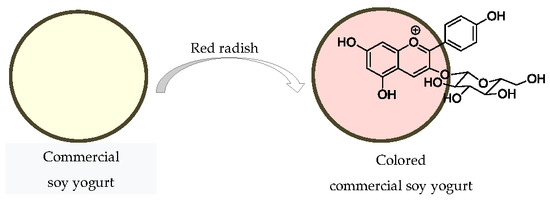
Natural Pigments of Anthocyanin and Betalain for Coloring Soy-Based Yogurt Alternative
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Preparation of Betalain-Rich Extracts
2.4. Preparation of Anthocyanin-Rich Extracts
2.5. Determination of Betalains Content
2.6. LRMS and UHPLC-ESI-Q-TOF-MS Analysis of Extracts
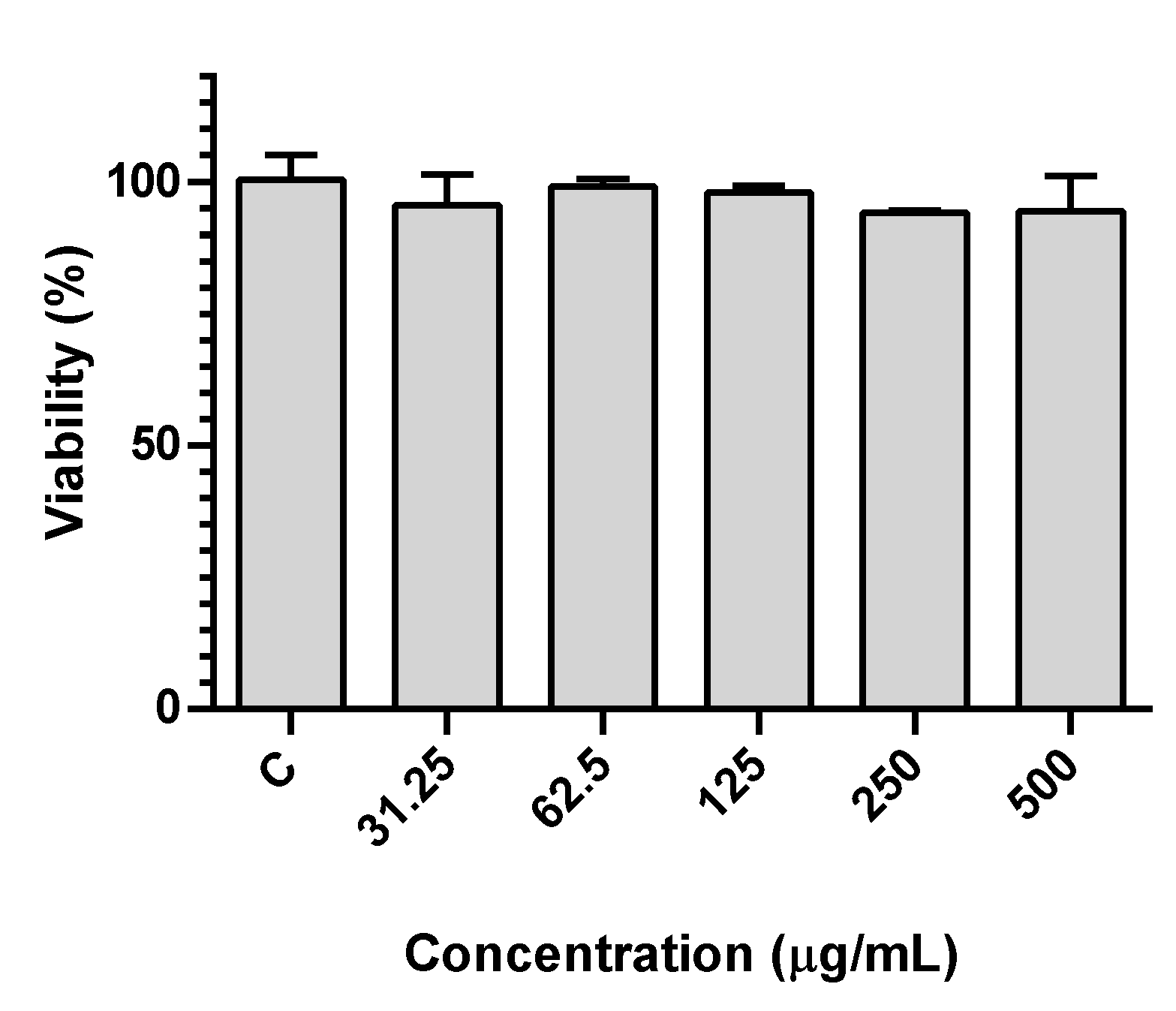
2.7. Human Gastric Adenocarcinoma Cell Line Assays
2.8. Nanoencapsulation Studies
2.9. Application of Betalain- and Anthocyanin-Rich Extracts to Soy-Based Yogurt Alternative
2.10. Color Measurement
2.11. Statistical Analysis
3. Results and Discussion
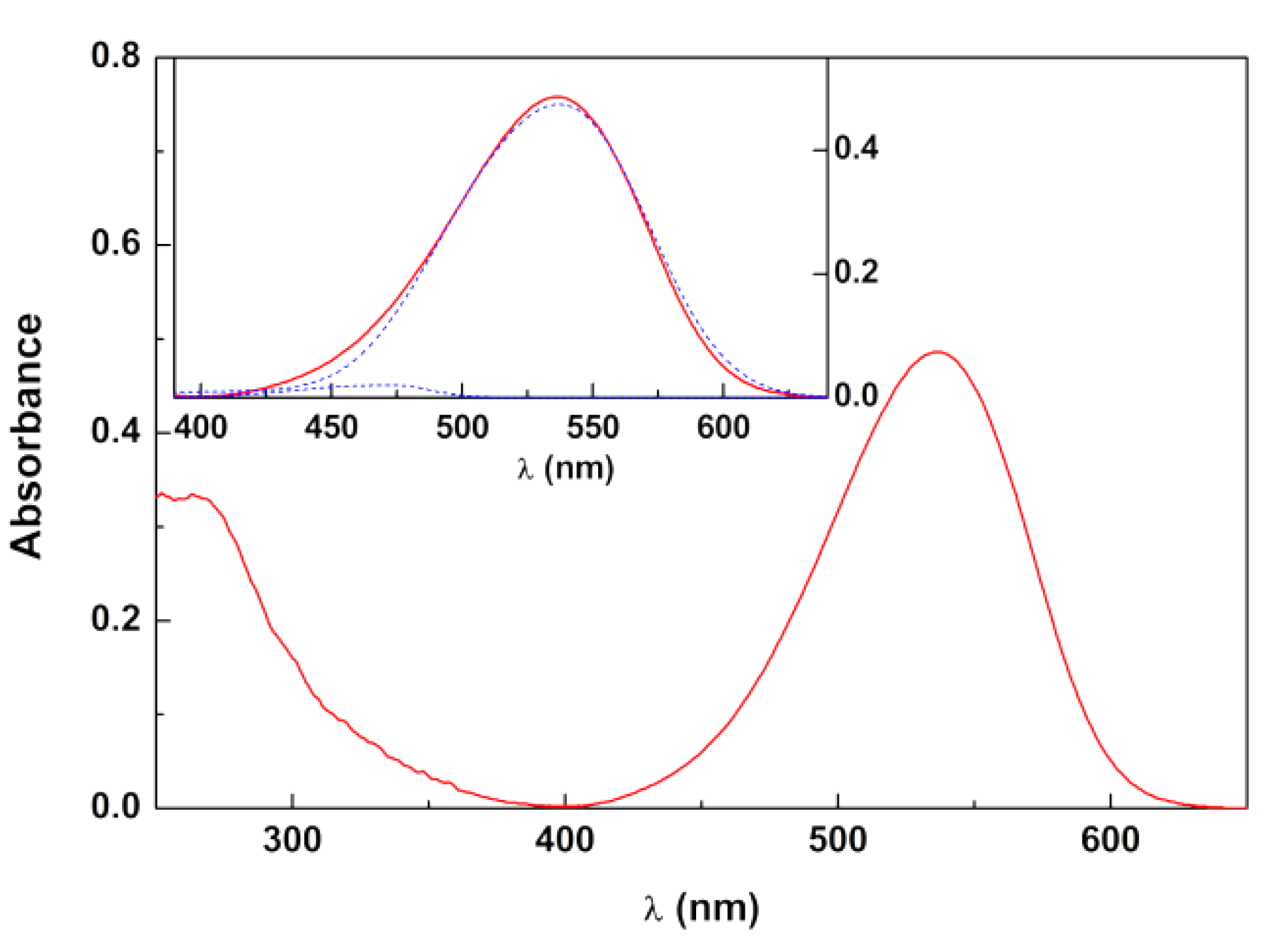
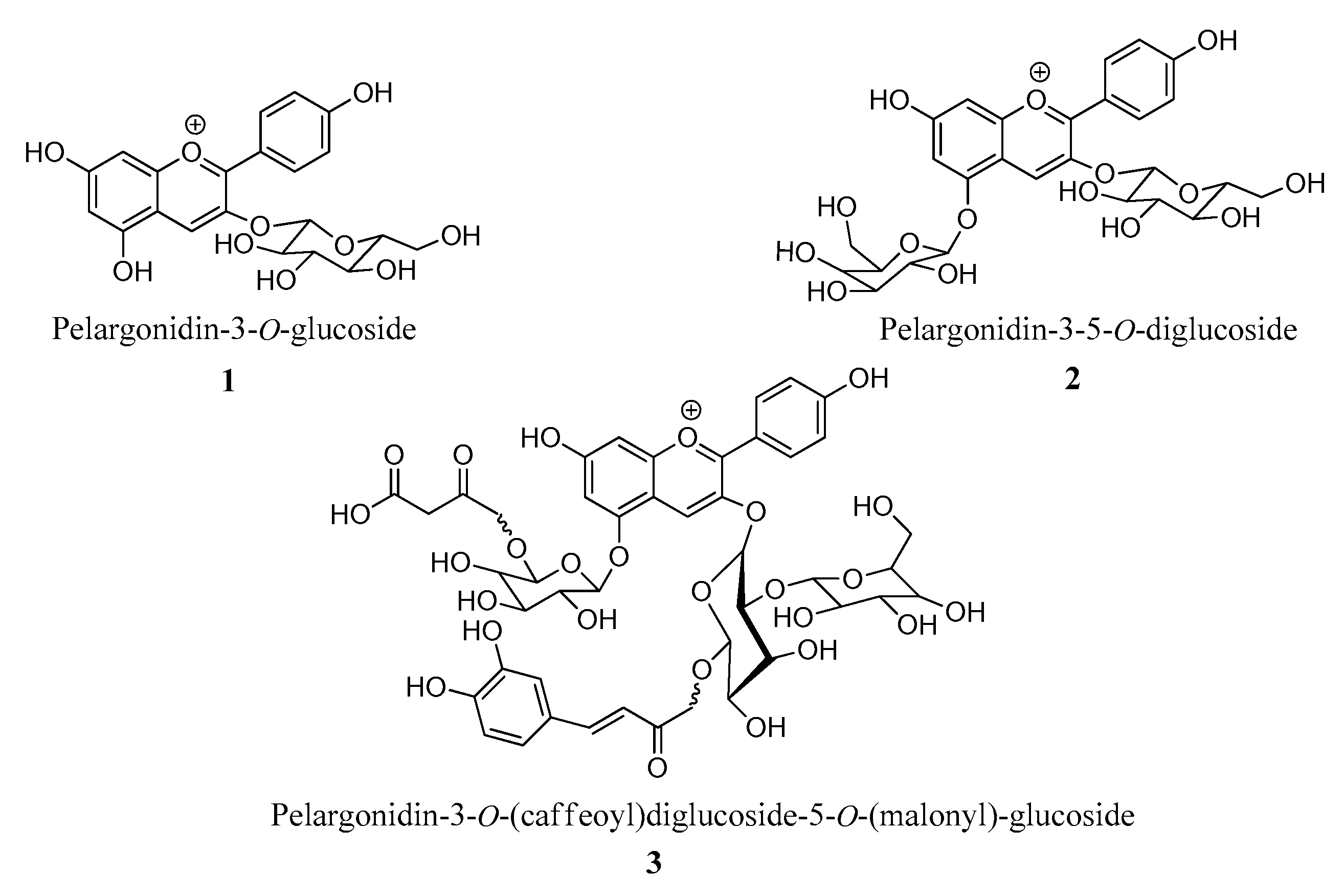
3.1. Betalain- and Anthocyanin-Rich Extracts
3.2. Application of the Extracts to Soy-Based Yogurt Alternative
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McClements, D.J.; Newman, E.; McClements, I.F. Plant-based milks: A review of the science underpinning their design, fabrication, and performance. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2047–2067. [Google Scholar] [CrossRef]
- McClements, D.J. Development of next-generation nutritionally fortified plant-based milk substitutes: Structural design principles. Foods 2020, 9, 421. [Google Scholar] [CrossRef] [PubMed]
- Munekata, P.E.S.; Domínguez, R.; Budaraju, S.; Roselló-Soto, E.; Barba, F.J.; Mallikarjunan, K.; Roohinejad, S.; Lorenzo, J.M. Effect of innovative food processing technologies on the physicochemical and nutritional properties and quality of non-dairy plant-based beverages. Foods 2020, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Grasso, N.; Alonso-Miravalles, L.; O’Mahony, J.A. Composition, physicochemical and sensorial properties of commercial plant-based yogurts. Foods 2020, 9, 252. [Google Scholar] [CrossRef]
- FONA International. Non-Dairy Yogurt 2018-Trend Insight Report; FONA International: Geneva, Switzerland, 2018. [Google Scholar]
- Fukushima, D. Soy Proteins. In Handbook of Food Proteins; Williams, P.A., Phillips, G.O., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 210–232. [Google Scholar]
- Gengatharan, A.; Dykes, G.; Choo, W. Betalains: Natural plant pigments with potential application in functional foods. LWT Food Sci. Technol. 2015, 64, 645–649. [Google Scholar] [CrossRef]
