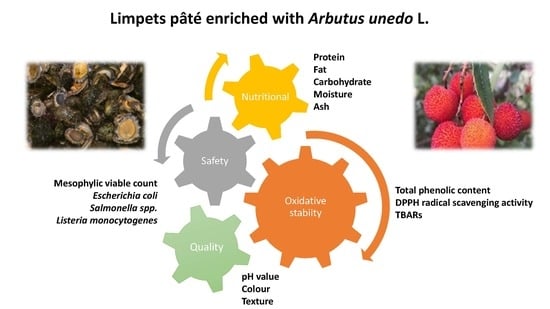
Impact of Aqueous Extract of Arbutus unedo Fruits on Limpets (Patella spp.) Pâté during Storage: Proximate Composition, Physicochemical Quality, Oxidative Stability, and Microbial Development
Abstract
1. Introduction
2. Materials and Methods
2.1. Location and Limpets Handling
2.2. Location and Preparation of Arbutus unedo Fruits Extracts
2.3. Evaluation of Proximate Composition, Physicochemical Properties, Oxidative Stability and Microbial Development of Limpets Pâté with Natural and Synthetic Antioxidant Additives
2.3.1. Processing of Limpets Pâté Enriched with Natural and Synthetic Antioxidant Additives
2.3.2. Proximate Composition
2.3.3. Physicochemical Quality
2.3.4. Antioxidant Capacity and Oxidative Stability
2.3.5. Microbial Development
2.4. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Proximate Composition of Limpets Pâté
3.2. Evaluation of Physicochemical Quality of Limpets Pâté
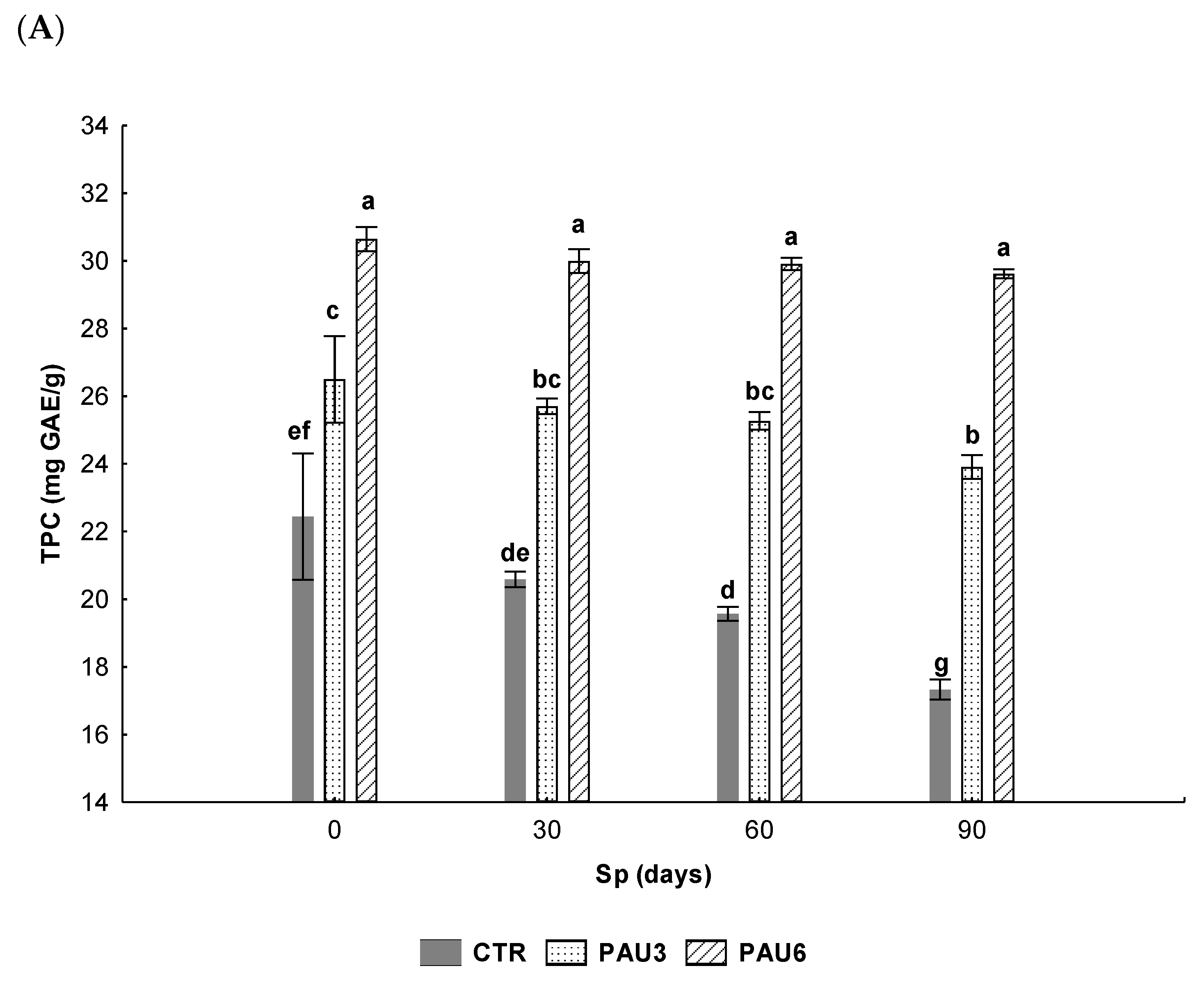
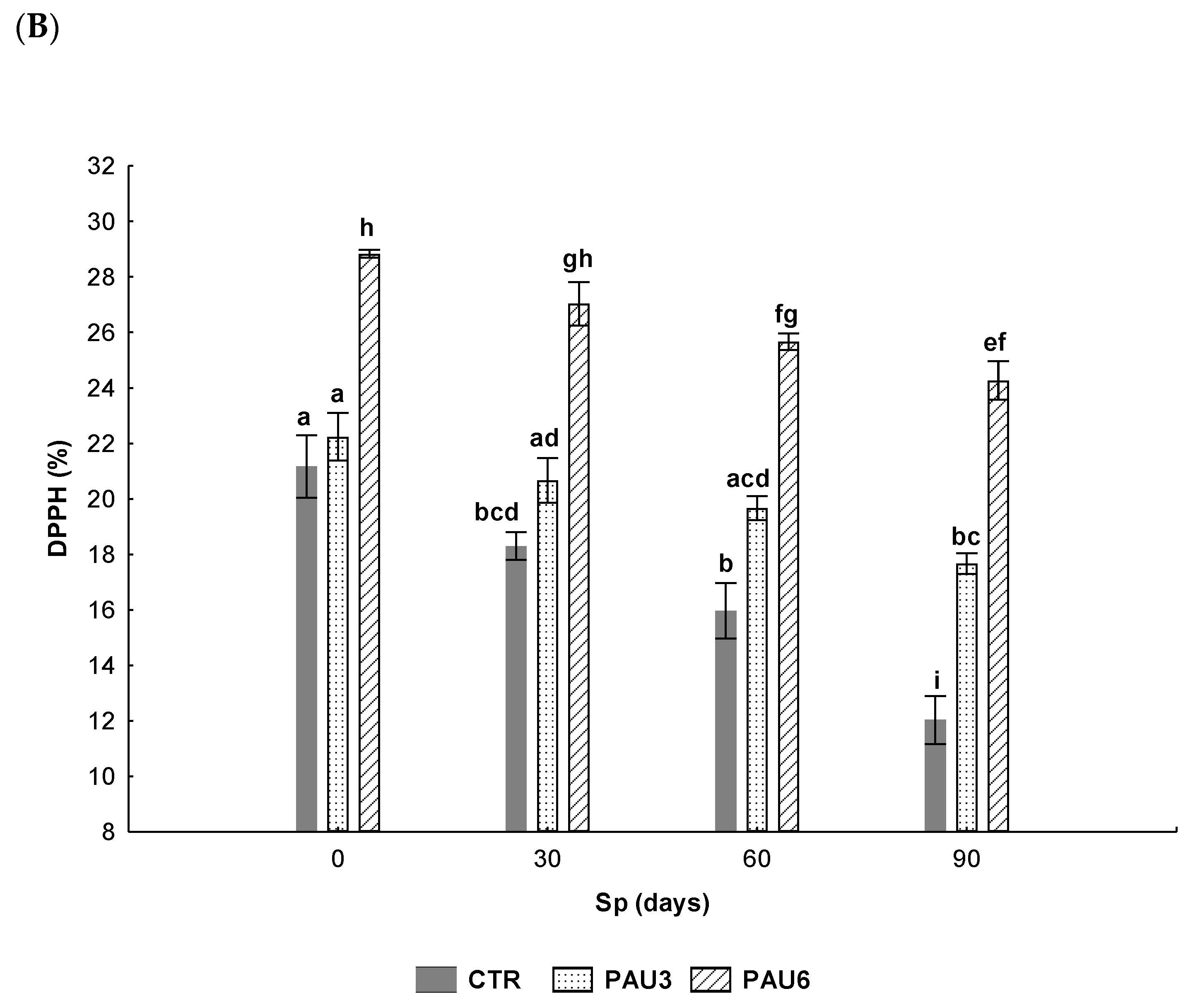
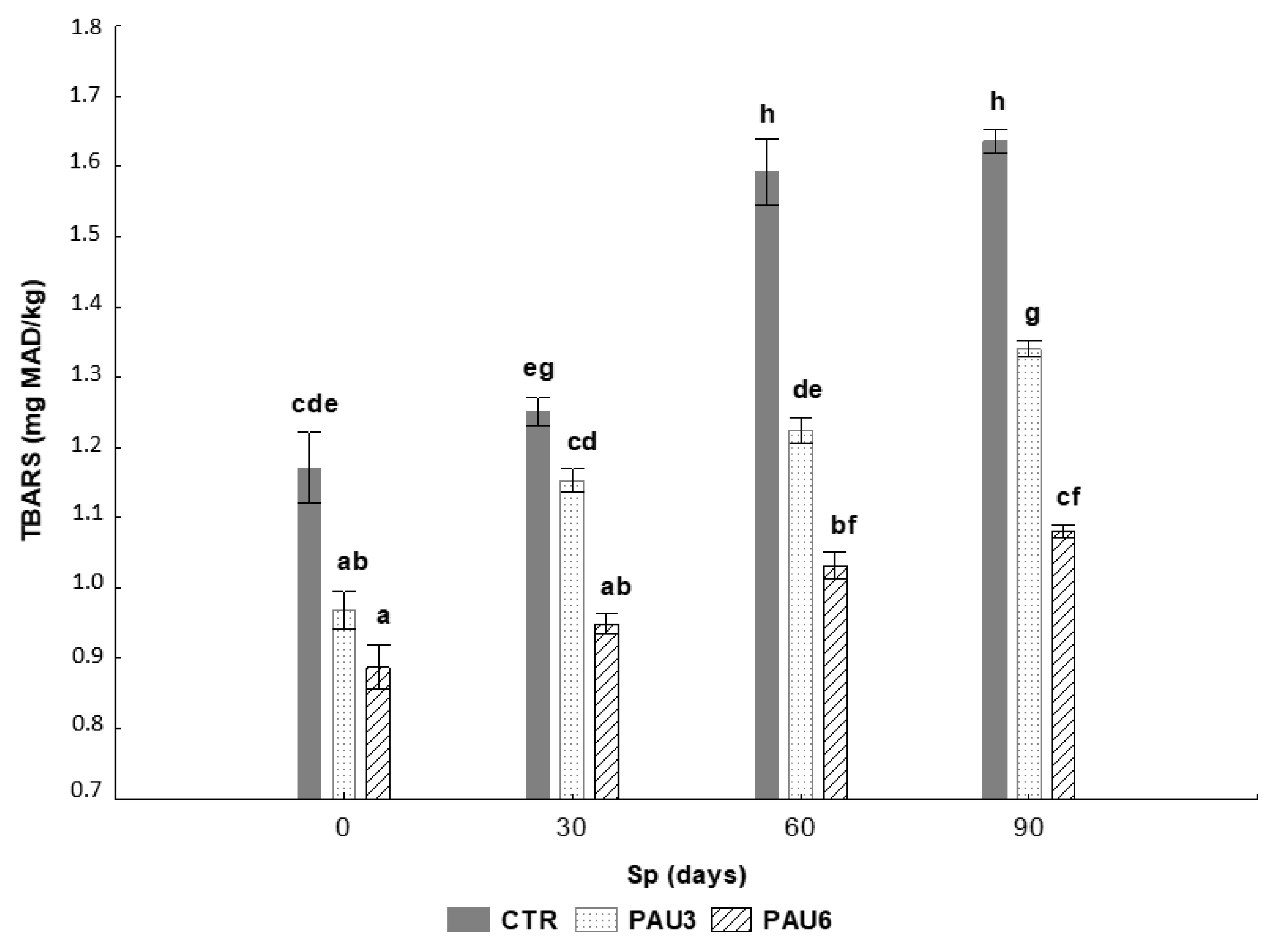
3.3. Evaluation of Antioxidant Capacity and Oxidative Stability of Limpets Pâté
3.4. Evaluation of Microbial Quality of Limpets Pâté
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References and Notes
- PCP. Facts and Figures on the Common Fisheries Policy–Basic Statistical Data–2016 Edition; Publications Office of the European Union: Luxembourg, 2016. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Altintzoglou, T.; Mendes, J.; Nunes, M.L.; Dinis, M.T.; Dias, J. Farmed fish as a functional food: Perception of fish fortification and the influence of origin–Insights from Portugal. Aquaculture 2019, 501, 22–31. [Google Scholar] [CrossRef]
