Effects of Chitosan Coating with Green Tea Aqueous Extract on Lipid Oxidation and Microbial Growth in Pork Chops during Chilled Storage
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extract Preparation
2.3. Coating Preparation
2.4. Polyphenols Content and Biological Activity
2.4.1. Total Phenolic Content
2.4.2. Antiradical Assay
2.4.3. Antimicrobial Assay
2.5. Pork Meat Preparation and Storage
2.6. Pork Quality Measurements
2.6.1. pH measurement
2.6.2. Colour Measurement
2.6.3. Lipid Oxidation
2.6.4. Microbial Growth
2.7. Statistical Analysis
3. Results
3.1. In vitro Polyphenol Content and Biological Properties
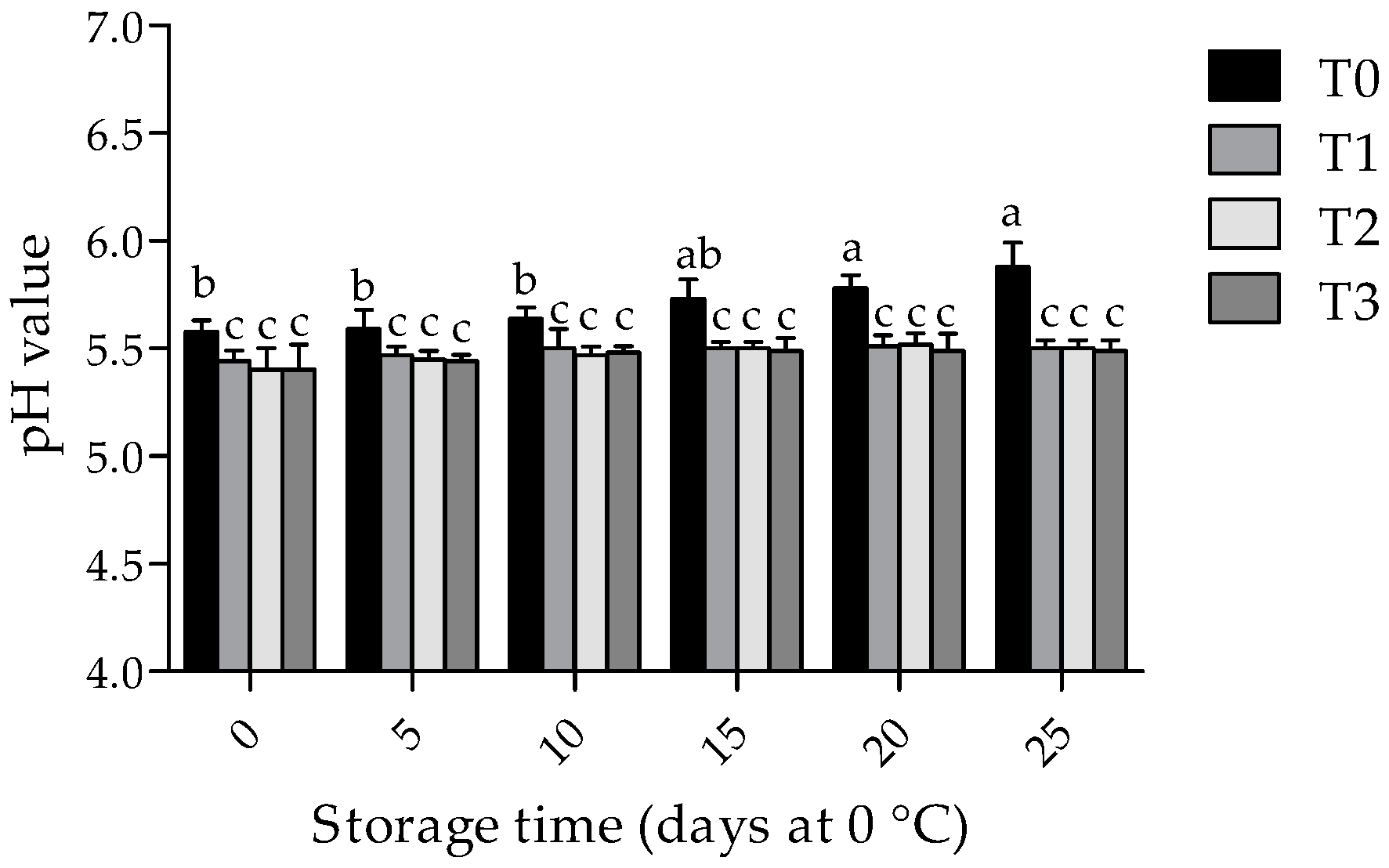
3.2. Physicochemical Changes in Pork Samples during Storage Times
