obtained by glutamic acid biosensor—Artificial neural network and the identification of its active central fragment. J. Funct. Foods 2016, 24, 37–47. [Google Scholar] [CrossRef]
- Li, W.; Xu, C.; Zhang, C.; Cao, W.; Qin, X.; Gao, J.; Zheng, H. The purification and identification of immunoregulatory peptides from oyster (: Crassostrea hongkongensis) enzymatic hydrolysate. RSC Adv. 2019, 9, 32854–32863. [Google Scholar] [CrossRef]
- Wald, M.; Schwarz, K.; Rehbein, H.; Bußmann, B.; Beermann, C. Detection of antibacterial activity of an enzymatic hydrolysate generated by processing rainbow trout by-products with trout pepsin. Food Chem. 2016, 205, 221–228. [Google Scholar] [CrossRef]
- Ennaas, N.; Hammami, R.; Beaulieu, L.; Fliss, I. Purification and characterization of four antibacterial peptides from protamex hydrolysate of Atlantic mackerel (Scomber scombrus) by-products. Biochem. Biophys. Res. Commun. 2015, 462, 195–200. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S.; Yesilsu, A.F. Antimicrobial Compounds from Crustaceans and Their Applications for Extending Shelf-Life of Marine-Based Foods. Turkish J. Fish. Aquat. Sci. 2020, 20. [Google Scholar] [CrossRef]
- Borkar, S.; Nandanwar, S.; Lee, J.; Kim, H. Characterization of Four Liver-Expressed Antimicrobial Peptides from Antarctic Fish and Their Antibacterial Activity. Appl. Sci. 2019, 9, 4299. [Google Scholar] [CrossRef]
- Shin, S.C.; Ahn, I.H.; Ahn, D.H.; Lee, Y.M.; Lee, J.; Lee, J.H.; Kim, H.W.; Park, H. Characterization of two antimicrobial peptides from antarctic fishes (notothenia coriiceps and parachaenichthys charcoti). PLoS ONE 2017, 12. [Google Scholar] [CrossRef][Green Version]
- Della Pelle, G.; Perà, G.; Belardinelli, M.C.; Gerdol, M.; Felli, M.; Crognale, S.; Scapigliati, G.; Ceccacci, F.; Buonocore, F.; Porcelli, F. Trematocine, a Novel Antimicrobial Peptide from the Antarctic Fish Trematomus bernacchii: Identification and Biological Activity. Antibiotics 2020, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-Y.; Liu, Z.-Y.; Zhao, J.; Xi, M.-Z.; Fu, Y.-H.; Zhang, T.; Ji, C.-F.; Zhu, B.-W. Antarctic Krill (Euphausia superba) Protein Hydrolysates Stimulate Cholecystokinin Release in STC-1 Cells and its Signaling Mechanism. J. Food Process. Preserv. 2017, 41, e12903. [Google Scholar] [CrossRef]
- Sae-leaw, T.; O’Callaghan, Y.C.; Benjakul, S.; O’Brien, N.M. Antioxidant, immunomodulatory and antiproliferative effects of gelatin hydrolysates from seabass (Lates calcarifer) skins. Int. J. Food Sci. Technol. 2016, 51, 1545–1551. [Google Scholar] [CrossRef]
- Sayari, N.; Sila, A.; Abdelmalek, B.E.; Abdallah, R.B.; Ellouz-Chaabouni, S.; Bougatef, A.; Balti, R. Chitin and chitosan from the Norway lobster by-products: Antimicrobial and anti-proliferative activities. Int. J. Biol. Macromol. 2016, 87, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, A.D.; Chen, X.; Yan, N.; Sperry, J. Towards the Shell Biorefinery: Sustainable Synthesis of the Anticancer Alkaloid Proximicin A from Chitin. ChemSusChem 2018, 11, 532–535. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Kim, E.A.; Lee, H.; Kim, H.S.; Lee, J.S.; Jeon, Y.J. Antihypertensive effect of surimi prepared from olive flounder (Paralichthys olivaceus) by angiotensin-I converting enzyme (ACE) inhibitory activity and characterization of ACE inhibitory peptides. Process Biochem. 2019, 80, 164–170. [Google Scholar] [CrossRef]
