Reliable Discrimination of Green Coffee Beans Species: A Comparison of UV-Vis-Based Determination of Caffeine and Chlorogenic Acid with Non-Targeted Near-Infrared Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Determination of Caffeine and Chlorogenic Acid Content by UV-Vis Spectroscopy
2.3. Determination of Species by Near-Infrared Spectroscopy
2.4. Statistical Procedures
3. Results
3.1. UV-Vis Spectroscopy
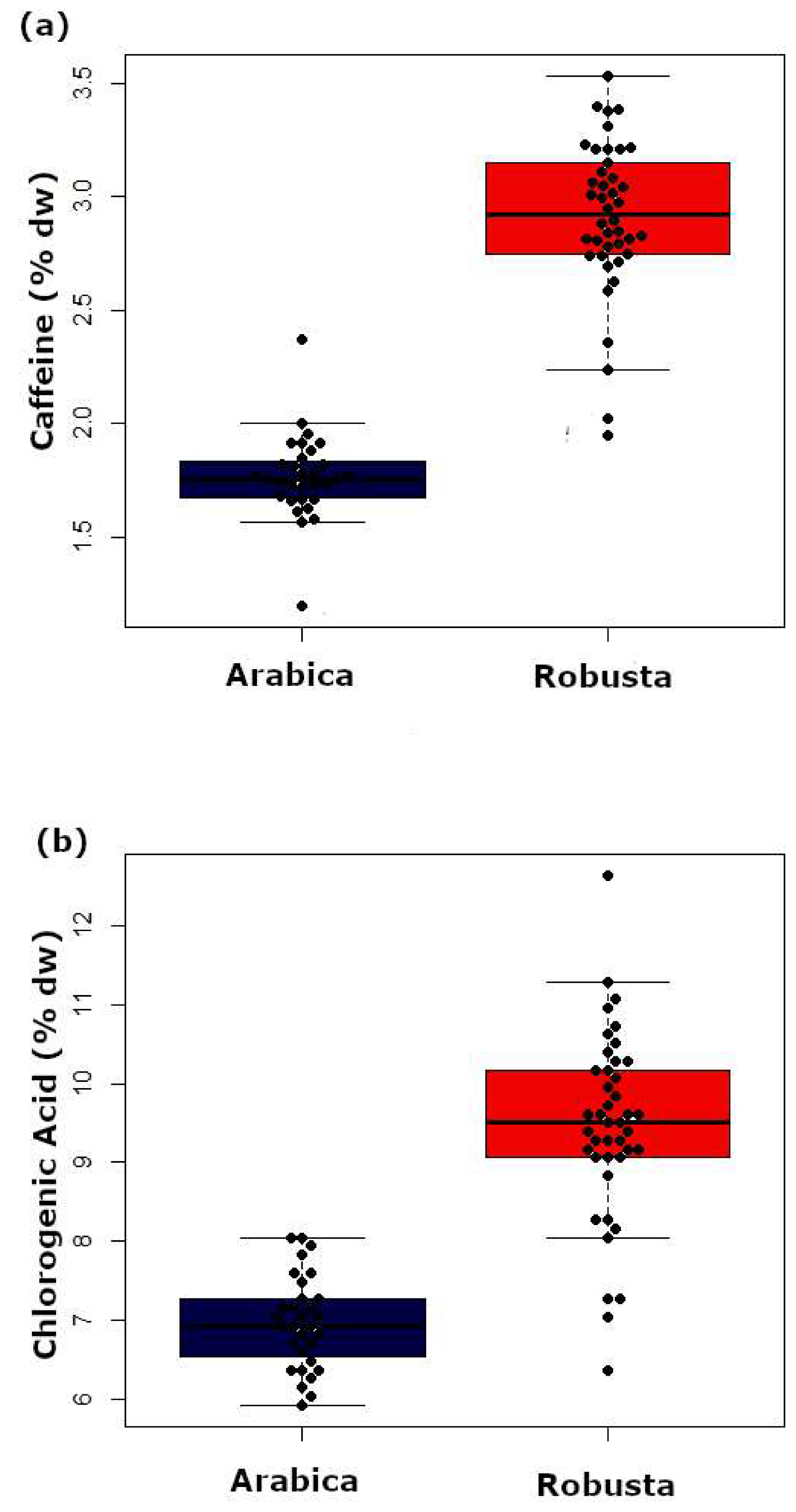
3.1.1. Caffeine Content
3.1.2. Chlorogenic Acid Content
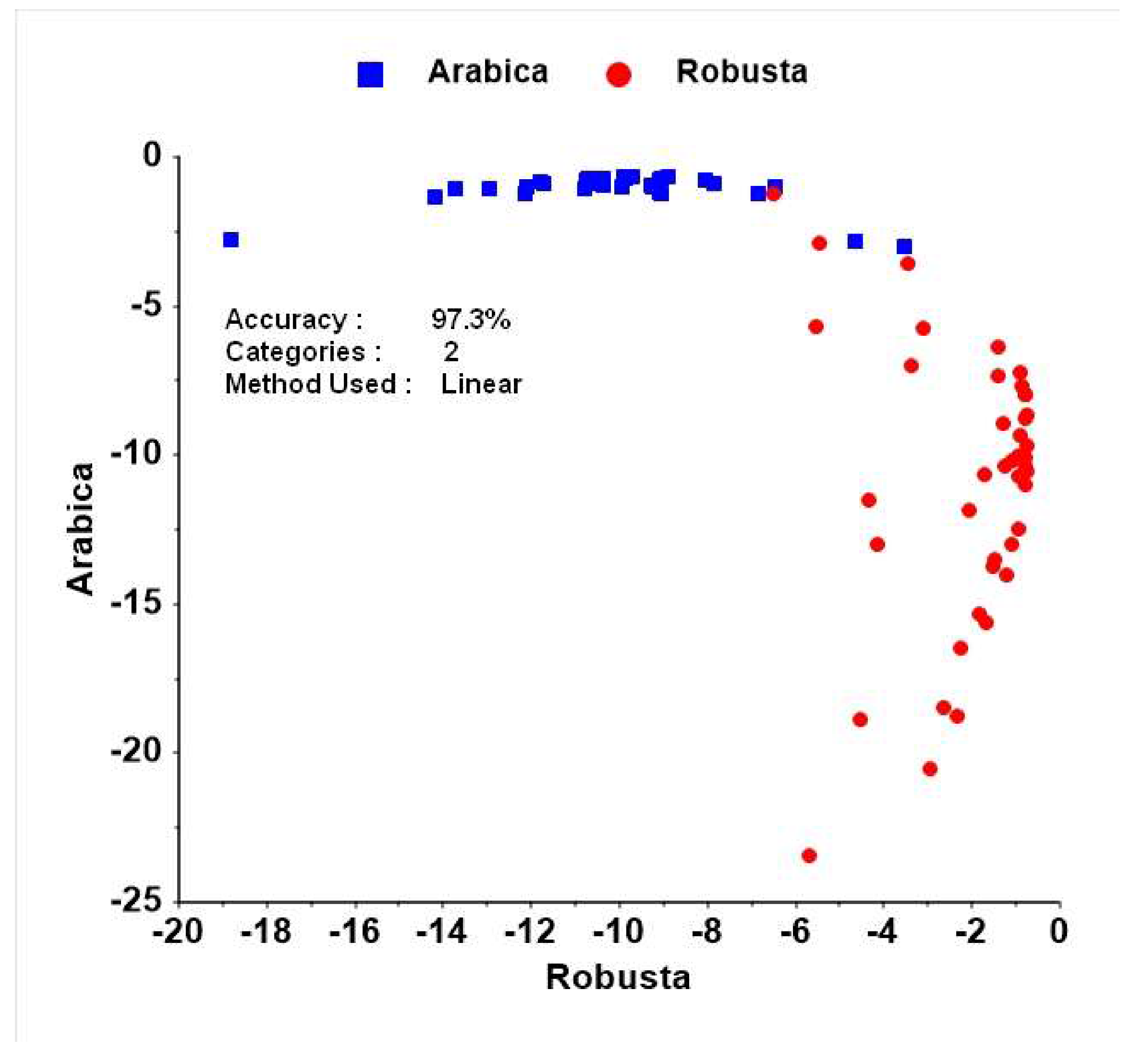
3.1.3. Discrimination among Species on the Basis of Caffeine and