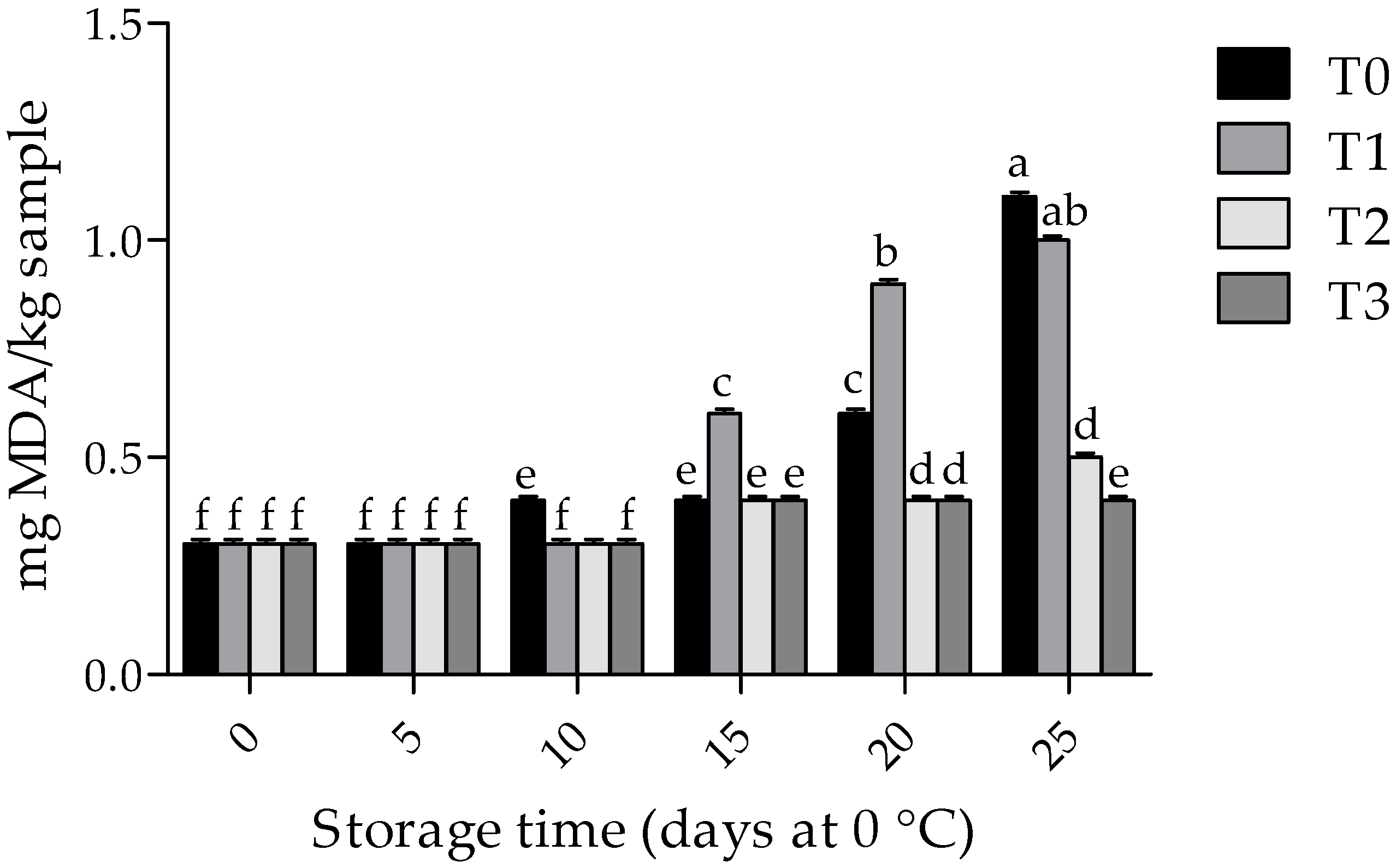
3.3. Lipid Oxidation
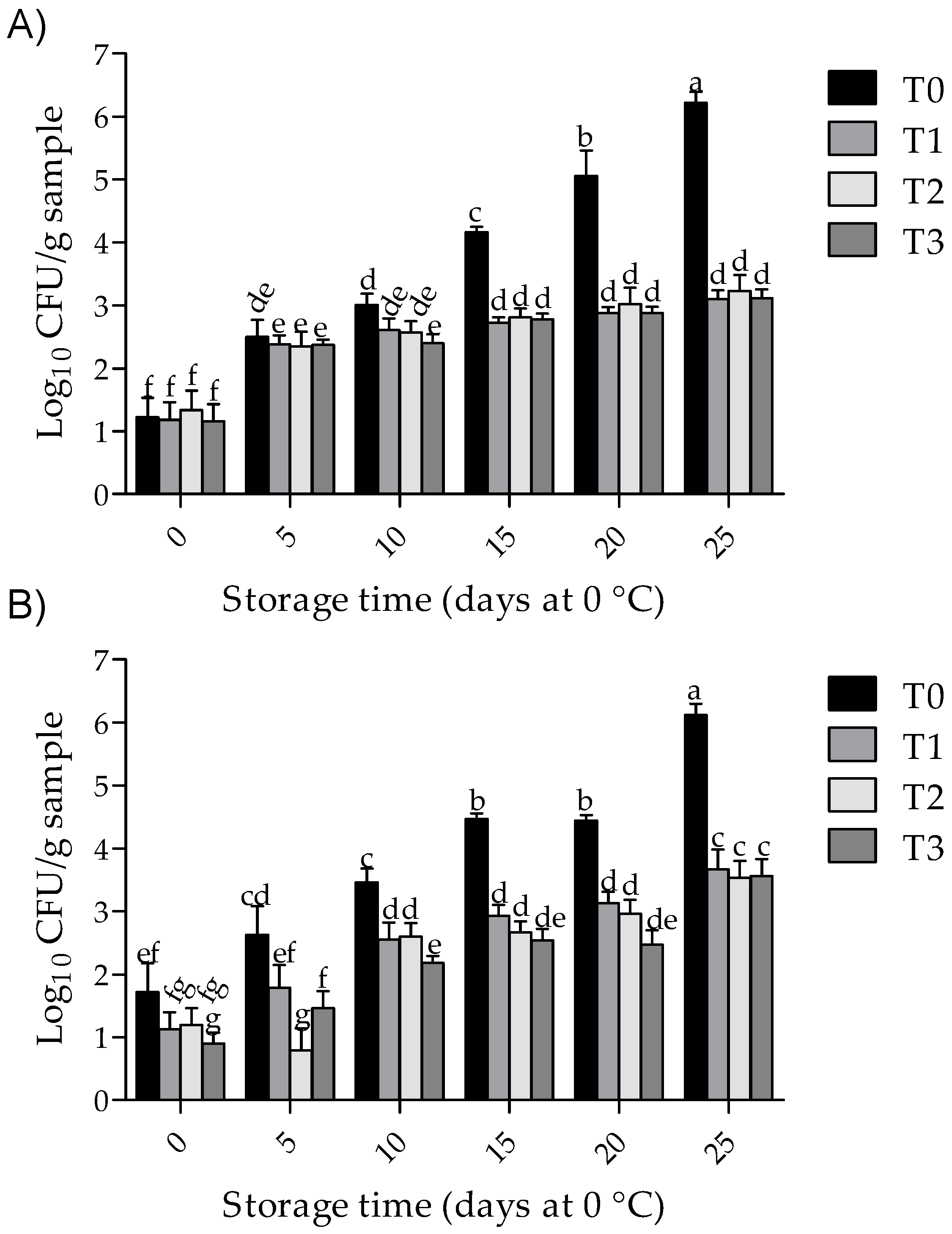
3.4. Total Aerobic Bacterial Counts
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- USDA Livestock and Poultry: World Markets and Trade. Available online: https://apps.fas.usda.gov/psdonline/circulars/livestock_poultry.pdf (accessed on 10 January 2019).