- Choo, W.S. Betalains: Application in functional foods. In Bioactive Molecules in Food; Reference Series in Phytochemistry; Mérillon, J.M., Ramawat, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Khan, M.I. Plant betalains: Safety, antioxidant activity, clinical efficacy, and bioavailability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 316–330. [Google Scholar] [CrossRef]
- Rahimi, P.; Abedimanesh, S.; Mesbah-Namin, S.A.; Ostadrahimi, A. Betalains, the nature-inspired pigments, in health and diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 2949–2978. [Google Scholar] [CrossRef]
- Georgiev, V.G.; Weber, J.; Kneschke, E.; Denev, P.N.; Bley, T.; Pavlov, A.I. Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit dark red. Plant Foods Hum. Nutr. 2010, 65, 105–111. [Google Scholar] [CrossRef]
- Kujala, T.S.; Vienola, M.S.; Klika, K.; Loponen, J.M.; Pihlaja, K. Betalain and phenolic compositions of four beetroot (Beta vulgaris) cultivars. Eur. Food Res. Technol. 2002, 214, 505–510. [Google Scholar] [CrossRef]
- Nemzer, B.; Pietrzkowski, Z.; Spórna, A.; Stalica, P.; Thresher, W.; Michalowski, T.; Wybraniec, S. Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem. 2011, 127, 42–53. [Google Scholar] [CrossRef]
- Chhikaraa, N.; Kushwahaa, K.; Sharmab, P.; Gata, Y.; Panghala, A. Bioactive compounds of beetroot and utilization in food processing industry: A critical review. Food Chem. 2019, 272, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, K.; Ravichandran, P.; Saw, N.M.T.; Gabr, A.M.M.; Ahmed, A.R.; Knorr, D.; Smetanska, I. Effects of different encapsulation agents and drying process on stability of betalains extract. J. Food Sci. Technol. 2012, 51, 2216–2221. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, J.A.; Angosto, J.M.; Giménez, P.J.; León, G.L. Thermal stability of selected natural red extracts used as food colorants. Plant Foods Hum. Nut. 2013, 68, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Šaponjac, V.T.; Canadanovic-Brunet, J.; Cetkovic, G.; Jakišic, M.; Djilas, S.; Vulic, J.; Stajcic, S. Encapsulation of beetroot pomace extract: RSM optimization, storage and gastrointestinal stability. Molecules 2016, 21, 584. [Google Scholar] [CrossRef]
- Ammar, I.; Ennouri, M.; Khem, B.K.; Yangui, T.; Attia, H. Variation in chemical composition and biological activities of two species of Opuntia flowers at four stages of flowering. Ind. Crops Prod. 2012, 37, 34–40. [Google Scholar] [CrossRef]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Opuntia (Cactaceae) plant compounds, biological activities and prospects—a comprehensive review. Food Res. Int. 2018, 112, 328–344. [Google Scholar] [CrossRef]