- Chen, J.; Ryu, B.; Zhang, Y.; Liang, P.; Li, C.; Zhou, C.; Yang, P.; Hong, P.; Qian, Z. Comparison of an angiotensin-I-converting enzyme inhibitory peptide from tilapia (Oreochromis niloticus) with captopril: Inhibition kinetics, in vivo effect, simulated gastrointestinal digestion and a molecular docking study. J. Sci. Food Agric. 2020, 100, 315–324. [Google Scholar] [CrossRef]
- Ishak, N.H.; Sarbon, N.M. A Review of Protein Hydrolysates and Bioactive Peptides Deriving from Wastes Generated by Fish Processing. Food Bioprocess Technol. 2018, 11, 2–16. [Google Scholar] [CrossRef]
- Thuanthong, M.; De Gobba, C.; Sirinupong, N.; Youravong, W.; Otte, J. Purification and characterization of angiotensin-converting enzyme-inhibitory peptides from Nile tilapia (Oreochromis niloticus) skin gelatine produced by an enzymatic membrane reactor. J. Funct. Foods 2017, 36, 243–254. [Google Scholar] [CrossRef]
- Cai, L.; Wu, X.; Zhang, Y.; Li, X.; Ma, S.; Li, J. Purification and characterization of three antioxidant peptides from protein hydrolysate of grass carp (Ctenopharyngodon idella) skin. J. Funct. Foods 2015, 16, 234–242. [Google Scholar] [CrossRef]
- Pal, G.K.; Suresh, P.V. Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredients. Innov. Food Sci. Emerg. Technol. 2016, 37, 201–215. [Google Scholar] [CrossRef]
- Singh, A.; Mittal, A.; Benjakul, S. Full Utilization of Squid Meat and Its Processing By-products: Revisit. Food Rev. Int. 2020, 1–25. [Google Scholar] [CrossRef]
- Nwachukwu, I.D.; Aluko, R.E. Anticancer and antiproliferative properties of food-derived protein hydrolysates and peptides. J. Food Bioact. 2019, 7. [Google Scholar] [CrossRef][Green Version]
- Yaghoubzadeh, Z.; Peyravii Ghadikolaii, F.; Kaboosi, H.; Safari, R.; Fattahi, E. Antioxidant Activity and Anticancer Effect of Bioactive Peptides from Rainbow Trout (Oncorhynchus mykiss) Skin Hydrolysate. Int. J. Pept. Res. Ther. 2020, 26, 625–632. [Google Scholar] [CrossRef]
- Zamora-Sillero, J.; Gharsallaoui, A.; Prentice, C. Peptides from Fish By-product Protein Hydrolysates and Its Functional Properties: An Overview. Mar. Biotechnol. 2018, 20, 118–130. [Google Scholar] [CrossRef]
- Liu, C.; Ren, D.; Li, J.; Fang, L.; Wang, J.; Liu, J.; Min, W. Cytoprotective e ff ect and puri fi cation of novel antioxidant peptides from hazelnut (C. heterophylla Fisch) protein hydrolysates. J. Funct. Foods 2018, 42, 203–215. [Google Scholar] [CrossRef]
- Hu, F.; Ci, A.; Wang, H.; Zhang, Y.; Zhang, J.; Thakur, K.; Wei, Z. Identi fication and hydrolysis kinetic of a novel antioxidant peptide from pecan meal using Alcalase. Food Chem. 2018, 261, 301–310. [Google Scholar] [CrossRef]