- Castañeda-Acuña, E.R.; Méndez-Miranda, M.V.; Rocha-Zavaleta, A.S. Carne de res en Cortes Empacada al alto Vacío a Beijing, China. Bachelor’s Thesis, Universidad Veracruzana, Xalapa-Enríquez Veracruz, Mexico, August 2011. [Google Scholar]
- Hales, K.C. Refrigerated transport on shipboard. In Advances in Food Research; Academic Press: Cambridge, UK, 1963; pp. 147–201. [Google Scholar]
- Williams, P. Nutritional composition of red meat. Nutr. Diet. 2007, 64, S113–S119. [Google Scholar] [CrossRef]
- Wood, J.; Richardson, I.; Nute, G.; Fisher, A.; Campo, M.M.; Kasapidou, E.; Sheard, P.R.; Enser, M. Effects of fatty acids on meat quality: A review. Meat Sci. 2004, 66, 21–32. [Google Scholar] [CrossRef]
- Nair, M.S.; Nair, D.V.; Johny, A.K.; Venkitanarayanan, K. Use of food preservatives and additives in meat and their detection techniques. In Meat Quality Analysis; Academic Press: Cambridge, UK, 2020; pp. 187–213. [Google Scholar]
- FDA Substances Added to Food (Formerly EAFUS). Available online: https://www.fda.gov/food/food-additives-petitions/substances-added-food-formerly-eafus (accessed on 10 January 2019).
- Kahl, R.; Kappus, H. Toxicology of the synthetic antioxidants BHA and BHT in comparison with the natural antioxidant vitamin E. Z. Lebens. Unters. F A 1993, 196, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Martinez, Z.; Aditya, A.; Biswas, D. Plant antioxidants, extraction strategies, and their application in meat. In Meat Quality Analysis; Academic Press: Cambridge, UK, 2020; pp. 241–264. [Google Scholar]
- Ladikos, D.; Lougovois, V. Lipid oxidation in muscle foods: A review. Food Chem. 1990, 35, 295–314. [Google Scholar] [CrossRef]
- Fabra, M.J.; Falcó, I.; Randazzo, W.; Sanchez, G.; López-Rubio, A.; López, A. Antiviral and antioxidant properties of active alginate edible films containing phenolic extracts. Food Hydrocoll. 2018, 81, 96–103. [Google Scholar] [CrossRef]
- Kabanov, V.L.; Novinyuk, L. Chitosan application in food technology: A review of rescent advances. Food Syst. 2020, 3, 10–15. [Google Scholar] [CrossRef]
- Perva-Uzunalić, A.; Škerget, M.; Knez, Ž.; Weinreich, B.; Otto, F.; Grüner, S. Extraction of active ingredients from green tea (Camellia sinensis): Extraction efficiency of major catechins and caffeine. Food Chem. 2006, 96, 597–605. [Google Scholar] [CrossRef]
- Vásconez, M.B.; Flores, S.; Campos, C.A.; Alvarado, J.; Gerschenson, L.N. Antimicrobial activity and physical properties of chitosan–tapioca starch based edible films and coatings. Food Res. Int. 2009, 42, 762–769. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Jorgensen, J.H.; Turnidge, J.D.; Washington, J.A. Antibacterial susceptibility tests: Dilution and disk diffusion methods. In Manual of Clinical Microbiology; Murray, P.R., Jo Baron, E., Pfaller, M.A., Tenover, F.C., Yolken, R.H., Eds.; ASM Press: Washington, DC, USA, 1999; pp. 1526–1543. [Google Scholar]