Chlorogenic Acid Content (by UV-Vis Spectroscopy)
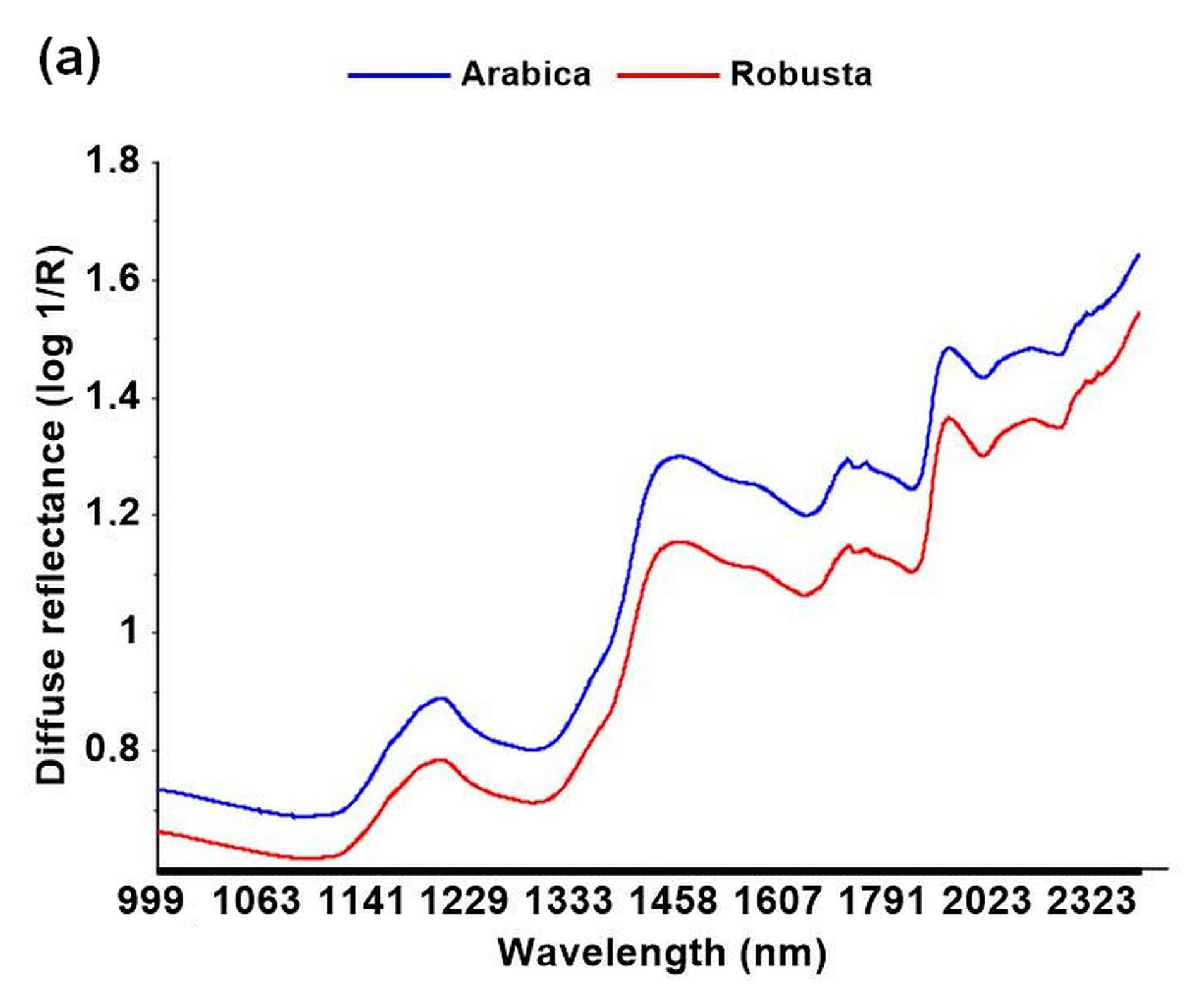
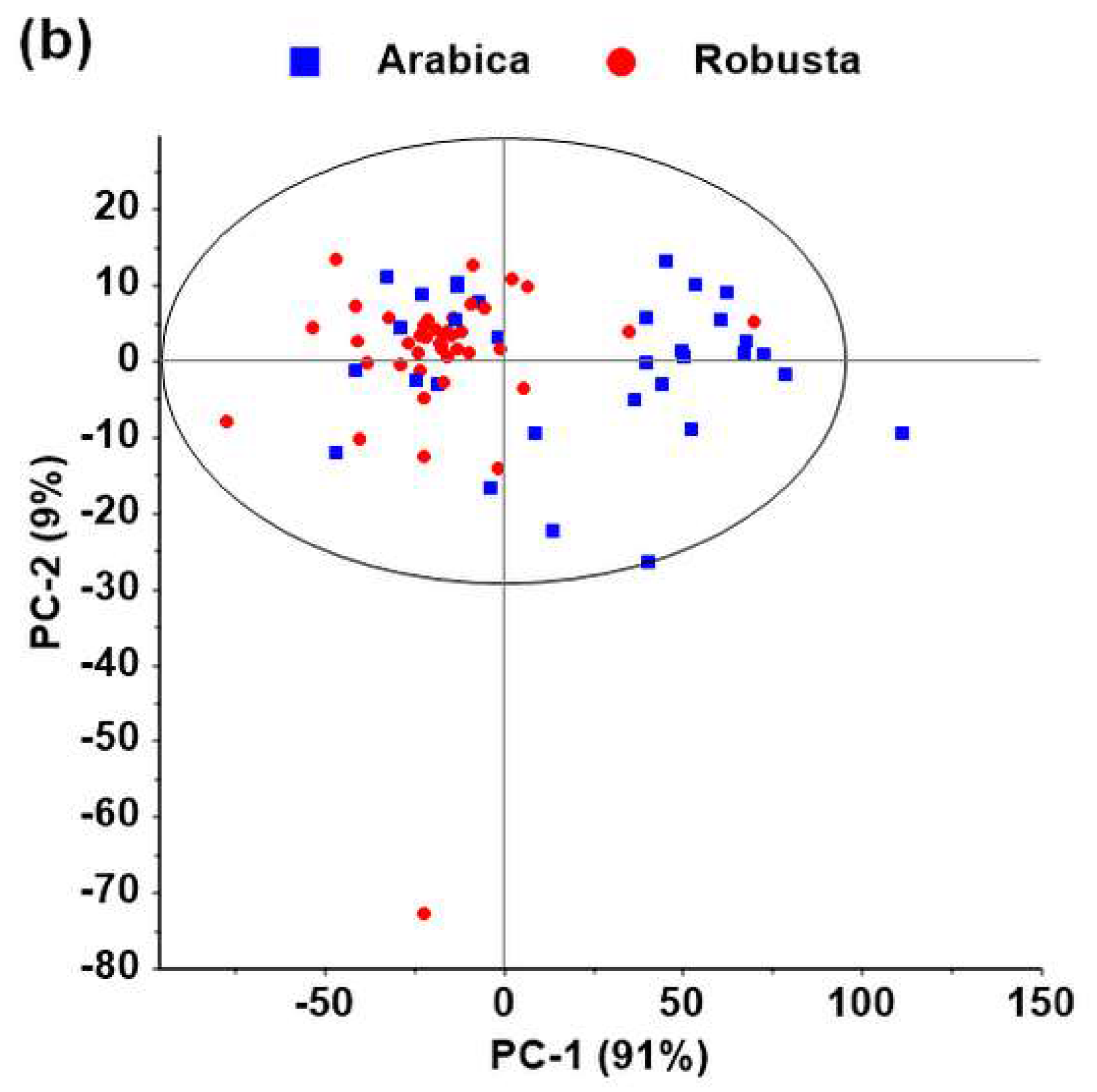
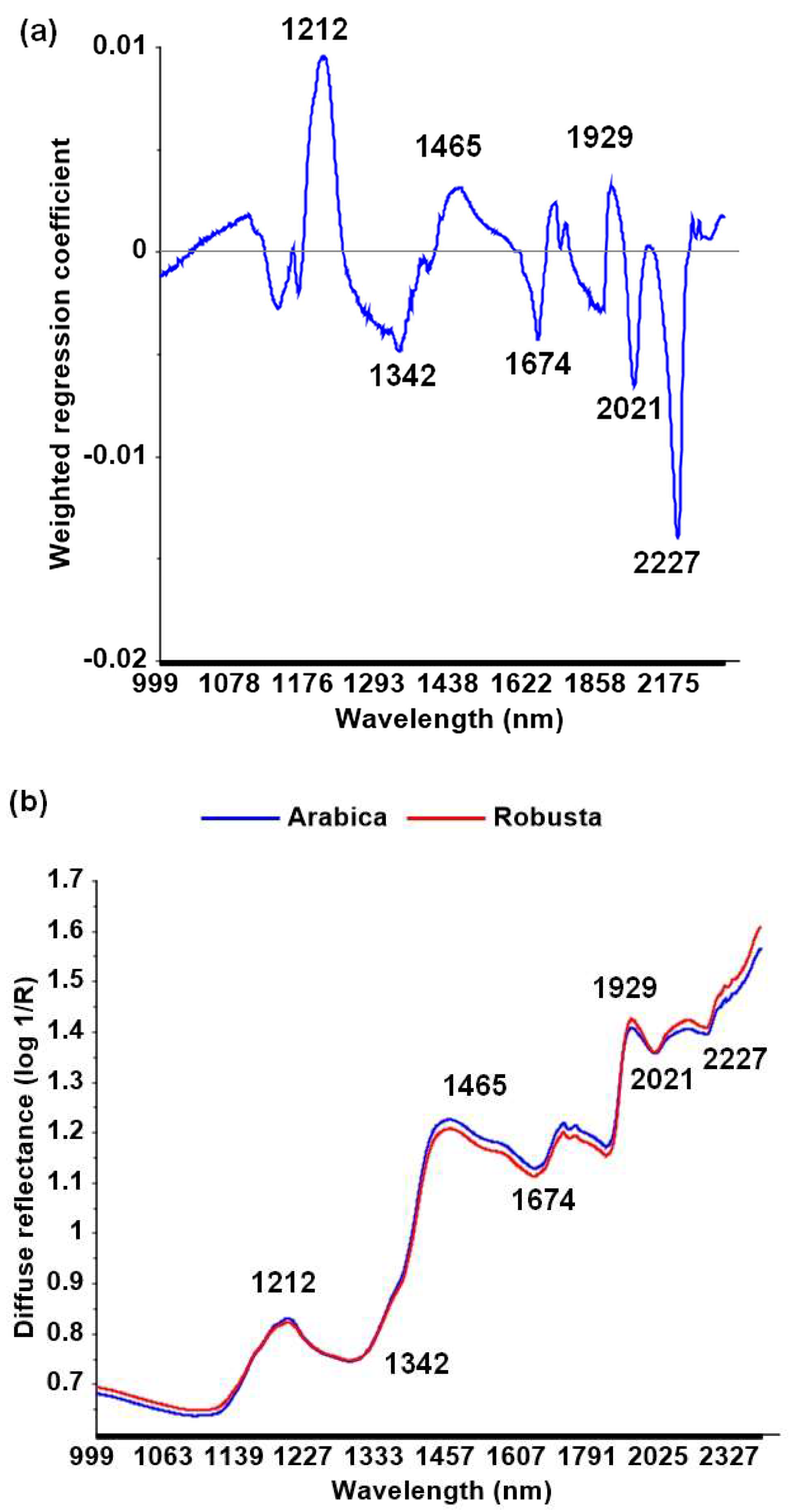
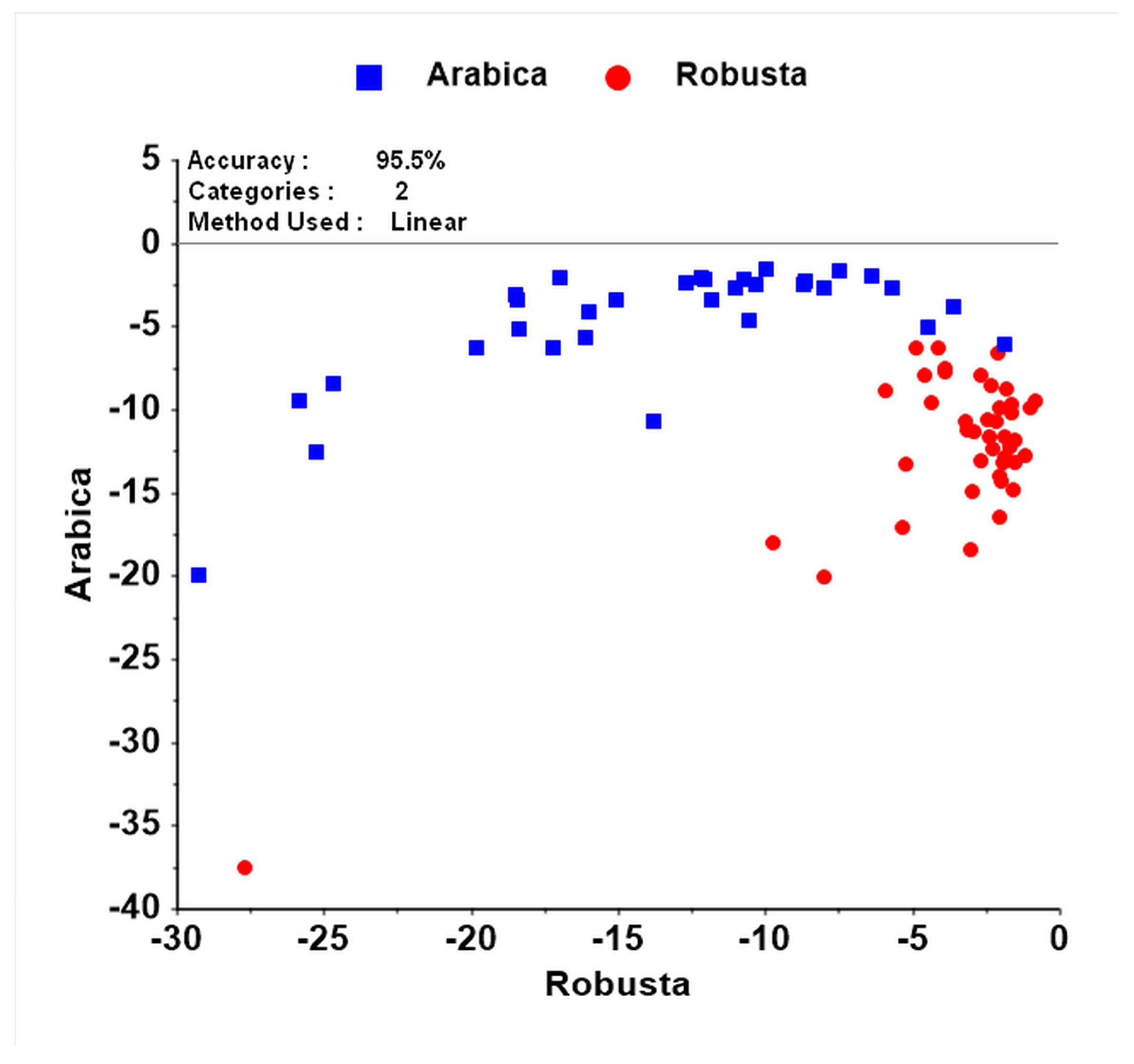
3.2. Discrimination among Species Using NIR Spectroscopy
4. Discussion
4.1. UV-Vis Spectroscopy
4.1.1. Caffeine Content
4.1.2. Chlorogenic Acid Content
4.1.3. Discrimination among Species on the Basis of Caffeine and Chlorogenic Acid Content (by UV-Vis Spectroscopy)
4.2. Discrimination among Species Using NIR Spectroscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Toci, A.T.; Farah, A.; Pezza, H.R.; Pezza, L. Coffee adulteration: More than two decades of research. Crit. Rev. Anal. Chem. 2016, 46, 83–92. [Google Scholar] [CrossRef] [PubMed]
- International Coffee Organization. Historical Data on the Global Coffee Trade. Available online: http://www.ico.org/new_historical.asp (accessed on 1 September 2019).