- Ramirez, J.A.; Uresti, R.M.; Velazquez, G.; Vázquez, M. Food Hydrocolloids as additives to improve the mechanical and functional properties of fish products. Food Hydrocoll. 2011, 25, 1842–1852. [Google Scholar] [CrossRef]
- Brazão, S.; Morais, S.; Boaventura, D.; Ré, P.; Narciso, L.; Hawkins, S.J. Spatial and temporal variation of the fatty acid composition of Patella spp. (Gastropoda: Prosobranchia) soft bodies and gonads). Comp. Biochem. Physiol. Part B 2003, 136, 425–441. [Google Scholar] [CrossRef]
- Sánchez-Zapata, E.; Zaragoza, E.F.; de Vera, C.N.R.; Sayas, E.; Sendra, E.; López, J.F.; Alvarez, J.F.P. Effects of tuna pâté thickness and background on CIEL*a*b*color parameters and reflectance spectra. Food Control 2011, 22, 1226–1232. [Google Scholar] [CrossRef]
- Agregán, R.; Franco, D.; Carballo, J.; Tomasevic, I.; Barba, F.J.; Gómez, B.; Muchenje, V.; Lorenzo, J.M. Shelf life study of healthy pork liver pâté with added seaweed extracts from Ascophyllum nodosum, Fucus vesiculosus and Bifurcaria bifurcata. Food Res. Int. 2018, 112, 400–411. [Google Scholar] [CrossRef]
- Ladikos, D.; Lougovois, V. Lipid oxidation in muscle foods: A review. Food Chem. 1990, 35, 295–314. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein oxidation: Basic principles and implications for meat quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef]
- Andersen, M.L.; Lauridsen, R.K.; Skibsted, L.H. Optimising the use of phenolic compounds in foods. In Phytochemical Functional Foods; Johnson, I., Williamson, G., Eds.; Woodhead Publishing: Cambridge, UK, 2003; pp. 315–346. [Google Scholar]