- Zhang, F.; Qu, J.; Thakur, K.; Zhang, J.; Mocan, A.; Wei, Z. Purification and identification of an antioxidative peptide from peony (Paeonia suff ruticosa Andr.) seed dreg. Food Chem. 2019, 285, 266–274. [Google Scholar] [CrossRef]
- González-garcía, E.; Puchalska, P.; Marina, M.L.; García, M.C. Fractionation and identification of antioxidant and angiotensin-converting enzyme-inhibitory peptides obtained from plum (Prunus domestica L.) stones. J. Funct. Foods 2015, 19, 376–384. [Google Scholar] [CrossRef]
- Wu, S.; Wang, X.; Qi, W.; Guo, Q. Bioactive protein/peptides of flaxseed: A review. Trends Food Sci. Technol. 2019, 92, 184–193. [Google Scholar] [CrossRef]
- Taniya, M.S.; Mv, R.; Ps, S.; Krishnan, G.; Priya, S. Food Bioscience Bioactive peptides from amaranth seed protein hydrolysates induced apoptosis and antimigratory e ff ects in breast cancer cells. Food Biosci. 2020, 35. [Google Scholar]
- Chim-Chi, Y.; Gallegos-Tintoré, S.; Jiménez-Martínez, C.; Dávila-Ortiz, G.; Chel-Guerrero, L. Antioxidant capacity of Mexican chia (Salvia hispanica L.) protein hydrolyzates. J. Food Meas. Charact. 2018, 12, 323–331. [Google Scholar] [CrossRef]
- Cotabarren, J.; Mabel, A.; Tellechea, M.; García-pardo, J.; Lorenzo, J.; David, W.; Graciela, M. Adding value to the chia (Salvia hispanica L.) expeller: Production of bioactive peptides with antioxidant properties by enzymatic hydrolysis with Papain. Food Chem. 2019, 274. [Google Scholar] [CrossRef]
| Sequence of Peptide | Source of Peptide | Activity | IC50 | Reference |
|---|---|---|---|---|
| WESLSRLLG | Ostrich egg white protein | ACE inhibitory | 46.7 µg/mL | Asoodeh et al. [65] |
| Antiradical against DPPH | 15 µg/mL | |||
| Antiradical against ABTS | 130 µg/mL | |||
| Anti-superoxide radical | 150 µg/mL | |||
| Anti-hydroxyl radical | 160 µg/mL | |||
| GAA GFVG GIISHR ELLI KFPE | Spotless smoothhound muscle | Antiradical against ABTS | 1.75 mg/mL 1.30 mg/mL, 0.34 mg/mL 0.32 mg/mL 0.46 mg/mL | Wang et al. [66] |
| SSEDIKE | Amaranth proteins | Anti-inflammatory activity | nd | Moronta et al. [67] |
| NMAINPSKENLCSTFCK | Casein proteins | ACE inhibitory | 129.07 μM | Tu et al. [40] |
| NLEIILR TQMVDEEIMELFR | Mare whey protein | Dipeptidyl peptidase-IV inhibitory | 86.34 μM 69.84 μM | Song et al. [68] |
| GGSK ELS | Red seaweed | α-amylase inhibitory | 2.58 mM 2.62 mM | Admassu et al. [68] |
| KKFFRAWWAPRFLK | Synthetic peptides | Inhibition of the yeast Zygosaccharomyces rouxii | MIC (400 μg/mL) | Shwaiki et al. [69] |
| TTFHTSGY GYDTQAIVQ | Whey protein | ACE inhibitory | 142 μM 1 mM | Villadóniga et al. [70] |
| YAP VIIF MAW | Cuttlefish muscle (Sepia officinalis) | ACE inhibitory | 6.1 µM 8.7 µM 16.32 µM | Balti et al. [71] |
| ASPYAFGL | Mushrooms | ACE inhibitory | 1.080 × 10−7 mol/L | Zhang et al. [72] |
| AREGEM | Synthetic peptide | Antioxidant | nd | Cao et al. [73] |
| LAHMIVAGA VAHPVF | Quinoa yoghurt beverages | α-glucosidase inhibitory | 127 mg/mL 10.39 mg/mL | Ujiroghene et al. [74] |