- AOAC. Official methods of analysis. In Association of Official Analytical Chemists, 18th ed.; Association of Official Analytical Chemists: Gaitherburg, MD, USA, 2005. [Google Scholar]
- Robertson, A.R.; Lozano, R.D.; Alman, D.H.; Orchard, S.E.; Keitch, J.A.; Connely, R.; Graham, L.A.; Acree, W.L.; John, R.S.; Hoban, R.F.; et al. CIE recommendations on uniform color spaces, color-difference equations, and metric color terms. Color Res. Appl. 1977, 2, 5–6. [Google Scholar]
- Pfalzgraf, A.; Frigg, M.; Steinhart, H. Alpha.-tocopherol contents and lipid oxidation in pork muscle and adipose tissue during storage. J. Agric. Food Chem. 1995, 43, 1339–1342. [Google Scholar] [CrossRef]
- NOM-110-SSA1-1994 Preparación y Dilución de Muestras de Alimentos Para su Análisis Microbiológico. Available online: http://www.salud.gob.mx/unidades/cdi/nom/110ssa14.html. (accessed on 5 February 2020).
- Graham, H.N. Green tea composition, consumption, and polyphenol chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Soh, E.Y.; Tie, P.P.; Law, Y.P. Antioxidant and antibacterial properties of green, black, and herbal teas of Camellia sinensis. Pharmacogn. Res. 2011, 3, 266–272. [Google Scholar] [CrossRef]
- Tsai, T.-H.; Tsai, T.-H.; Chien, Y.-C.; Lee, C.-W.; Tsai, P.-J. In vitro antimicrobial activities against cariogenic streptococci and their antioxidant capacities: A comparative study of green tea versus different herbs. Food Chem. 2008, 110, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Atarés, L.; Vargas, M.; Chiralt, A. Edible films and coatings to prevent the detrimental effect of oxygen on food quality: Possibilities and limitations. J. Food Eng. 2012, 110, 208–213. [Google Scholar] [CrossRef]
- Cutter, C.N. The effectiveness of triclosan-incorporated plastic against bacteria on beef surfaces. J. Food Prot. 1999, 62, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Ruengwilysup, C.; Fung, D.Y.C. Antibacterial effect of water-soluble tea extracts on foodborne pathogens in laboratory medium and in a food model. J. Food Prot. 2004, 67, 2608–2612. [Google Scholar] [CrossRef] [PubMed]
- Carlson, R.P.; Taffs, R.; Davison, W.M.; Stewart, P.S. Anti-biofilm properties of chitosan-coated surfaces. J. Biomater. Sci. Polym. Ed. 2008, 19, 1035–1046. [Google Scholar] [CrossRef]
- Liu, X.F.; Guan, Y.; Yang, D.Z.; Li, Z.; De Yao, K. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2000, 79, 1324–1335. [Google Scholar] [CrossRef]