- Martins, V.D.C.; Godoy, R.L.D.O.; Gouvêa, A.C.M.S.; Santiago, M.C.P.D.A.; Borguini, R.G.; Braga, E.C.D.O.; Pacheco, S.; Nascimento, L.D.S.D.M.D. Fraud investigation in commercial coffee by chromatography. Food Qual. Saf. 2018, 2, 121–133. [Google Scholar] [CrossRef]
- Davis, A.P.; Govaerts, R.; Bridson, D.M.; Stoffelen, P. An annotated taxonomic conspectus of the genus Coffea (Rubiaceae). Bot. J. Linn. Soc. 2006, 152, 465–512. [Google Scholar] [CrossRef]
- Keidel, A.; von Stetten, D.; Rodrigues, C.; Máguas, C.; Hildebrandt, P. Discrimination of green Arabica and Robusta coffee beans by Raman Spectroscopy. J. Agric. Food Chem. 2010, 58, 11187–11192. [Google Scholar] [CrossRef] [PubMed]
- Feria-Morales, A.M. Examining the case of green coffee to illustrate the limitations of grading systems/expert tasters in sensory evaluation for quality control. Food Qual. Prefer. 2002, 13, 355–367. [Google Scholar] [CrossRef]
- Kim, S.-E.; Lee, S.M.; Kim, K.-O. Consumer acceptability of coffee as affected by situational conditions and involvement. Food Qual. Prefer. 2016, 52, 124–132. [Google Scholar] [CrossRef]
- Mendonça, J.C.F.; Franca, A.S.; Oliveira, L.S. Physical characterization of non-defective and defective Arabica and Robusta coffees before and after roasting. J. Food Eng. 2009, 92, 474–479. [Google Scholar] [CrossRef]
- Salamanca, C.A.; Fiol, N.; González, C.; Saez, M.; Villaescusa, I. Extraction of espresso coffee by using gradient of temperature. Effect on physicochemical and sensorial characteristics of espresso. Food Chem. 2017, 214, 622–630. [Google Scholar] [CrossRef]
- Di Donfrancesco, B.; Gutierrez Guzman, N.; Chambers, E. Comparison of results from cupping and descriptive sensory analysis of colombian brewed coffee: Coffee: Comparing Cupping and Descriptive Analysis. J. Sens. Stud. 2014, 29, 301–311. [Google Scholar] [CrossRef]
- Esteban-Díez, I.; González-Sáiz, J.M.; Pizarro, C. Prediction of sensory properties of espresso from roasted coffee samples by near-infrared spectroscopy. Anal. Chim. Acta 2004, 525, 171–182. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Sentkowska, A.; Pyrzyńska, K.; De Peña, M.P. Chlorogenic acids, caffeine content and antioxidant properties of green coffee extracts: Influence of green coffee bean preparation. Eur. Food Res. Technol. 2016, 242, 1403–1409. [Google Scholar] [CrossRef]
- DePaula, J.; Farah, A. Caffeine consumption through coffee: Content in the beverage, metabolism, health benefits and risks. Beverages 2019, 5, 37. [Google Scholar] [CrossRef]
- Tajik, N.; Tajik, M.; Mack, I.; Enck, P. The potential effects of chlorogenic acid, the main phenolic components in coffee, on health: A comprehensive review of the literature. Eur. J. Nutr. 2017, 56, 2215–2244. [Google Scholar] [CrossRef] [PubMed]
- Górnaś, P.; Siger, A.; Pugajeva, I.; Czubinski, J.; Waśkiewicz, A.; Polewski, K. New insights regarding tocopherols in Arabica and Robusta species coffee beans: RP-UPLC-ESI/MSn and NP-HPLC/FLD study. J. Food Compos. Anal. 2014, 36, 117–123. [Google Scholar] [CrossRef]
- Oestreich-Janzen, S. Chemistry of coffee. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Waltham, MA, USA, 2013; ISBN 978-0-12-409547-2. [Google Scholar]
- Tomaszewska, E.; Soliwoda, K.; Kadziola, K.; Tkacz-Szczesna, B.; Celichowski, G.; Cichomski, M.; Szmaja, W.; Grobelny, J. Detection Limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J. Nanomater. 2013, 2013, 1–10. [Google Scholar] [CrossRef]
- Belay, A.; Ture, K.; Redi, M.; Asfaw, A. Measurement of caffeine in coffee beans with UV/vis spectrometer. Food Chem. 2008, 108, 310–315. [Google Scholar] [CrossRef]
- Belay, A.; Gholap, A.V. Characterization and determination of chlorogenic acids (CGA) in coffee beans by UV-Vis spectroscopy. Afr. J. Pure Appl. Chem. 2009, 3, 34–240. [Google Scholar]
- Dankowska, A.; Domagała, A.; Kowalewski, W. Quantification of Coffea arabica and Coffea canephora var. robusta concentration in blends by means of synchronous fluorescence and UV-Vis spectroscopies. Talanta 2017, 172, 215–220. [Google Scholar] [CrossRef]