| HGSEPFGPR RGDPFPWPWYSH RPRYPWRYT | Amaranth proteins | LOX inhibitory | 11.5 µM >50 µM 17.3 µM | Montoya-Rodríguez et al. [75] |
| Sequences of Peptide | Antioxidant Methods | Source of Peptide | Antioxidant Activity Expressed as: IC 50; % or Trolox Equivalent | Reference |
|---|---|---|---|---|
| LDDPVFIH VAAGRTDAGVH | DPPH radical scavenging ABTS radical scavenging reducing power | fermented anchovy fish (Budu) extract | 0.84 mg/mL 1.45 mg/mL 0.617 mg/mL 0.795 mg/mL 0.702 0.422 | Najafian and Babji [72] |
| VVEVYLPR, VEVYLPR, VYLPR | ORAC | egg-white | 36.09 µM 41.05 µM 44.37 µM | Zhang et al. [76] |
| IREADIDGDGQVN, PEILPDGDHD, ASDEQDSVRL, APLEEPSSPH | DPPH radical scavenging DPPH radical scavenging DPPH radical scavenging Fe2+ chelating ability | crucian carp | 1.78 mM 1.18 mM 1.45 mM 0.09 mM | Zhang et al. [66] |
| TSSSLNMAVRGGLTR, STTVGLGISMRSASVR | DPPH radical scavenging | finger millet | 80.55% 75.1% | Agrawal et al. [79] |
| SYPTECRMR | DPPH radical scavenging ABTS radical scavenging | sesame | 0.105 mg/mL 0.004 mg/mL | Lu et al. (2019) [71] |
| QMDDQ | DPPH radical scavenging, hydroxyl radical-scavenging activities | shrimp | 0.5 mg/mL 1.0 mg/mL | Wu et al. [74] |
| EVGK, RCLQ | Fe2+ chelating ability reducing power, ABTS radical scavenging DPPH radical scavenging | duck plasma | 16.35% 0.62, 274.83 mM 95.12% | Yang et al. [73] |
| LAGNPHQQQQN and HNLDTQTESDV | hydroxyl radical scavenging or ROS reduction | walnut meal | - | Sheng et al. [66] |
| SF and QY | protective effects on 385 H2O2-induced Chang liver cells. | M. oleifera seed | - | Liang et al. [70] |
| LY, RALP and GHS | inhibited the production of ROS and lipid peroxide | rapeseed | - | He et al. [84] |
| WDHHAPQLR | model of Caco-2 cell monolayers and oxidative stress in HUVECs | rapeseed | - | Xu et al. [83] |
| NTVPAKSCQAQPTTM, EDELQDKIHPF, QGPIVLNPWDQVKR, APSFSDIPNPIGSENSE | model of Caco-2 cell | fermented milk | - | Tonolo et al. [85] |
| AGPSIVH, FLLPH, LLCVAV | DPPH radical scavenging ABTS radical scavenging reducing power | duck breast | 56.41% 0.6393 mmol TE/g 0.0651 | Li et al. [80] |
| LLSGTQNQPSFLSGF, NSLTLPILRYL, TLEPNSVFLPVLLH | ORAC | lentil storage proteins | 0.013 μmol TE/μmol 1.432 μmol TE/μmol 0.139 μmol TE/μmol | García-Mora et al. [75] |
| AYL AYI | ORAC | Jiuzao | 1.35 μmol TE/μmol 1.37 μmol TE/μmol | Jiang et al. [70] |
| Peptide fractions < 1 kDa | DPPH, ABTS, hydroxyl radical-scavenging activities | brown rice | 0.19 mM TE, 2.28 mM TE, 24.64 mM TE, | Selamassakul et al. [63] |
| Sequence of Peptide (Name) | Source of Peptide | Antimicrobial Activity | Reference |
|---|---|---|---|
| RYRRKKKMKKALQYIKLLKE (peptide 35,409) | synthetic peptide, analog from peptide 20,628 (321RYRRKKKMKKKLQYIKLLKE340) | inhibit growth of E. coli, S. aureus, P.aeruginosa | Barreto-Santamaría et al. [104] |
| ASHLGHHALDHLLK (H2) | Holothuria tubulosa | inhibit growth of L. monocytogenes | Cusimano et al. [105] |
| MRGSHHHHHHGSSGENLYFQSL (Tag) | synthetic peptide | inhibit growth of L. monocytogenes | Cusimano et al. [105] |