- Heinonen, M. Antioxidant activity and antimicrobial effect of berry phenolics-a Finnish perspective. Mol. Nutr. Food Res. 2007, 51, 684–691. [Google Scholar] [CrossRef]
- Vuorela, S.; Salminen, H.; Mäkelä, M.; Kivikari, R.; Karonen, M.; Heinonen, M. Effect of plant phenolics on protein and lipid oxidation in cooked pork meat patties. J. Agric. Food Chem. 2005, 53, 8492–8497. [Google Scholar] [CrossRef]
- Alexandre, A.M.R.C.; Matias, A.; Duarte, C.M.M.; Bronze, M.R. High-pressure CO2 assisted extraction as a tool to increase phenolic content of strawberry-tree (Arbutus unedo) extracts. J. CO2 Util 2018, 27, 73–80. [Google Scholar] [CrossRef]
- Delgado-Pelayo, R.; Gallardo-Guerrero, L.; Hornero-Méndez, D. Carotenoid composition of strawberry tree (Arbutus unedo L.) fruits. Food Chem. 2016, 199, 165–175. [Google Scholar] [CrossRef]
- Ganhão, R.; Estévez, M.; Kylli, P.; Heinonen, M.; Morcuende, D. Characterization of selected wild Mediterranean fruits and comparative efficacy as inhibitors of oxidative reactions in emulsified raw pork burger patties. J. Agric. Food Chem. 2010, 58, 8854–8861. [Google Scholar] [CrossRef] [PubMed]
- Ganhão, R.; Estévez, M.; Morcuende, D. Suitability of the TBA method for assessing lipid oxidation in a meat system with added phenolic-rich materials. Food Chem. 2011, 126, 772–778. [Google Scholar] [CrossRef]
- Malheiro, R.; Sá, O.; Pereira, E.; Aguiar, C.; Baptista, P.; Pereira, J.A. Arbutus unedo L. leaves as source of phytochemicals with bioactive properties. Ind. Crops Prod. 2012, 37, 473–478. [Google Scholar] [CrossRef]