- Diaz, M.S.S.; Rosa, A.P.B.; Héliès-Toussaint, C.; Guéraud, F.; Nègre-Salvayre, A. Opuntia spp.: Characterization and benefits in chronic diseases. Oxid. Med. Cell. Longev. 2017, 1–7. [Google Scholar] [CrossRef]
- Castellar, M.R.; Solano, F.; Obón, J.M. Betacyanin and other antioxidants production during growth of Opuntia stricta (Haw.) Fruits. Plant Foods Hum. Nut. 2012, 67, 337–343. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Carle, R. Functional properties of anthocyanins and betalains in plants, food and in human nutrition. Food Sci. Technol. 2004, 15, 19–38. [Google Scholar] [CrossRef]
- Obón, J.M.; Castellar, M.R.; Alacid, M.; Fernández-Lopes, J.A. Production of a red-purple food colorant from Opuntia stricta fruits by spray drying and its application in food model systems. J. Food Eng. 2009, 90, 471–479. [Google Scholar] [CrossRef]
- Castellar, R.; Obón, J.M.; Alacid, M.; Fernandez-Lopez, J.A. Color properties and stability of betacyanins from Opuntia stricta fruits. J. Agric. Food Chem. 2003, 51, 2772–2776. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, E.; Sáenz, C.; Aliaga, E.; Aceituno, C. Extraction and characterization of mucilage in Opuntia stricta spp. J. Arid Environ. 2007, 68, 534–545. [Google Scholar] [CrossRef]
- Robert, P.; Torres, V.; García, P.; Vergara, C.; Sáenz, C. The encapsulation of purple cactus pear (Opuntia stricta ficus-indica) pulp by using polysaccharide-proteins as encapsulating agent. LWT Food Sci. Technol. 2014, 60, 1039–1045. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Barragán-Huerta, B.E.; Yáñez-Fernández, J. Use of gelatin-maltodextrin composite as an encapsulation support for clarified juice from purple cactus pear (Opuntia stricta). LWT Food Sci. Technol. 2015, 62, 242–248. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Amperawati, S.; Hastuti, P.; Pranoto, Y.; Santoso, U. The anthocyanins content, colour changes and thermal stability of roselle (Hibiscus sabdariffa L.) petal extract. Int. J. Sci. Res. 2019, 8, 428–435. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.M.; Hucl, P.; Rabalski, I. Compositional and antioxidant properties of anthocyanin-rich products prepared from purple wheat. Food Chem. 2018, 254, 13–19. [Google Scholar] [CrossRef]
- Grajeda-Iglesias, C.; Figueroa-Espinoza, M.C.; Barouh, N.; Baréa, B.; Fernandes, A.; Freitas, V.; Salas, E. Isolation and characterization of anthocyanins from Hibiscus sabdariffa flowers. J. Nat. Prod. 2016, 79, 1709–1718. [Google Scholar] [CrossRef]