- Navarra, G.; Moschetti, M.; Guarrasi, V.; Mangione, M.R.; Militello, V.; Leone, M. Simultaneous determination of caffeine and chlorogenic acids in green coffee by UV/Vis spectroscopy. J. Chem. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Buratti, S.; Sinelli, N.; Bertone, E.; Venturello, A.; Casiraghi, E.; Geobaldo, F. Discrimination between washed Arabica, natural Arabica and Robusta coffees by using near infrared spectroscopy, electronic nose and electronic tongue analysis: NIR and e-devices for coffee classification. J. Sci. Food Agric. 2015, 95, 2192–2200. [Google Scholar] [CrossRef]
- Esteban-Díez, I.; González-Sáiz, J.M.; Sáenz-González, C.; Pizarro, C. Coffee varietal differentiation based on near infrared spectroscopy. Talanta 2007, 71, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Kitzberger, C.S.G.; dos Santos Scholz, M.B.; de Toledo Benassi, M. Bioactive compounds content in roasted coffee from traditional and modern Coffea arabica cultivars grown under the same edapho-climatic conditions. Food Res. Int. 2014, 61, 61–66. [Google Scholar] [CrossRef]
- Adnan, A.; Hörsten, D.V.; Pawelzik, E.; Mörlein, D. Rapid prediction of moisture content in intact green coffee beans using near infrared spectroscopy. Foods 2017, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Krzywinski, M.; Altman, N. Points of significance: Visualizing samples with box plots. Nat. Methods 2014, 11, 119–120. [Google Scholar] [CrossRef]
- Puth, M.-T.; Neuhäuser, M.; Ruxton, G.D. Effective use of Pearson’s product–moment correlation coefficient. Anim. Behav. 2014, 93, 183–189. [Google Scholar] [CrossRef]
- Bassbasi, M.; De Luca, M.; Ioele, G.; Oussama, A.; Ragno, G. Prediction of the geographical origin of butters by partial least square discriminant analysis (PLS-DA) applied to infrared spectroscopy (FTIR) data. J. Food Compos. Anal. 2014, 33, 210–215. [Google Scholar] [CrossRef]
- Bylesjö, M.; Rantalainen, M.; Cloarec, O.; Nicholson, J.K.; Holmes, E.; Trygg, J. OPLS discriminant analysis: Combining the strengths of PLS-DA and SIMCA classification. J. Chemom. 2006, 20, 341–351. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Ferreira, M.M.C.; Salva, T.J.G. Chemometric models for the quantitative descriptive sensory analysis of Arabica coffee beverages using near infrared spectroscopy. Talanta 2011, 83, 1352–1358. [Google Scholar] [CrossRef]
- Tran, H.T.M.; Vargas, C.A.C.; Slade Lee, L.; Furtado, A.; Smyth, H.; Henry, R. Variation in bean morphology and biochemical composition measured in different genetic groups of arabica coffee (Coffea arabica L.). Tree Genet. Genomes 2017, 13. [Google Scholar] [CrossRef]
- Couto, C.C.; Santos, T.F.; Mamede, A.M.G.N.; Oliveira, T.C.; Souza, A.M.; Freitas-Silva, O.; Oliveira, E.M.M. Coffea arabica and C. canephora discrimination in roasted and ground coffee from reference material candidates by real-time PCR. Food Res. Int. 2019, 115, 227–233. [Google Scholar] [CrossRef]
- Babova, O.; Occhipinti, A.; Maffei, M.E. Chemical partitioning and antioxidant capacity of green coffee (Coffea arabica and Coffea canephora) of different geographical origin. Phytochemistry 2016, 123, 33–39. [Google Scholar] [CrossRef]
- Joët, T.; Laffargue, A.; Descroix, F.; Doulbeau, S.; Bertrand, B.; Kochko, A.D.; Dussert, S. Influence of environmental factors, wet processing and their interactions on the biochemical composition of green Arabica coffee beans. Food Chem. 2010, 118, 693–701. [Google Scholar] [CrossRef]
- Barbosa, J.N.; Borem, F.M.; Cirillo, M.A.; Malta, M.R.; Alvarenga, A.A.; Alves, H.M.R. Coffee quality and its interactions with environmental factors in Minas Gerais, Brazil. J. Agric. Sci. 2012, 4. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant. Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Badmos, S.; Fu, M.; Granato, D.; Kuhnert, N. Classification of Brazilian roasted coffees from different geographical origins and farming practices based on chlorogenic acid profiles. Food Res. Int. 2020, 134, 109218. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Furtado, A.; Smyth, H.E.; Henry, R.J. Influence of genotype and environment on coffee quality. Trends Food Sci. Technol. 2016, 57, 20–30. [Google Scholar] [CrossRef]