- Tavares, L.; Fortalezas, S.; Carrilho, C.; McDougall, G.J.; Stewart, D.; Ferreira, R.B.; Santos, C.N. Antioxidant and antiproliferative properties of strawberry tree tissues. J. Berry Res. 2010, 1, 3–12. [Google Scholar] [CrossRef]
- Morgado, S.; Morgado, M.; Plácido, A.I.; Roque, F.; Duarte, A.P. Review–Arbutus unedo L.: From traditional medicine to potential uses in modern pharmacotherapy. J. Ethnopharmacol. 2018, 225, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rodríguez, B.M.; Morales, P.; Fernández-Ruiz, V.; Sánchez-Mata, M.C.; Cámara, M.; Díez-Marqués, C.; Pardo-de-Santayana, M.; Molina, M.; Tardío, J. Valorization of wild strawberry-tree fruits (Arbutus unedo L.) through nutritional assessment and natural production data. Food Res. Int. 2011, 44, 1244–1253. [Google Scholar] [CrossRef]
- Estévez, M.; Ramírez, R.; Ventana, S.; Cava, R. Sage and rosemary essential oils versus BHT for the inhibition of lipid oxidative reactions in liver pâté. Food Sci. Technol. 2007, 40, 58–65. [Google Scholar] [CrossRef]
- Nielsen, N.S.; Jacobsen, C. Oxidative stability of fish oil-enriched fish pâté. J. Food Biochem. 2013, 37, 88–97. [Google Scholar] [CrossRef]
- Commission Regulation (EU) Nº 1129/2011 of 11 November 2011-Amending Annex II to Regulation (EC) No 1333/2008 of the European Parliament and of the Council by Establishing a Union List of Food Additives. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32011R1129&from=EN (accessed on 10 January 2019).
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis of the Association of the Analytical Chemists; AOAC: Rockville, VA, USA, 2016. [Google Scholar]