- Sabzghabaee, A.M.; Ataei, E.; Kelishadi, R.; Ghannadi, A.; Soltani, R.; Badri, S.; Hirani, S. Effect of Hibiscus sabdariffa calices on dyslipidemia in obese adolescents: A triplemasked randomized controlled trial. Mater. Sociomed. 2013, 25, 76–79. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Muñoz, A.Z.; Beltrán-Rodríguez, U.; Díaz-Díaz, E.; Martínez-Memije, R.; Lans, V.G. Modification of the liver fatty acids by Hibiscus sabdariffa Linnaeus (Malvaceae) infusion, its possible effect on vascular reactivity in a metabolic syndrome model. Clin. Exp. Hypertens. 2014, 36, 123–131. [Google Scholar] [CrossRef]
- Riaz, G.; Chopra, R. A review on phytochemistry and therapeutic uses of Hibiscus sabdariffa L. Biomed. Pharmacother. 2018, 102, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Characterization of red radish anthocyanins. J. Food Sci. 1996, 61, 322–326. [Google Scholar] [CrossRef]
- Giusti, M.M.; Ghanadan, H.; Wrolstad, R.E. Elucidation of the structure and conformation of red radish (Raphanus sativus) anthocyanins using one- and two-dimensional nuclear magnetic resonance techniques. J. Agric. Food Chem. 1998, 46, 4858–4863. [Google Scholar] [CrossRef]
- Prado, M.A.; Godoy, H.T. Corantes artificiais em alimentos. Alim. Nutr. Araraquara 2003, 14, 237–250. [Google Scholar]
- Tavakolifar, F.; Givianrad, M.H.; Saber-Tehrani, M. Extraction of anthocyanins from hibiscus sabdariffa and assessment of its antioxidant properties in extra virgin olive oil. Fresenius Environ. Bull. 2016, 25, 3709–3713. [Google Scholar]
- Tamura, S.; Tsuji, K.; Yongzhen, P.; Ohnishi-Kameyama, M.; Murakami, N. Six new acylated anthocyanins from red radish (Raphanus sativus). Chem. Pharm. Bull. 2010, 58, 1259–1262. [Google Scholar] [CrossRef]
- Dias, S.; Castanheira, E.M.S.; Fortes, A.G.; Pereira, D.M.; Rodrigues, A.R.O.; Pereira, R.; Gonçalves, M.S.T. Application of natural pigments in ordinary cooked ham. Molecules 2020, 25, 2241. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Herbach, K.M.; Mosshammer, M.R.; Carle, R.; Yi, W.; Sellappan, S.; Akoh, C.C.; Bunch, R.; Felker, P. Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia stricta spp.) clones. J. Agric. Food Chem. 2005, 53, 442–451. [Google Scholar] [CrossRef]
- Silva, H.R.P.; Silva, C.; Bolanho, B.C. Ultrasonic-assisted extraction of betalains from red beet (Beta vulgaris L.). J. Food Proc. Eng. 2018, 41, e12833. [Google Scholar] [CrossRef]
- Gonçalves, L.C.P.; Trassi, M.A.S.; Lopes, N.B.; Dörr, F.A.; Santos, M.T.; Baader, W.J.; Oliveira, V.X., Jr.; Bastos, E.L. A comparative study of the purification of betanin. Food Chem. 2012, 131, 231–238. [Google Scholar] [CrossRef]