- Farah, A.; Monteiro, M.C.; Calado, V.; Franca, A.S.; Trugo, L.C. Correlation between cup quality and chemical attributes of Brazilian coffee. Food Chem. 2006, 98, 373–380. [Google Scholar] [CrossRef]
- Martín, M.J.; Pablos, F.; González, A.G. Discrimination between arabica and robusta green coffee varieties according to their chemical composition. Talanta 1998, 46, 1259–1264. [Google Scholar] [CrossRef]
- Monteiro, P.I.; Santos, J.S.; Rodionova, O.Y.; Pomerantsev, A.; Chaves, E.S.; Rosso, N.D.; Granato, D. Chemometric Authentication of Brazilian Coffees Based on Chemical Profiling. J. Food Sci. 2019, 84, 3099–3108. [Google Scholar] [CrossRef]
- Westad, F.; Marini, F. Validation of chemometric models—A tutorial. Anal. Chim. Acta 2015, 893, 14–24. [Google Scholar] [CrossRef]
- Munawar, A.A.; von Hörsten, D.; Wegener, J.K.; Pawelzik, E.; Mörlein, D. Rapid and non-destructive prediction of mango quality attributes using Fourier transform near infrared spectroscopy and chemometrics. Eng. Agric. Environ. Food 2016, 9, 208–215. [Google Scholar] [CrossRef]
- Brereton, R.G. Consequences of sample size, variable selection, and model validation and optimisation, for predicting classification ability from analytical data. TrAC Trends Anal. Chem. 2006, 25, 1103–1111. [Google Scholar] [CrossRef]
- Barbin, D.F.; Felicio, A.L.D.S.M.; Sun, D.W.; Nixdorf, S.L.; Hirooka, E.Y. Application of infrared spectral techniques on quality and compositional attributes of coffee: An overview. Food Res. Int. 2014, 61, 23–32. [Google Scholar] [CrossRef]
- Severa, L.; Buchar, J.; Nedomová, Š. Shape and size variability of roasted arabica coffee beans. Int. J. Food Prop. 2012, 15, 426–437. [Google Scholar] [CrossRef]
- Downey, G.; Boussion, J.; Beauchêne, D. Authentication of whole and ground coffee beans by near infrared reflectance spectroscopy. J. Infrared Spectrosc. 1994, 2, 85–92. [Google Scholar] [CrossRef]
- Monteiro, P.I.; Santos, J.S.; Alvarenga Brizola, V.R.; Pasini Deolindo, C.T.; Koot, A.; Boerrigter-Eenling, R.; van Ruth, S.; Georgouli, K.; Koidis, A.; Granato, D. Comparison between proton transfer reaction mass spectrometry and near infrared spectroscopy for the authentication of Brazilian coffee: A preliminary chemometric study. Food Control 2018, 91, 276–283. [Google Scholar] [CrossRef]
| Preprocessing Method | LVs | R2 of Calibration Model (%) | RMSEC | R2 of Validation Model (%) | RMSEP |
|---|---|---|---|---|---|
| Raw | 7 | 89.0 | 0.3266 | 71.5 | 0.6005 |
| EMSC | 6 | 91.4 | 0.2884 | 90.5 | 0.3641 |
| Normalization (area) | 7 | 93.2 | 0.2570 | 90.3 | 0.3745 |
| Normalization (mean) | 6 | 93.2 | 0.2570 | 90.3 | 0.3745 |
| Smoothing (Moving average, 3 segments) | 7 | 89.0 | 0.3266 | 88.9 | 0.3270 |
| MSC | 3 | 85.3 | 0.3774 | 81.3 | 0.4734 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adnan, A.; Naumann, M.; Mörlein, D.; Pawelzik, E. Reliable Discrimination of Green Coffee Beans Species: A Comparison of UV-Vis-Based Determination of Caffeine and Chlorogenic Acid with Non-Targeted Near-Infrared Spectroscopy. Foods 2020, 9, 788. https://doi.org/10.3390/foods9060788
Adnan A, Naumann M, Mörlein D, Pawelzik E. Reliable Discrimination of Green Coffee Beans Species: A Comparison of UV-Vis-Based Determination of Caffeine and Chlorogenic Acid with Non-Targeted Near-Infrared Spectroscopy. Foods. 2020; 9(6):788. https://doi.org/10.3390/foods9060788
Chicago/Turabian StyleAdnan, Adnan, Marcel Naumann, Daniel Mörlein, and Elke Pawelzik. 2020. "Reliable Discrimination of Green Coffee Beans Species: A Comparison of UV-Vis-Based Determination of Caffeine and Chlorogenic Acid with Non-Targeted Near-Infrared Spectroscopy" Foods 9, no. 6: 788. https://doi.org/10.3390/foods9060788
APA StyleAdnan, A., Naumann, M., Mörlein, D., & Pawelzik, E. (2020). Reliable Discrimination of Green Coffee Beans Species: A Comparison of UV-Vis-Based Determination of Caffeine and Chlorogenic Acid with Non-Targeted Near-Infrared Spectroscopy. Foods, 9(6), 788. https://doi.org/10.3390/foods9060788