- Zheng, L.-Y.; Zhu, J. Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr. Polym. 2003, 54, 527–530. [Google Scholar] [CrossRef]
- Anesini, C.; Ferraro, G.E.; Filip, R. Total polyphenol content and antioxidant capacity of commercially available tea (Camellia sinensis) in argentina. J. Agric. Food Chem. 2008, 56, 9225–9229. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; He, J.; Ban, X.; Zeng, H.; Yao, X.; Wang, Y. Antioxidant activity of bovine and porcine meat treated with extracts from edible lotus (Nelumbo nucifera) rhizome knot and leaf. Meat Sci. 2011, 87, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Custódio, F.B.; Vasconcelos-Neto, M.C.; Theodoro, K.H.; Chisté, R.; Gloria, M.B.A. Assessment of the quality of refrigerated and frozen pork by multivariate exploratory techniques. Meat Sci. 2018, 139, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.W.; Chao, J.Y. Quality characteristics of reduced-fat Chinese-style sausage as related to chitosan’s molecular weight. Meat Sci. 2001, 59, 343–351. [Google Scholar] [CrossRef]
- Lanari, M.; Schaefer, D.; Scheller, K. Dietary vitamin E supplementation and discoloration of pork bone and muscle following modified atmosphere packaging. Meat Sci. 1995, 41, 237–250. [Google Scholar] [CrossRef]
- Mancini, R.; Hunt, M. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, M.R.; Mohamed, M.A.; Mohamed, H.M.; Emara, M.M.; Mohmaed, M.A. Improving the sensory, physicochemical and microbiological quality of pastirma (A traditional dry cured meat product) using chitosan coating. LWT 2017, 86, 247–253. [Google Scholar] [CrossRef]
- Salueña, B.H.; Sáenz, C.; Rubial, J.M.D.; Odriozola, C.A. Cielab color paths during meat shelf life. Meat Sci. 2019, 157, 107889. [Google Scholar] [CrossRef] [PubMed]
- Raines, C.; Dikeman, M.E.; Grobbel, J.; Yancey, E. Effects of ascorbic acid and Origanox™ in different packaging systems to prevent pork lumbar vertebrae discoloration. Meat Sci. 2006, 74, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Nicolalde, C.; Stetzer, A.; Tucker, E.; McKeith, F.; Brewer, M. Antioxidant and modified atmosphere packaging prevention of discoloration in pork bones during retail display. Meat Sci. 2006, 72, 713–718. [Google Scholar] [CrossRef]
- Qin, Y.; Yang, J.-Y.; Lu, H.-B.; Wang, S.-S.; Yang, J.; Yang, X.-C.; Chai, M.; Li, L.; Cao, J.-X. Effect of chitosan film incorporated with tea polyphenol on quality and shelf life of pork meat patties. Int. J. Biol. Macromol. 2013, 61, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, P.; Tajik, H.; Rohani, S.M.R.; Moradi, M.; Hashemi, M.; Aliakbarlu, J. Effect of functional chitosan coating and gamma irradiation on the shelf-life of chicken meat during refrigerated storage. Radiat. Phys. Chem. 2017, 141, 103–109. [Google Scholar] [CrossRef]