- Otálora, M.C.; Carriazo, J.G.; Iturriaga, L.; Nazareno, M.A.; Osorio, C. Microencapsulation of betalains obtained from cactus fruit (Opuntia ficus-indica) by spray drying using cactus cladode mucilageand maltodextrin as encapsulating agentes. Food Chem. 2015, 87, 174–181. [Google Scholar] [CrossRef] [PubMed]
- List, G.R. Soybean lecithin: Food, industrial uses, and other applications. In Polar Lipids: Biology, Chemistry, and Technology; Ahmad, M.U., Xu, X., Eds.; Academic Press and AOCS Press: Urbana, IL, USA, 2015; pp. 1–33. [Google Scholar]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Thin-film hydration followed by extrusion method for liposome preparation. Methods Mol. Biol. 2017, 1522, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Malien-Aubert, C.; Dangles, O.; Amiot, M.J. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition protective effects by intra and intermolecular copigmentation. J. Agric. Food Chem. 2001, 49, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Matsufuji, H.; Kido, H.; Misawa, G.; Yaguchi, J.; Otsuki, T.; Chino, M.; Takeda, M.; Yamagata, K. Stability to light, heat, and hydrogen peroxide at different pH values and dpph radical scavenging activity of acylated anthocyanins from red radish extract. J. Agric. Food Chem. 2007, 55, 3692–3701. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.S.; von Elbe, J.H. Effect of pH on the degradation and regeneration of betanine. J. Food Sci. 1987, 52, 1689–1693. [Google Scholar] [CrossRef]
- Jing, P.; Zhao, S.; Ruan, S.; Xie, Z.; Dong, Y.; Yu, L. Anthocyanin and glucosinolate occurrences in the roots of chinese red radish (Rhapanus sativus L.), and their stability to heat and pH. Food Chem. 1987, 133, 1569–1576. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Trigueros, L.; Wojdyło, A.; Carbonell-Barrachina, Á.A.; Sendra, E. Anthocyanins decay in pomegranate enriched fermented milks as a function of bacterial strain and processing conditions. LWT 2017, 80, 193–199. [Google Scholar] [CrossRef]
- Sánchez-Bravo, P.; Zapata, P.J.; Martínez-Esplá, A.; Carbonell-Barrachina, Á.A.; Sendra, E. Antioxidant and anthocyanin content in fermented milks with sweet cherry is affected by the starter culture and the ripening stage of the cherry. Beverages 2018, 4, 57. [Google Scholar] [CrossRef]
 : red beetroot;
: red beetroot;  : opuntia;
: opuntia;  : hibiscus;
: hibiscus;  : red radish;
: red radish; : commercial strawberry flavor yogurt.
