Changes in Physical Meat Traits, Protein Solubility, and the Microstructure of Different Beef Muscles during Post-Mortem Aging
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Muscle Samples Preparation
2.2. pH Measurement
2.3. Myofibril Fragmentation Index (MFI)
2.4. Protein Solubility
2.5. Microstructure Analysis
2.6. Statistical Analysis
3. Results and Discussions
3.1. Changes in pH Value
3.2. Changes in Myofibril Fragmentation Index (MFI)
3.3. Changes in Protein Solubility
3.4. Pearson Correlations
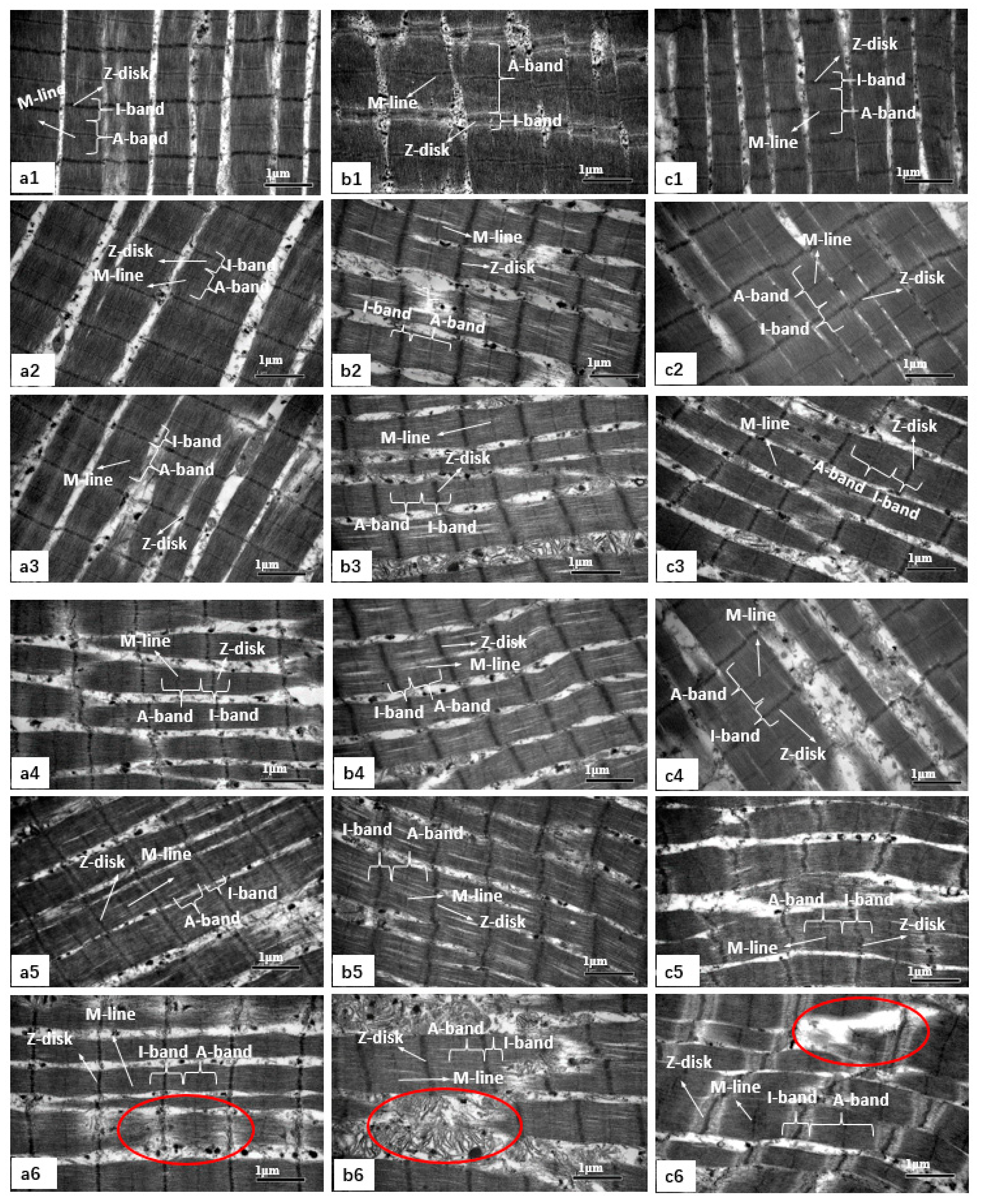
3.5. Changes in Muscle Microstructure
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiménez-Colmenero, F.; Herrero, A.; Cofrades, S.; Ruiz-Capillas, C. Meat: Eating Quality and Preservation. In The Encyclopedia of Food and Health; Caballero, B., Finglas, P., Toldrá, F., Eds.; Oxford Academic Press: Kidlington, UK, 2016; Volume 3, pp. 685–692. [Google Scholar]
- Dobbs, L.M.; Jensen, K.L.; Leffew, M.B.; English, B.C.; Lambert, D.M.; Clark, C.D. Consumer willingness to pay for Tennessee beef. J. Food Distrib. Res. 2016, 47, 38–61. [Google Scholar]
- O’Quinn, T.G.; Legako, J.F.; Brooks, J.C.; Miller, M.F. Evaluation of the contribution of tenderness, juiciness, and flavor to the overall consumer beef eating experience. Trans. Anim. Sci. 2018, 2, 26–36. [Google Scholar] [CrossRef]
- Nair, M.N.; Canto, A.C.; Rentfrow, G.; Suman, S.P. Muscle-specific effect of aging on beef tenderness. LWT Food Sci. Technol. 2019, 100, 250–252. [Google Scholar] [CrossRef]
- Von Seggern, D.D.; Calkins, C.R.; Johnson, D.D.; Brickler, J.E.; Gwartney, B.L. Muscle profiling: Characterizing the muscles of the beef chuck and round. Meat Sci. 2005, 71, 39–51. [Google Scholar] [CrossRef]
- Neath, K.E.; Del Barrio, A.N.; Lapitan, R.M.; Herrera, J.R.V.; Cruz, L.C.; Fujihara, T.; Muroya, S.; Chikuni, K.; Hirabayashi, M.; Kanai, Y. Difference in tenderness and pH decline between water buffalo meat and beef during postmortem aging. Meat Sci. 2007, 75, 499–505. [Google Scholar] [CrossRef]
- Hanzelková, Š.; Simeonovová, J.; Hampel, D.; Dufek, A.; Šubrt, J. The effect of breed, sex and aging time on tenderness of beef meat. Acta Vet. Brno 2011, 80, 191–196. [Google Scholar] [CrossRef]
- Jiménez-Colmenero, F.; Herrero, A.M.; Ruiz-Capillas, C.; Cofrades, S. Meat and functional foods. In Handbook of Meat and Meat Processing; Hui, Y.H., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2012; pp. 225–248. [Google Scholar]
- Lee, S.H.; Joo, S.T.; Ryu, Y.C. Skeletal muscle fiber type and myofibrillar proteins in relation to meat quality. Meat Sci. 2010, 86, 166–170. [Google Scholar] [CrossRef]