- Estévez, M.; Ventanas, S.; Cava, C. Protein oxidation in frankfurters with increasing levels of added rosemary essential oil: Effect on colour and texture deterioration. J. Food Sci. 2005, 70, 427–432. [Google Scholar] [CrossRef]
- Yu, L.; Haley, S.; Perret, J.; Harris, M.; Wilson, J.; Qian, M. Free radical scavenging properties of wheat extracts. J. Agr. Food Chem. 2002, 50, 1619–1624. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Carvalho, A.M.; Morais, J.S.; Ferreira, I.C.F.R. Strawberry-tree, blackthorn and rose fruits: Detailed characterization in nutrients and phytochemicals with antioxidant properties. Food Chem. 2010, 120, 247–254. [Google Scholar] [CrossRef]
- Duffy, C.F.; Power, R.F. Antioxidant and antimicrobial properties of some chinese plant extracts. Int. J. Antimicrob. Agents 2001, 17, 527–529. [Google Scholar] [CrossRef]
- Rosmini, M.R.; Perlo, F.; Pérez-Alvarez, J.A.; Pagán-Moreno, M.J.; Gago-Gago, A.; López Santoveña, F. TBA test by an extractive method applied to “Paté”. Meat Sci. 1996, 42, 103–110. [Google Scholar] [CrossRef]
- Commission Regulation (EC) Nº 2073 (2005) concerning microbiological criteria for foodstuffs. Off. J. Eur. Union 2005, 338, 1–26.
- NP 4405 (2002) General rules for counting microorganisms-counting colonies at 30 °C.
- ISO 16649-2 (2001)-Microbiology of food and animal feeding stuffs--Horizontal method for the enumeration of beta-glucuronidase-positive Escherichia coli--Part 2: Colony-count technique at 44 degrees C using 5-bromo-4-chloro-3-indolyl beta-D-glucuronide.