: commercial strawberry flavor yogurt.
 : red beetroot;
: red beetroot;  : opuntia;
: opuntia;  : hibiscus;
: hibiscus;  : red radish;
: red radish; : commercial strawberry flavor yogurt.
: commercial strawberry flavor yogurt.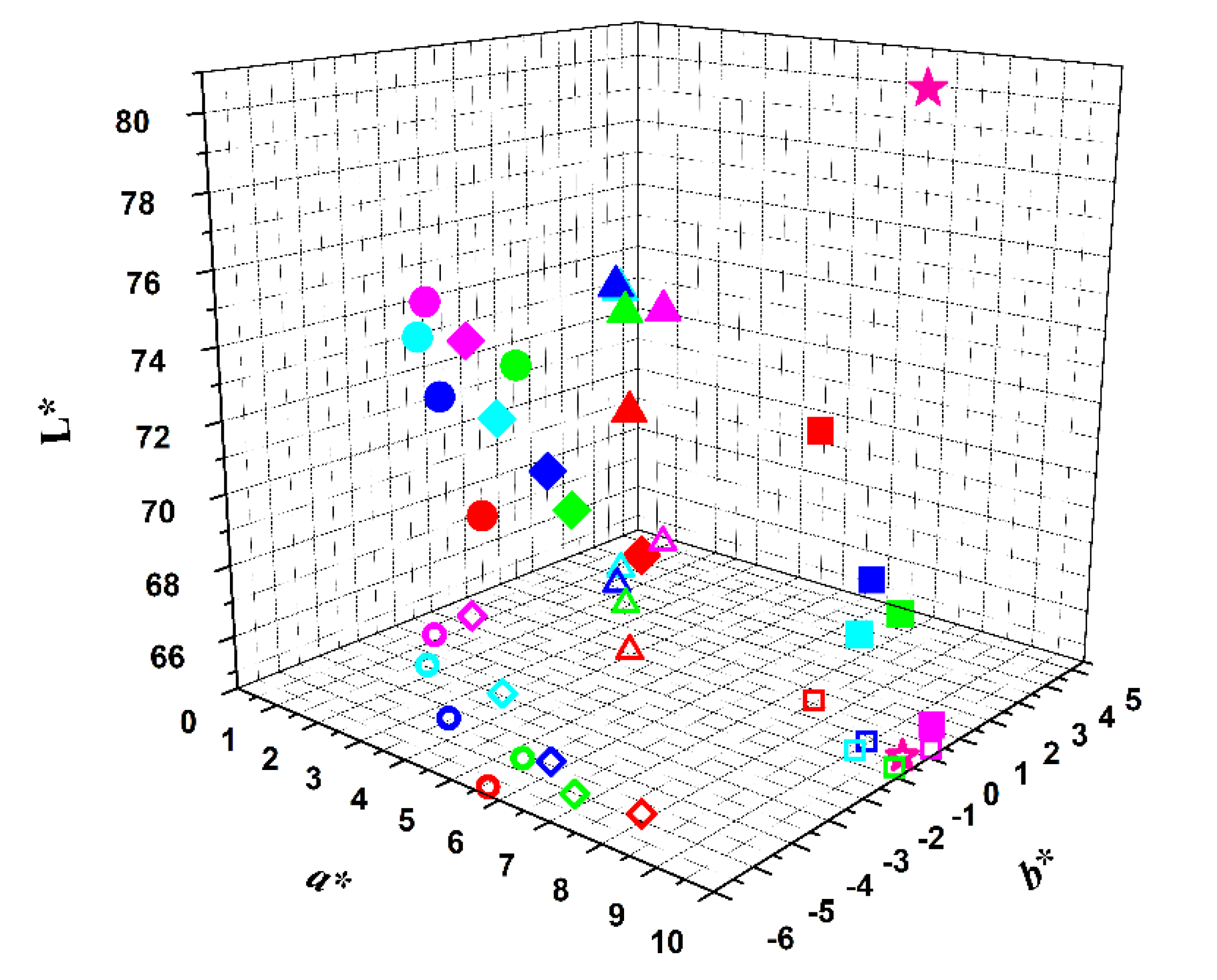
 : red beetroot;
: red beetroot;  : opuntia;
: opuntia;  : hibiscus;
: hibiscus;  : red radish.
: red radish.
 : red beetroot;
: red beetroot;  : opuntia;
: opuntia;  : hibiscus;
: hibiscus;  : red radish.