- Choi, Y.M.; Kim, B.C. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livest. Sci. 2009, 122, 105–118. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Jeong, T.C.; Yang, H.S.; Kim, G.D. Multivariate analysis of muscle fiber characteristics, intramuscular fat content and fatty acid composition in porcine longissimus thoracis muscle. Livest. Sci. 2017, 202, 13–20. [Google Scholar] [CrossRef]
- Hwang, Y.H.; Kim, G.D.; Jeong, J.Y.; Hur, S.J.; Joo, S.T. The relationship between muscle fiber characteristics and meat quality traits of highly marbled Hanwoo (Korean native cattle) steers. Meat Sci. 2010, 86, 456–461. [Google Scholar] [CrossRef]
- Kim, G.D.; Yang, H.S.; Jeong, J.Y. Comparison of characteristics of myosin heavy chain-based fiber and meat quality among four bovine skeletal muscles. Korean J. Food Sci. Anim. Resour. 2016, 36, 819. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.M.; Carmona, P.; Jiménez-Colmenero, F.; Ruiz-Capillas, C. Applications of vibrational spectroscopy to study protein structural changes in muscle and meat batter systems. In Applications of Vibrational Spectroscopy to Food Science; Chalmers, J., Griffiths, P., Li-Chan, E., Eds.; John Wiley & Sons: West Sussex, UK, 2010; pp. 315–328. [Google Scholar]
- Smuder, A.J.; Kavazis, A.N.; Hudson, M.B.; Nelson, W.B.; Powers, S.K. Oxidation enhances myofibrillar protein degradation via calpain and caspase-3. Free Radic. Biol. Med. 2010, 49, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Veiseth-Kent, E.; Hollung, K.; Ofstad, R.; Aass, L.; Hildrum, K.I. Relationship between muscle microstructure, the calpain system, and shear force in bovine longissimus dorsi muscle. J. Anim. Sci. 2010, 88, 3445–3451. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, D.L.; Thompson, J.M. The degradation of myofibrillar proteins in beef and lamb using denaturing electrophoresis—An overview. J. Muscle Foods 2002, 13, 81–102. [Google Scholar] [CrossRef]
- Marcos, B.; Kerry, J.P.; Mullen, A.M. High pressure induced changes on sarcoplasmic protein fraction and quality indicators. Meat Sci. 2010, 85, 115–120. [Google Scholar] [CrossRef]
- Santos, M.D.; Delgadillo, I.; Saraiva, J.A. Extended preservation of raw beef and pork meat by hyperbaric storage at room temperature. Int. J. Food Sci. 2020, 55, 1171–1179. [Google Scholar] [CrossRef]
- Joo, S.; Kauffman, R.; Kim, B.; Park, G. The relationship of sarcoplasmic and myofibrillar protein solubility to colour and water-holding capacity in porcine longissimus muscle. Meat Sci. 1999, 52, 291–297. [Google Scholar] [CrossRef]
- Dai, Y.; Miao, J.; Yuan, S.Z.; Liu, Y.; Li, X.M.; Dai, R.T. Colour and sarcoplasmic protein evaluation of pork following water bath and ohmic cooking. Meat Sci. 2013, 93, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Arner, A.; Puolanne, E.; Ertbjerg, P. On the water-holding of myofibrils: Effect of sarcoplasmic protein denaturation. Meat Sci. 2016, 119, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.G., III; van Laack, R.L.J.M. Sarcoplasmic proteins influence water-holding capacity of pork myofibrils. J. Sci. Food Agric. 1999, 79, 1939–1942. [Google Scholar] [CrossRef]