- Kanatt, S.R.; Rao, M.; Chawla, S.P.; Sharma, A. Effects of chitosan coating on shelf-life of ready-to-cook meat products during chilled storage. LWT 2013, 53, 321–326. [Google Scholar] [CrossRef]
- Yen, M.-T.; Yang, J.-H.; Mau, J. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelub, K.; Kumara, N. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
| Treatment 1 | TPHC (mg GAE/g) | FRSA (%) | Antimicrobial Assay (%) | |||
|---|---|---|---|---|---|---|
| S. aureus | L. innocua | E. coli | S. typhimurium | |||
| GTWE | 582.7 ± 24.0 a | 90.7 ± 1.0 a | 86.8 ± 0.2 c | 95.1 ± 0.22 c | 94.6 ± 0.10 d | 91.7 ± 0.17 c |
| ChC—0% | 0.03 ± 0.01 d | 0.16 ± 0.01 c | 58.6 ± 1.2 d | 64.1 ± 1.3 e | 49.7 ± 0.11 e | 82.2 ± 1.3 d |
| ChC—0.1% | 0.67 ± 0.05 c | 71.9 ± 2.2 b | 86.6 ± 0.10 c | 94.9 ± 0.29 d | 96.1 ± 0.06 c | 93.1 ± 0.13 c |
| ChC—0.5% | 3.3 ± 0.23 b | 92.5 ± 1.1 a | 96.6 ± 0.10 b | 97.6 ± 0.22 b | 97.7 ± 0.17 b | 97.4 ± 0.19 b |
| Asc ac | - | 94.0 ± 2.5 a | - | - | - | - |
| Gentamicin | - | - | 99.2 ± 0.10 a | 99.1 ± 0.11 a | 99.5 ± 0.06 a | 99.5 ± 0.06 a |
| Item | Treatment | Storage Time (Days) | |||||
|---|---|---|---|---|---|---|---|
| 0 | 5 | 10 | 15 | 20 | 25 | ||
| L *1 | T0 | 52.50 ± 1.93 b | 59.02 ± 1.22 a | 58.48 ± 1.72 a | 61.08 ± 1.28 a | 61.03 ± 1.09 a | 60.40 ± 1.36 a |
| T1 | 52.31 ± 2.68 b | 52.57 ± 2.16 b | 54.12 ± 1.64 b | 55.54 ± 1.25 b | 58.50 ± 1.48 a | 58.76 ± 1.98 a | |
| T2 | 53.47 ± 2.16 b | 54.34 ± 2.31 b | 54.27 ± 1.99 b | 55.80 ± 1.51 b | 55.24 ± 1.38 b | 56.04 ± 1.40 b | |
| T3 | 54.33 ± 1.85 b | 55.79 ± 2.17 b | 55.91 ± 0.78 b | 56.33 ± 1.54 b | 54.50 ± 1.63 b | 54.84 ± 1.17 b | |
| a *1 | T0 | 2.56 ± 0.43 a | 2.05 ± 0.38 a | 1.55 ± 0.33 b | 1.20 ± 0.29 c | 1.22 ± 0.32 c | 1.19 ± 0.02 c |
| T1 | 1.76 ± 0.60 b | 1.60 ± 0.57 b | 1.54 ± 0.43 b | 1.25 ± 0.24 c | 1.26 ± 0.18 c | 1.14 ± 0.49 c | |
| T2 | 1.78 ± 0.52 b | 1.71 ± 0.36 b | 1.65 ± 0.18 b | 1.61 ± 0.32 b | 1.60 ± 0.18 b | 1.61 ± 0.10 b | |
| T3 | 1.78 ± 0.36 b | 1.70 ± 0.24 b | 1.72 ± 0.07 b | 1.62 ± 0.22 b | 1.60 ± 0.12 b | 1.59 ± 0.11 b | |
| b *1 | T0 | 11.83 ± 0.58 c | 12.78 ± 0.19 b,c | 12.07 ± 0.63 b,c | 12.69 ± 0.21 b,c | 13.30 ± 0.75 a,b | 13.84 ± 0.35 a |
| T1 | 10.44 ± 1.01 c | 10.10 ± 1.25 c | 10.94 ± 1.02 c | 11.36 ± 0.78 c | 13.13 ± 0.90 a,b | 13.22 ± 0.22 a | |
| T2 | 10.49 ± 1.21 c | 11.22 ± 0.95 c | 11.35 ± 1.05 c | 11.50 ± 1.04 c | 11.46 ± 0.80 c | 11.18 ± 0.79 c | |
| T3 | 10.51 ± 1.57 c | 11.73 ± 0.89 c | 11.03 ± 1.34 c | 11.12 ± 1.32 c | 11.84 ± 0.83 c | 11.85 ± 0.65 c | |
| L *2 | T0 | 38.60 ± 1.37 d | 38.96 ± 0.79 d | 43.73 ± 1.39 c | 54.37 ± 1.77 a | 53.61 ± 1.42 a | 52.34 ± 1.55 a |