: red radish.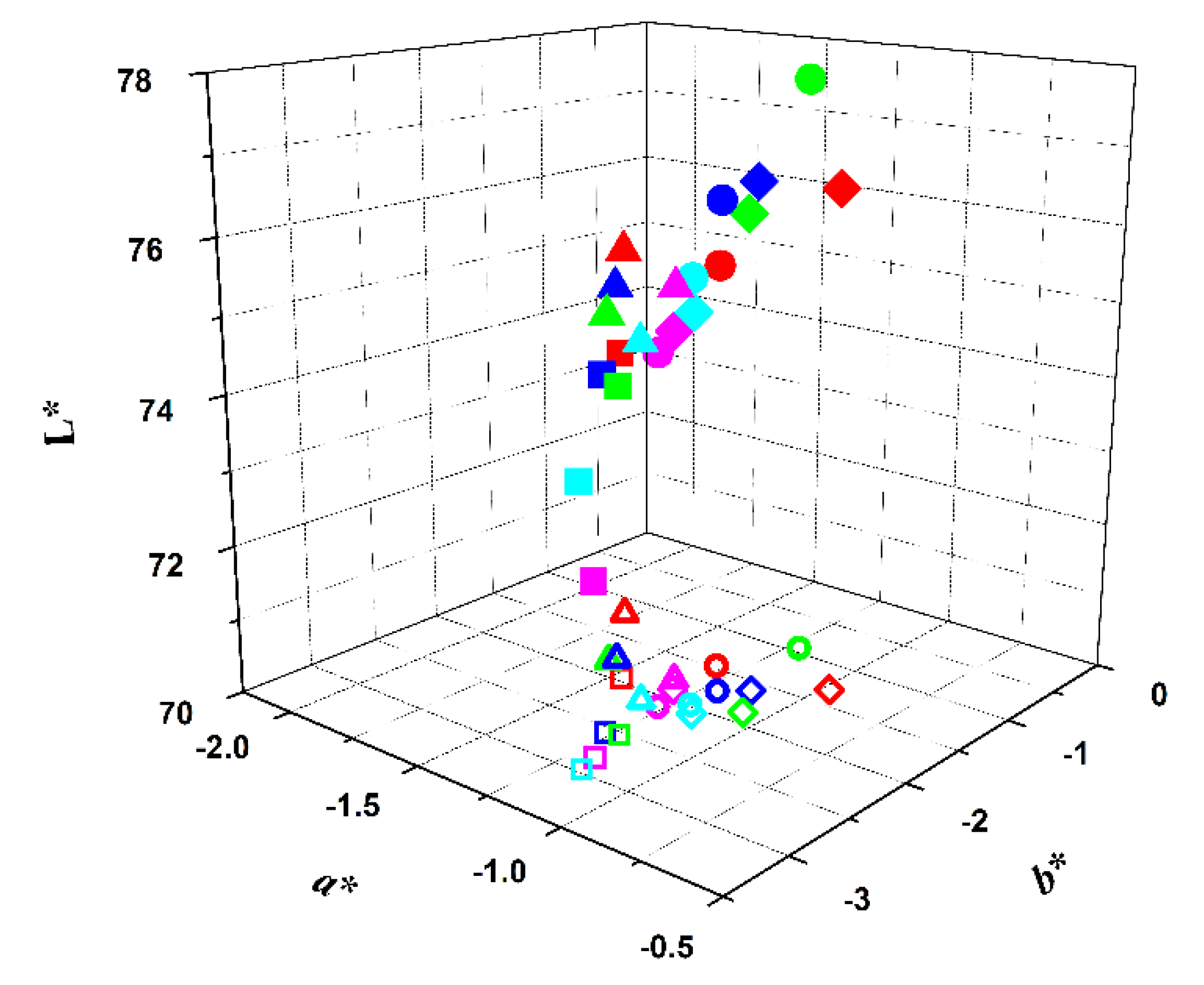
 : red beetroot;
: red beetroot;  : opuntia;
: opuntia;  : hibiscus;
: hibiscus;  : red radish.
: red radish.
 : red beetroot;
: red beetroot;  : opuntia;
: opuntia;  : hibiscus;
: hibiscus;  : red radish.
: red radish.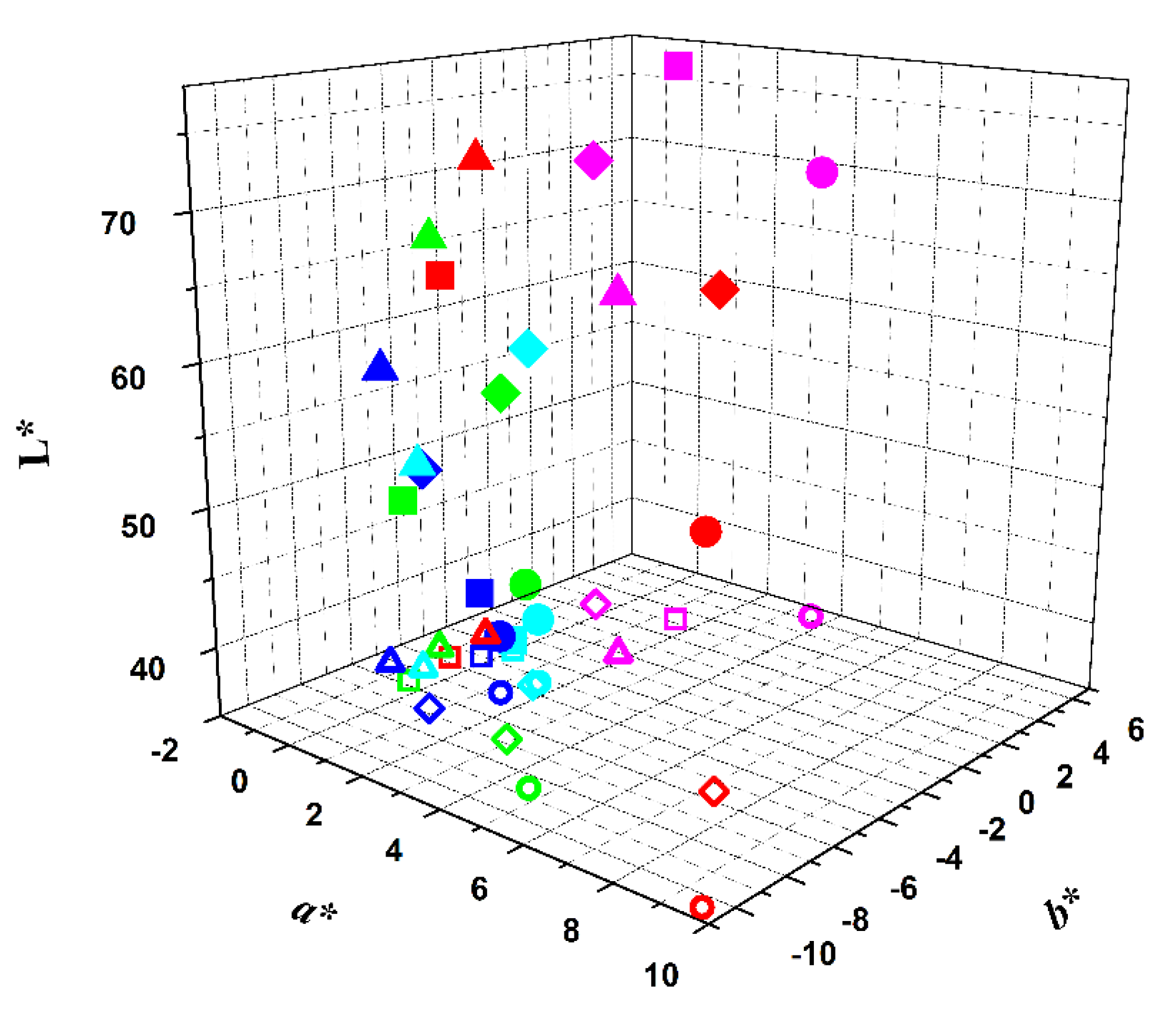
| Extract | Betalains (mg/L) | Anthocyanins (g/L) | |
|---|---|---|---|
| Betacyanins | Betaxanthins | ||
| Red Beetroot | 20.1 1 | 4.27 1 | - |
| Opuntia | 4.10 | 0.13 | - |
| Hibiscus | - | - | 13.01 1 |
| Red radish | - | - | 1.27 1 |
| Entry | M+ | Compound |
|---|---|---|
| 1 | 403 | - |
| 2 | 271 | Pelargonidin |
| 3 | 433 | Pelargonidin-3-O-glucoside |
| 4 | 1181 | Pelargonidin-3-O-(caffeoyl, feruloyl)-diglucoside-5-O-(malonyl)-glucoside |
| 5 | 989 | Pelargonidin-3-O-(p-coumaroyl)diglucoside-5-O-(malonyl)-glucoside |
| 6 | 1005 | Pelargonidin-3-O-(caffeoyl)diglucoside-5-O-glucoside |
| 7 | 1019 | Pelargonidin-3-O-(feruloyl)sophoroside-5-O-glucoside |
| 8 | 1135 | Pelargonidin-(p-coumaroyl,p-coumaroyl)-diglucoside-5-O-(malonyl)-glucoside |
| 9 | 1165 | Pelargonidin-3-O-(feruloyl,p-coumaroyl)-diglucoside-5-O-(malonyl)-glucoside |
| Species | Ethanolic Injection | Thin Film Hydration |