| GIWKKWIKKVVNVLKNLF-NH2 (KU2) | hybride peptides (KABT-AMP/Uperin 3.6) | inhibit growth of C. albicans | Lum et al. [110] |
| GIWKKWIKKWLNVLKNLF-NH2 (KU3) | hybride peptides (KABT-AMP/Uperin 3.6) | inhibit growth of C. albicans | Lum et al. [110] |
| KTCENLADTYKGPPPFFTTG (phaseococcin) | Phaseolus coccineus | inhibit HIV reverse transcriptase activity | Patrick et al. [111] |
| KTCENLADTY (sesquins) | Vigna sesquipedalis | inhibit HIV reverse transcriptase activity | Wong and Ng [112] |
| Sequence of Peptide | Source of Peptide | Activity | Reference |
|---|---|---|---|
| KHV | Bombyx mori | ACE inhibitory | Jia et al. [126] |
| ASL | Bombyx mori | ACE inhibitory | Wu et al. [127] |
| GNPWM | Bombyx mori | ACE inhibitory | Tao et al. [124] |
| Sequence of Peptide | Source of Peptide | Activity | Reference |
|---|---|---|---|
| Seafood by-products | |||
| GASSGMPG LAYA | Pacific cod (G. macrocephalus) | ACE inhibitory | Ngo et al. [161] |
| IVDR WYK VSAVI | olive flounder (P. olivaceus) surimi | ACE inhibitory | Oh et al. [175] |
| LSGYGP | tilapia (O. niloticus) skin | ACE inhibitory | Chen et al. [176] |
| LWHTH | tunicate (S. clava) | ACE inhibitory | Kang et al. [160] |
| YP | Atlantic salmon (S. salar) | DPP-IV inhibitory | Neves et al. [159] |
| WEGPK GPP GVPLT | Bluefin leatherjacket (N. septentrionalis) head | antioxidant | Chi et al. [152] |
| GSGGL GPGGFI FIGP | N. septentrionalis skin | antioxidant | Chi et al. [153] |
| GPDGR GADIVA GAPGPQMV AGPK GAEGFIF | skipjack tuna (K. pelamis) bones | antioxidant | Yang et al. [150] |
| GIV GAP*GF GFA*GPA SGNIGFP*GPK GIPGPIGPP*GRP | tilapia (O. niloticus) skin | antioxidant | Thuanthong et al. [178] |
| GIPGAP | thornback ray (R. clavata) skin | antioxidant | Lassoued et al. [154] |
| PYSFK GFGPEL VGGRP | grass carp (C. idella) skin | antioxidant | Cai et al. [157] Cai et al. [179] |
| Plants and seeds | |||
| ADGF AGGF AWDPE DWDPK ETTL SGAF | Wild hazelnut (C. heterophylla) | antioxidant | Liu et al. [185] |
| LAYLQYTDFETR | pecan meal | antioxidant | Hu et al. [186] |
| SMRKPPG | peony (P. suffruticos) seed | antioxidant | Zhang et al. [187] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jakubczyk, A.; Karaś, M.; Rybczyńska-Tkaczyk, K.; Zielińska, E.; Zieliński, D. Current Trends of Bioactive Peptides—New Sources and Therapeutic Effect. Foods 2020, 9, 846. https://doi.org/10.3390/foods9070846
Jakubczyk A, Karaś M, Rybczyńska-Tkaczyk K, Zielińska E, Zieliński D. Current Trends of Bioactive Peptides—New Sources and Therapeutic Effect. Foods. 2020; 9(7):846. https://doi.org/10.3390/foods9070846
Chicago/Turabian StyleJakubczyk, Anna, Monika Karaś, Kamila Rybczyńska-Tkaczyk, Ewelina Zielińska, and Damian Zieliński. 2020. "Current Trends of Bioactive Peptides—New Sources and Therapeutic Effect" Foods 9, no. 7: 846. https://doi.org/10.3390/foods9070846
APA StyleJakubczyk, A., Karaś, M., Rybczyńska-Tkaczyk, K., Zielińska, E., & Zieliński, D. (2020). Current Trends of Bioactive Peptides—New Sources and Therapeutic Effect. Foods, 9(7), 846. https://doi.org/10.3390/foods9070846