| T1 | 45.80 ± 1.94 c | 43.17 ± 2.25 c | 43.69 ± 1.23 c | 51.24 ± 0.94 b | 54.45 ± 1.19 a | 54.27 ± 1.87 a | |
| T2 | 43.82 ± 1.35 c | 45.04 ± 1.71 c | 46.47 ± 2.89 b,c | 51.88 ± 1.03 b | 49.33 ± 1.22 b | 48.13 ± 0.66 b | |
| T3 | 43.74 ± 2.32 c | 43.70 ± 0.70 c | 48.83 ± 0.82 b,c | 50.67 ± 1.27 b | 50.04 ± 1.75 b | 49.65 ± 1.52 b | |
| a *2 | T0 | 20.17 ± 1.70 a | 22.82 ± 1.49 a | 18.21 ± 0.03 b | 6.14 ± 1.37 e | 6.94 ± 1.16 e,f | 5.08 ± 0.46 f |
| T1 | 13.64 ± 0.82 c | 12.56 ± 0.87 c | 8.76 ± 0.66 e | 6.44 ± 0.71 e | 6.39 ± 0.55 e | 5.05 ± 0.83 e,f | |
| T2 | 12.30 ± 0.54 c | 11.43 ± 1.73 c,d | 11.83 ± 0.37 d | 8.23 ± 1.82 e | 7.51 ± 0.47 e | 6.20 ± 0.52 e | |
| T3 | 14.52 ± 0.98 c | 13.80 ± 1.51 c | 10.88 ± 1.62 d | 7.92 ± 0.31 e | 7.37 ± 0.53 e | 6.76 ± 0.53 e | |
| b *2 | T0 | 17.71 ± 1.84 a | 17.44 ± 1.55 a | 17.19 ± 0.70 a | 12.73 ± 0.54 b | 10.74 ± 1.52 c | 10.35 ± 1.28 c |
| T1 | 17.59 ± 1.56 a | 16.11 ± 1.33 a | 12.15 ± 0.70 b | 9.71 ± 0.72 c | 9.91 ± 0.78 c | 9.69 ± 0.87 c | |
| T2 | 14.29 ± 1.58 a,b | 14.26 ± 1.82 a,b | 11.09 ± 1.00 b,c | 10.91 ± 1.69 c | 10.69 ± 0.58 c | 10.82 ± 0.30 c | |
| T3 | 14.43 ± 1.41 a,b | 12.31 ± 1.19 b | 11.52 ± 0.95 b,c | 10.72 ± 1.87 c | 10.79 ± 0.33 c | 10.42 ± 1.21 c | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montaño-Sánchez, E.; Torres-Martínez, B.d.M.; Vargas-Sánchez, R.D.; Huerta-Leidenz, N.; Sánchez-Escalante, A.; Beriain, M.J.; Torrescano-Urrutia, G.R. Effects of Chitosan Coating with Green Tea Aqueous Extract on Lipid Oxidation and Microbial Growth in Pork Chops during Chilled Storage. Foods 2020, 9, 766. https://doi.org/10.3390/foods9060766
Montaño-Sánchez E, Torres-Martínez BdM, Vargas-Sánchez RD, Huerta-Leidenz N, Sánchez-Escalante A, Beriain MJ, Torrescano-Urrutia GR. Effects of Chitosan Coating with Green Tea Aqueous Extract on Lipid Oxidation and Microbial Growth in Pork Chops during Chilled Storage. Foods. 2020; 9(6):766. https://doi.org/10.3390/foods9060766
Chicago/Turabian StyleMontaño-Sánchez, Eduardo, Brisa del Mar Torres-Martínez, Rey David Vargas-Sánchez, Nelson Huerta-Leidenz, Armida Sánchez-Escalante, María J. Beriain, and Gastón R. Torrescano-Urrutia. 2020. "Effects of Chitosan Coating with Green Tea Aqueous Extract on Lipid Oxidation and Microbial Growth in Pork Chops during Chilled Storage" Foods 9, no. 6: 766. https://doi.org/10.3390/foods9060766
APA StyleMontaño-Sánchez, E., Torres-Martínez, B. d. M., Vargas-Sánchez, R. D., Huerta-Leidenz, N., Sánchez-Escalante, A., Beriain, M. J., & Torrescano-Urrutia, G. R. (2020). Effects of Chitosan Coating with Green Tea Aqueous Extract on Lipid Oxidation and Microbial Growth in Pork Chops during Chilled Storage. Foods, 9(6), 766. https://doi.org/10.3390/foods9060766