- Kim, H.J.; Sujiwo, J.; Kim, H.J.; Jang, A. Effects of dipping chicken breast meat inoculated with Listeria monocytogenes in lyophilized scallion, garlic, and kiwi extracts on its physicochemical quality. Food Sci. Anim. Resour. 2019, 39, 418. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Liang, R.; Mao, Y.; Zhang, Y.; Niu, L.; Wang, R.; Liu, C.; Liu, Y.; Luo, X. Effect of suspension method and aging time on meat quality of Chinese fattened cattle M. Longissimus Dorsi. Meat Sci. 2014, 96, 640–645. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem. 1949, 177, 751–766. [Google Scholar] [PubMed]
- Li, Y.J.; Li, J.L.; Zhang, L.; Gao, F.; Zhou, G.H. Effects of dietary starch types on growth performance, meat quality and myofibre type of finishing pigs. Meat Sci. 2017, 131, 60–67. [Google Scholar] [CrossRef]
- Anderson, M.J.; Lonergan, S.M.; Fedler, C.A.; Prusa, K.J.; Binning, J.M.; Huff-Lonergan, E. Profile of biochemical traits influencing tenderness of muscles from the beef round. Meat Sci. 2012, 91, 247–254. [Google Scholar] [CrossRef]
- Melody, J.L.; Lonergan, S.M.; Rowe, L.J.; Huiatt, T.W.; Mayes, M.S.; Huff-Lonergan, E. Early postmortem biochemical factors influence tenderness and water-holding capacity of three porcine muscles. J. Anim. Sci. 2004, 82, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Ilian, M.A.; Morton, J.D.; Kent, M.P.; Le Couteur, C.E.; Hickford, J.; Cowley, R.; Bickerstaffe, R. Intermuscular variation in tenderness: Association with the ubiquitous and muscle-specific calpains. J. Anim. Sci. 2001, 79, 122–132. [Google Scholar] [CrossRef][Green Version]
- Shen, L.Y.; Luo, J.; Lei, H.G.; Jiang, Y.Z.; Bai, L.; Li, M.Z.; Tang, G.Q.; Li, X.W.; Zhang, S.H.; Zhu, L. Effects of muscle fiber type on glycolytic potential and meat quality traits in different Tibetan pig muscles and their association with glycolysis-related gene expression. Genet. Mol. Res. 2015, 14, 14366–14378. [Google Scholar] [CrossRef]
- McDonagh, M.B.; Fernandez, C.; Oddy, V.H. Hind-limb protein metabolism and calpain system activity influence post-mortem change in meat quality in lamb. Meat Sci. 1999, 52, 9–18. [Google Scholar] [CrossRef]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.U. Applications and ultrasonics in food potential of processing. Trends Food Sci. Technol. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- Xiong, G.; Fu, X.; Pan, D.; Qi, J.; Xu, X.; Jiang, X. Influence of ultrasound-assisted sodium bicarbonate marination on the curing efficiency of chicken breast meat. Ultrason. Sonochem. 2020, 60, 104808. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, Y.; Mao, Y.; Cornforth, D.; Dong, P.; Wang, R.; Zhu, H.; Luo, X. Effect of very fast chilling and aging time on ultra-structure and meat quality characteristics of Chinese Yellow cattle M. Longissimus Lumborum. Meat Sci. 2012, 92, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Aroeira, C.N.; Torres Filho, R.A.; Fontes, P.R.; Ramos, A.L.; Castillo, C.J.C.; Hopkins, D.L.; Ramos, E.M. Comparison of different methods for determining the extent of myofibrillar fragmentation of chilled and frozen/thawed beef across postmortem aging periods. Meat Sci. 2020, 160, 107955. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.M.; Lee, S.H.; Choe, J.H.; Rhee, M.S.; Lee, S.K.; Joo, S.T.; Kim, B.C. Protein solubility is related to myosin isoforms, muscle fiber types, meat quality traits, and postmortem protein changes in porcine longissimus dorsi muscle. Livest. Sci. 2010, 127, 183–191. [Google Scholar] [CrossRef]
- Li, F.; Wang, B.; Liu, Q.; Chen, Q.; Zhang, H.; Xia, X.; Kong, B. Changes in myofibrillar protein gel quality of porcine longissimus muscle induced by its stuctural modification under different thawing methods. Meat Sci. 2019, 147, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Li, X.; Wang, H.; Mehmood, W.; Zhong, M.; Zhang, C.; Blecker, C. Effects of low voltage electrostatic field thawing on the changes in physicochemical properties of myofibrillar proteins of bovine Longissimus dorsi muscle. J. Food Eng. 2019, 261, 140–149. [Google Scholar] [CrossRef]
- Li, S.; Zheng, Y.; Xu, P.; Zhu, X.; Zhou, C. L-Lysine and L-arginine inhibit myosin aggregation and interact with acidic amino acid residues of myosin: The role in increasing myosin solubility. Food Chem. 2018, 242, 22–28. [Google Scholar] [CrossRef]
- Maity, I.; Rasale, D.B.; Das, A.K. Sonication induced peptide-appended bolaamphiphile hydrogels for in situ generation and catalytic activity of Pt nanoparticles. Soft Matter 2012, 8, 5301–5308. [Google Scholar] [CrossRef]
- Pan, B.S.; Yeh, W.T. Biochemical and morphological changes in grass shrimp (Penaeus monodon) muscle following freezing by air blast and liquid nitrogen methods. J. Food Biochem. 1993, 17, 147–160. [Google Scholar] [CrossRef]
- Zhang, M.; Li, F.; Diao, X.; Kong, B.; Xia, X. Moisture migration, microstructure damage and protein structure changes in porcine longissimus muscle as influenced by multiple freeze-thaw cycles. Meat Sci. 2017, 133, 10–18. [Google Scholar] [CrossRef]
- Alahakoon, A.U.; Faridnia, F.; Bremer, P.J.; Silcock, P.; Oey, I. Pulsed electric fields effects on meat tissue quality and functionality. In Handbook of Electroporation; Miklavcic, D., Ed.; Springer: Cham, Switzerland, 2016; Volume 4, pp. 2455–2475. [Google Scholar]