- ISO 11290-2 (1998)-Microbiology of food and animal feeding stuffs--Horizontal method for the detection and enumeration of Listeria monocytogenes--Part 2: Enumeration method.
- ISO 6579 (2002)-Microbiology of food and animal feeding stuffs--Horizontal method for the detection of Salmonella spp.
- Zar, J. Biostatistical Analysis; Prentice-Hall/Pearson: New York, NY, USA, 2010. [Google Scholar]
- StatSoft Inc. STATISTICA (Data Analysis Software System), Version 8; StatSoft Inc.: Tulsa, OK, USA, 2007. [Google Scholar]
- Aquerreta, Y.; Astiasara, I.; Mohino, A.; Bello, J. Composition of pâtés elaborated with mackerel flesh (Scomber scombrus) and tuna liver (Thunnus thynnus): Comparison with commercial fish pâtés. Food Chem. 2002, 77, 147–153. [Google Scholar] [CrossRef]
- Écharte, M.; Conchillo, A.; Ansorena, D.; Astiasaran, I. Evaluation of the nutritional aspects and cholesterol oxidation products of pork liver and fish patés. Food Chem. 2004, 86, 47–53. [Google Scholar] [CrossRef]
- Estévez, M.; Ventanas, S.; Cava, R. Effect of natural and synthetic antioxidants on protein oxidation and colour and texture changes in refrigerated stored porcine liver pâté. Meat Sci. 2006, 74, 396–403. [Google Scholar] [CrossRef]
- Terrasa, A.M.; Staffolo, M.D.; Tomás, M.C. Nutritional improvement and physicochemical evaluation of liver pâté formulations. LWT–Food Sci. Technol. 2016, 66, 678–684. [Google Scholar] [CrossRef]
- Fernández-López, J.; Fernández-Ginés, J.M.; Aleson-Carbonell, L.; Sendra, E.; Sayas-Barberá, E.; Pérez-Alvarez, J.A. Application of functional citrus by-products to meat products. Trends Food Sci. Tech. 2004, 15, 176–185. [Google Scholar] [CrossRef]
- Amaral, D.S.; Silva, F.A.P.; Bezerra, T.K.A.; Arcanjo, N.M.O.; Guerra, I.C.D.; Dalmás, P.S.; Madruga, M.S. Effect of storage time and packaging on the quality of lamb pâté prepared with “variety meat”. Food Packag. Shelf Life 2015, 3, 39–46. [Google Scholar] [CrossRef]
- Miguel, M.G.; Faleiro, M.L.; Guerreiro, A.C.; Antunes, M.D. Review-Arbutus unedo L.; Chemical and Biological Properties. Molecules 2014, 19, 15799–15823. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.N.; Mu, T.H.; Xi, L.S. Effect of pH, heat, and light treatments on the antioxidant activity of sweet potato leaf polyphenols. Int. J. Food Prop. 2017, 318–332. [Google Scholar] [CrossRef]
- Pateiro, M.; Lorenzo, J.M.; Amado, I.R.; Franco, D. Effect of addition of green tea, chestnut, and grape extract on the shelf-life of pig liver pâté. Food Chem. 2014, 147, 386–394. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Domínguez, R.; Campagnol, P.C.B.; Franco, D.; Trindade, M.A.; Lorenzo, J.M. Effect of natural antioxidants on physicochemical properties and lipid stability of pork liver pâté manufactured with healthy oils during refrigerated storage. J. Food Sci. Tec. Mys. 2017, 54, 4324–4334. [Google Scholar] [CrossRef]