|---|---|---|
| Red Beetroot | 98.4 ± 3.1 1 | 99.3 ± 3.6 1 |
| Opuntia | 90.9 ± 1.8 | 92.8 ± 2.0 |
| Hibiscus | 99.2 ± 4.8 1 | 98.1 ± 2.9 1 |
| Red radish | 99.9 ± 6.2 1 | 99.6 ± 4.2 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dias, S.; Castanheira, E.M.S.; Fortes, A.G.; Pereira, D.M.; Gonçalves, M.S.T. Natural Pigments of Anthocyanin and Betalain for Coloring Soy-Based Yogurt Alternative. Foods 2020, 9, 771. https://doi.org/10.3390/foods9060771
Dias S, Castanheira EMS, Fortes AG, Pereira DM, Gonçalves MST. Natural Pigments of Anthocyanin and Betalain for Coloring Soy-Based Yogurt Alternative. Foods. 2020; 9(6):771. https://doi.org/10.3390/foods9060771
Chicago/Turabian StyleDias, Sandra, Elisabete M. S. Castanheira, A. Gil Fortes, David M. Pereira, and M. Sameiro T. Gonçalves. 2020. "Natural Pigments of Anthocyanin and Betalain for Coloring Soy-Based Yogurt Alternative" Foods 9, no. 6: 771. https://doi.org/10.3390/foods9060771
APA StyleDias, S., Castanheira, E. M. S., Fortes, A. G., Pereira, D. M., & Gonçalves, M. S. T. (2020). Natural Pigments of Anthocyanin and Betalain for Coloring Soy-Based Yogurt Alternative. Foods, 9(6), 771. https://doi.org/10.3390/foods9060771