- Moczkowska, M.; Półtorak, A.; Montowska, M.; Pospiech, E.; Wierzbicka, A. The effect of the packaging system and storage time on myofibrillar protein degradation and oxidation process in relation to beef tenderness. Meat Sci. 2017, 130, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Purchas, R.W.; Zou, M. Composition and quality differences between the longissimus and infraspinatus muscles for several groups of pasture-finished cattle. Meat Sci. 2008, 80, 470–479. [Google Scholar] [CrossRef] [PubMed]
| Items | Aging (Days) | SEM | |||||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 7 | 9 | 11 | 14 | ||
| ST | 5.65 a,y | 5.45 ab,x | 5.54 b,xy | 5.50 a,xy | 5.45 ab,x | 5.38 a,x | 0.028 |
| LT | 5.64 a,y | 5.38 a,x | 5.41 a,x | 5.39 a,x | 5.37 a,x | 5.40 ab,x | 0.027 |
| RH | 5.76 ab,y | 5.55 bc,x | 5.53 b,x | 5.62 ab,x | 5.56 b,x | 5.55 ab,x | 0.028 |
| GN | 5.72 ab,y | 5.46 ab,x | 5.53 b,x | 5.51 a,x | 5.38 a,x | 5.45 ab,x | 0.032 |
| IN | 5.87 b,z | 5.66 c,xy | 5.70 c,xy | 5.76 b,yz | 5.73 c,xyz | 5.56 b,x | 0.029 |
| PM | 5.59 a | 5.51 ab | 5.53 b | 5.58 ab | 5.51 ab | 5.55 ab | 0.022 |
| BF | 5.67 a,y | 5.45 ab,x | 5.42 a,x | 5.47 a,x | 5.43 ab,x | 5.51 ab,x | 0.026 |
| SEM | 0.026 | 0.023 | 0.022 | 0.038 | 0.030 | 0.022 | |
| Items | Aging (days) | SEM | |||||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 7 | 9 | 11 | 14 | ||
| ST | 102.57 d,x | 107.93 c,x | 154.13 d,y | 157.53 e,y | 156.50 e,y | 189.93 e,z | 7.47 |
| LT | 81.87 bc,x | 83.10 a,x | 86.97 a,xy | 88.27 a,xy | 88.90 a,xy | 92.60 a,y | 1.17 |
| RH | 122.23 e,x | 129.93 d,xy | 131.73 c,xy | 132.97 cd,xy | 133.77 c,xy | 143.43 cd,y | 2.11 |
| GN | 87.97 c,x | 105.87 c,y | 128.56 c,z | 131.70 c,z | 131.37 c,z | 136.93 c,z | 4.41 |
| IN | 68.80 a,x | 76.87 a,xy | 79.77 a,xy | 84.37 a,y | 87.63 a,y | 101.83 ab,z | 2.62 |
| PM | 75.90 ab,x | 93.47 b,y | 98.90 b,y | 111.87 b,z | 110.93 b,z | 110.07 b,z | 3.27 |
| BF | 99.43 d,w | 122.87 d,x | 130.67 c,x | 141.80 d,y | 144.33 d,yz | 152.47 d,z | 4.33 |
| SEM | 3.95 | 4.19 | 5.77 | 5.76 | 5.62 | 7.07 | |
| Items | Aging (days) | SEM | |||||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 7 | 9 | 11 | 14 | ||
| Total Protein Solubility, mg/g | |||||||
| ST | 172.77 ab,x | 190.83 bc,yz | 207.59 c,z | 183.39 ab,xy | 192.81 ab,yz | 193.16 bc,yz | 3.19 |
| LT | 181.08 bcd,x | 198.36 c,xy | 193.45 b,xy | 195.42 ab,xy | 200.21 b,y | 200.06 c,y | 2.42 |
| RH | 169.00 a | 174.24 a | 176.05 a | 183.80 ab | 181.94 ab | 185.91 ab | 2.25 |
| GN | 189.47 d | 187.76 abc | 196.72 bc | 198.46 b | 185.01 ab | 194.29 bc | 2.01 |
| IN | 176.24 abc,x | 185.24 abc,x | 195.85 bc,y | 178.47 a,x | 177.82 a,x | 179.02 a,x | 1.95 |
| PM | 183.20 cd | 191.27 bc | 187.48 ab | 184.91 ab | 187.58 ab | 189.43 abc | 1.30 |
| BF | 179.64 bc,x | 179.67 ab,x | 208.62 c,y | 183.42 ab,x | 186.39 ab,x | 187.92 ab,x | 2.94 |