- Carli, C.; Moraes-Lovison, M.; Pinho, S.C. Production, physicochemical stability of quercetin-loaded nanoemulsions and evaluation of antioxidant activity in spreadable chicken pâtés. LWT–Food Sci. Tech. 2018, 98, 154–161. [Google Scholar] [CrossRef]
- Pallauf, K.; Rivas-Gonzalo, J.C.; Castillo, M.D.; Cano, M.P.; Pascual-Teresa, S. Characterization of the antioxidant composition of strawberry tree (Arbutus unedo L.) fruits. J. Food Compos. Anal. 2008, 21, 273–281. [Google Scholar] [CrossRef]
- Arshad, M.S.; Anjum, F.M.; Khan, M.I.; Shahid, M. Wheat Germ Oil Enrichment in Broiler Feed With α-Lipoic Acid to Enhance the Antioxidant Potential and Lipid Stability of Meat. Lipids Health Dis. 2013, 12, 164. [Google Scholar] [CrossRef]
- Szydłowska-Czerniak, A.; Tułodziecka, A.; Szłyk, E. Determination of antioxidant capacity of unprocessed and processed food products by spectrophotometric methods. Food Anal. Methods 2012, 5, 807–813. [Google Scholar] [CrossRef][Green Version]
- Saha, J.; Debnath, M.; Saha, A.; Ghosh, T.; Sarkar, P.K. Response surface optimisation of extraction of antioxidants from strawberry fruit, and lipid peroxidation inhibitory potential of the fruit extract in cooked chicken patties. J. Sci Food Agric. 2011, 91, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Ganhão, R.; Estévez, M.; Armenteros, M.; Morcuende, D. Mediterranean berries as inhibitors of lipid oxidation in porcine burger patties subjected to cooking and chilled storage. J. Integr. Agric. 2013, 12, 1982–1992. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Puupponen-Pimiä, R.; Nohynek, L.; Hartmann-Schmidlin, S.; Kähkönen, M.; Heinonen, M.; Määttä-Riihinen, K.; Oksman-Caldentey, K.M. Berry phenolics selectively inhibit the growth of intestinal pathogens. J. Appl. Microb. 2005, 98, 991–1000. [Google Scholar]
- Salem, I.B.; Ouesleti, S.; Mabrouk, Y.; Landolsi, A.; Saidi, M.; Boulilla, A. Exploring the nutraceutical potential and biological activities of Arbutus unedo L. (Ericaceae) fruits. Ind. Crops Prod. 2018, 122, 726–731. [Google Scholar] [CrossRef]
| Proximate Composition | CTR | PAU3 | PAU6 |
|---|---|---|---|
| Moisture (%) | 65.5 ± 0.2 | 65.5 ± 0.2 | 65.2 ± 0.1 |
| Fat (%) | 12.6 ± 0.2 | 12.7 ± 0.5 | 12.9 ± 0.4 |
| Protein (%) | 11.9 ± 0.0 | 11.8 ± 0.0 | 11.9 ± 0.1 |
| Carbohydrate (%) | 7.8 ± 0.4 | 8.1 ± 0.5 | 8.0 ± 0.6 |
| Ash | 2.2 ± 0.2 | 1.9 ± 0.2 | 2.0 ± 0.2 |
| Fibre | 0.0 ± 0.0 a | 0.2 ± 0.0 b | 0.8 ± 0.0 c |
| Energy value (kcal/100 g) | 192.2 ± 1.3 | 193.7 ± 2.5 | 195.5 ± 1.0 |
| Limpets Pâté | Storage Period (days) | Colour | Texture | ||||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | Maximum Force (N) | Adhesiveness (N/s) | pH | ||
| CTR | 0 | 34.5 ± 1.3 c | 1.3 ± 0.0 ac | 28.4 ± 0.9 ab | 4.3 ± 0.0 b | −2.1 ± 0.1 c | 6.8 ± 0.0 c |
| 30 | 42.7 ± 0.5 a | 1.4 ± 0.0 ab | 27.1 ± 0.5 a | 4.5 ± 0.1 b | −2.1 ± 0.1 c | 6.9 ± 0.0 d | |
| 60 | 42.6 ± 0.8 a | 1.5 ± 0.1 ab | 28.8 ± 0.6 ab | 4.7 ± 0.1 f | −2.5 ± 0.1 f | 6.9 ± 0.0 cd | |
| 90 | 47.0 ± 0.5 d | 1.6 ± 0.0 bde | 31.6 ± 0.5 cd | 5.3 ± 0.1 g | −3.1 ± 0.1 e | 6.8 ± 0.0 c | |
| PAU3 | 0 | 33.2 ± 0.8 bc | 1.4 ± 0.1 ac | 27.7 ± 0.9 ab | 3.8 ± 0.1 d | −1.4 ± 0.1 ad | 6.6 ± 0.0 b |
| 30 | 43.2 ± 0.5 a | 1.7 ± 0.0 de | 27.7 ± 0.2 ab | 3.8 ± 0.1 de | −1.4 ± 0.1 a | 6.6 ± 0.0 ab | |
| 60 | 44.4 ± 0.6 a | 1.7 ± 0.0 ef | 28.7 ± 0.6 ab | 4.0 ± 0.1 e | −1.5 ± 0.0 a | 6.5 ± 0.0 ab | |
| 90 | 48.3 ± 0.7 d | 1.8 ± 0.0 f | 31.2 ± 0.4 d | 4.3 ± 0.1 b | −1.9 ± 0.1 g | 6.6 ± 0.0 ab | |
| PAU6 | 0 | 31.3 ± 0.9 b | 1.2 ± 0.1 c | 29.3 ± 0.7 b | 2.8 ± 0.1 a | −1.0 ± 0.1 b | 6.6 ± 0.0 ab |
| 30 | 51.2 ± 0.4 e | 1.3 ± 0.0 ac | 33.0 ± 0.2 c | 2.9 ± 0.1 a | −1.1 ± 0.1 b | 6.6 ± 0.0 ab | |
| 60 | 52.3 ± 0.4 e | 1.5 ± 0.0 abd | 33.1 ± 0.3 c | 3.0 ± 0.0 ab | −1.2 ± 0.0 bd | 6.5 ± 0.0 a | |
| 90 | 55.2 ± 0.7 f | 1.6 ± 0.0 bde | 32.9 ± 0.5 c | 3.2 ± 0.1 b | −1.5 ± 0.1 a | 6.6 ± 0.0 ab | |
| Limpets Pâté | Storage Period (days) | Total Viable Mesophilic Counts | Escherichia coli | Listeria monocytogenes | Salmonella spp. |
|---|---|---|---|---|---|
| CTR | 0 | 0.0 ± 0.0 a | <102 a | <102 a | ND |
| 30 | 0.0 ± 0.0 a | <102 a | <102 a | ND | |
| 60 | 4.8 ± 0.1 d | <102 a | <102 a | ND | |
| 90 | 5.3 ± 0.1 e | <102 a | <102 a | ND | |
| PAU3 | 0 | 0.0 ± 0.0 a | <102 a | <102 a | ND |
| 30 | 0.0 ± 0.0 a | <102 a | <102 a | ND | |
| 60 | 4.3 ± 0.0 b | <102 a | <102 a | ND | |
| 90 | 4.4 ± 0.1 b | <102 a | <102 a | ND | |
| PAU6 | 0 | 0.0 ± 0.0 a | <102 a | <102 a | ND |
| 30 | 0.0 ± 0.0 a | <102 a | <102 a | ND | |
| 60 | 4.2 ± 0.0 c | <102 a | <102 a | ND | |
| 90 | 4.3 ± 0.0 b | <102 a | <102 a | ND |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinheiro, J.; Rodrigues, S.; Mendes, S.; Maranhão, P.; Ganhão, R. Impact of Aqueous Extract of Arbutus unedo Fruits on Limpets (Patella spp.) Pâté during Storage: Proximate Composition, Physicochemical Quality, Oxidative Stability, and Microbial Development. Foods 2020, 9, 807. https://doi.org/10.3390/foods9060807
Pinheiro J, Rodrigues S, Mendes S, Maranhão P, Ganhão R. Impact of Aqueous Extract of Arbutus unedo Fruits on Limpets (Patella spp.) Pâté during Storage: Proximate Composition, Physicochemical Quality, Oxidative Stability, and Microbial Development. Foods. 2020; 9(6):807. https://doi.org/10.3390/foods9060807
Chicago/Turabian StylePinheiro, Joaquina, Sidónio Rodrigues, Susana Mendes, Paulo Maranhão, and Rui Ganhão. 2020. "Impact of Aqueous Extract of Arbutus unedo Fruits on Limpets (Patella spp.) Pâté during Storage: Proximate Composition, Physicochemical Quality, Oxidative Stability, and Microbial Development" Foods 9, no. 6: 807. https://doi.org/10.3390/foods9060807
APA StylePinheiro, J., Rodrigues, S., Mendes, S., Maranhão, P., & Ganhão, R. (2020). Impact of Aqueous Extract of Arbutus unedo Fruits on Limpets (Patella spp.) Pâté during Storage: Proximate Composition, Physicochemical Quality, Oxidative Stability, and Microbial Development. Foods, 9(6), 807. https://doi.org/10.3390/foods9060807