| SEM | 1.69 | 2.25 | 2.67 | 2.31 | 2.48 | 1.77 | |
| Sarcoplasmic Protein Solubility, mg/g | |||||||
| ST | 59.71 abc,xy | 57.20 ab,xy | 60.66 b,y | 49.57 ab,x | 50.90 xy | 50.53 xy | 1.51 |
| LT | 68.31 c,y | 65.49 b,y | 50.83 a,x | 48.75 ab,x | 51.99 x | 51.51 x | 2.17 |
| RH | 51.35 a,xy | 59.33 ab,y | 48.77 a,x | 42.00 a,x | 46.14 x | 44.02 x | 1.70 |
| GN | 62.45 bc,yz | 58.45 ab,yz | 67.01 b,z | 55.05 b,xy | 48.67 x | 55.15 xy | 1.73 |
| IN | 55.69 ab,yz | 52.04 a,xy | 62.61 b,z | 52.65 b,xy | 45.06 x | 46.51 xy | 1.76 |
| PM | 51.93 a,y | 59.08 ab,z | 47.68 a,x | 48.06 ab,x | 47.51 x | 44.08 w | 1.21 |
| BF | 55.29 ab,x | 60.24 ab,xy | 70.66 b,y | 48.20 ab,x | 49.23 x | 57.48 xy | 2.31 |
| SEM | 1.55 | 1.19 | 2.14 | 1.15 | 1.02 | 1.68 | |
| Myofibrillar Protein Solubility, mg/g | |||||||
| ST | 113.07 a,x | 133.63 b,y | 146.93 b,y | 133.81 ab,y | 141.91 y | 142.63 abc,y | 3.28 |
| LT | 112.77 a,x | 132.87 b,y | 142.62 ab,y | 146.67 b,y | 148.22 y | 148.55 c,y | 3.59 |
| RH | 117.66 ab,x | 114.90 a,x | 127.28 a,xy | 141.80 ab,y | 135.80 y | 141.89 abc,y | 3.10 |
| GN | 127.01 de,x | 129.31 b,xy | 129.71 a,xy | 143.41 ab,y | 136.34 xy | 139.14 abc,xy | 2.15 |
| IN | 120.55 bc | 133.20 b | 133.23 ab | 125.82 a | 132.75 | 132.52 ab | 1.91 |
| PM | 131.27 e,x | 132.18 b,x | 139.80 ab,xy | 136.85 ab,xy | 140.06 xy | 145.34 bc,y | 1.62 |
| BF | 124.35 cd | 119.44 ab | 137.96 ab | 135.22 ab | 137.16 | 130.44 a | 2.55 |
| SEM | 1.57 | 2.09 | 2.17 | 2.37 | 2.33 | 1.86 | |
| MFI | TPS | SPS | MPS | |
|---|---|---|---|---|
| pH | −0.314 ** | −0.419 ** | −0.032 | −0.377 ** |
| MFI | 0.086 | −0.144 | 0.183 * | |
| TPS | 0.300 ** | 0.743 ** | ||
| SPS | −0.416 ** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.-H.; Zhang, S.-S.; Sun, B.-Z.; Xie, P.; Wen, K.-X.; Xu, C.-C. Changes in Physical Meat Traits, Protein Solubility, and the Microstructure of Different Beef Muscles during Post-Mortem Aging. Foods 2020, 9, 806. https://doi.org/10.3390/foods9060806
Feng Y-H, Zhang S-S, Sun B-Z, Xie P, Wen K-X, Xu C-C. Changes in Physical Meat Traits, Protein Solubility, and the Microstructure of Different Beef Muscles during Post-Mortem Aging. Foods. 2020; 9(6):806. https://doi.org/10.3390/foods9060806
Chicago/Turabian StyleFeng, Yong-Hong, Song-Shan Zhang, Bao-Zhong Sun, Peng Xie, Kai-Xin Wen, and Chen-Chen Xu. 2020. "Changes in Physical Meat Traits, Protein Solubility, and the Microstructure of Different Beef Muscles during Post-Mortem Aging" Foods 9, no. 6: 806. https://doi.org/10.3390/foods9060806
APA StyleFeng, Y.-H., Zhang, S.-S., Sun, B.-Z., Xie, P., Wen, K.-X., & Xu, C.-C. (2020). Changes in Physical Meat Traits, Protein Solubility, and the Microstructure of Different Beef Muscles during Post-Mortem Aging. Foods, 9(6), 806. https://doi.org/10.3390/foods9060806





