Changes in 3-, 2-Monochloropropandiol and Glycidyl Esters during a Conventional Baking System with Addition of Antioxidants
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Fortification of Palm Olein and Soft Stearin with Antioxidants
2.3. Sample Preparation
2.4. Fat Extraction
2.5. Free Fatty Acid Analysis (Acidity Value)
2.6. Electron Spin Resonance Measurements
2.7. Total Chlorine Analysis
2.8. Oxidation of the Fats Portion
2.9. Aclyglycerol Composition
2.10. MCPD Esters and GE Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effects of Different Antioxidants on the Changes of MCPD Esters and GE
3.2. Electron Spin Resonance Measurement
3.3. Oxidation and Stability of the Fats Portion with Addition of Single Antioxidant
3.4. Synergistic Effect of Antioxidant on the Changes in MCPD Esters and GE
3.5. Synergistic Effect of Antioxidants on the Changes of Oxidation State and Stability
3.6. MCPD Esters and GE Formation with the Presence of Potential Precursors
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dian, N.L.; Hamid, R.A.; Kanagaratnam, S.; Isa, W.R.; Hassim, N.A.; Ismail, N.H.; Omar, Z.; Sahri, M.M. Palm oil and palm kernel oil: Versatile Ingredients for Food Applications. J. Oil Palm Res. 2018, 29, 487–511. [Google Scholar] [CrossRef]
- Clemens, R.; Hayes, A.W.; Sundram, K.; Pressman, P. Palm oil and threats to a critically important food source. Toxicol. Res. Appl. 2017, 1, 239784731769984. [Google Scholar] [CrossRef]
- Nor Aini, I.; Miskandar, M.S. Utilization of palm oil and palm products in shortenings and margarines. Eur. J. Lipid Sci. Technol. 2007, 109, 422–432. [Google Scholar] [CrossRef]
- Gesteiro, E.; Guijarro, L.; Sanchez-Muniz, F.J.; Vidal-Carou, M.D.C.; Troncoso, A.; Venanci, L.; Jimeno, V.; Quilez, J.; Anadon, A.; Gonzalez-Gross, M. Palm Oil on the Edge. Nutrients 2019, 11, 2008. [Google Scholar] [CrossRef]
- Huang, Z.; Stipkovits, L.; Zheng, H.; Serventi, L.; Brennan, C.S. Bovine Milk Fats and Their Replacers in Baked Goods: A Review. Foods 2019, 8, 383. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.M.; Wong, Y.H.; Abas, F.; Lai, O.M.; Cheong, L.Z.; Wang, Y.; Wang, Y.; Tan, C.P. Effects of shortening and baking temperature on quality, MCPD ester and glycidyl ester content of conventional baked cake. LWT 2019, 116, 108553. [Google Scholar] [CrossRef]
- Lakum, R.; Sonwai, S. Production of trans-free margarine fat by enzymatic interesterification of soy bean oil, palm stearin and coconut stearin blend. Int. J. Food Sci. Technol. 2018, 53, 2761–2769. [Google Scholar] [CrossRef]
- Wallace, H.; Jan, A.; Barregård, L.; Bignami, M.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Risks for human health related to the presence of 3-and 2-monochloropropanediol (MCPD), and their fatty acid esters, and glycidyl fatty acid esters in food. EFSA J. 2016, 14, 4426. [Google Scholar]
- Bakhiya, N.; Abraham, K.; Gurtler, R.; Appel, K.E.; Lampen, A. Toxicological assessment of 3-chloropropane-1,2-diol and glycidol fatty acid esters in food. Mol. Nutr. Food Res. 2011, 55, 509–521. [Google Scholar] [CrossRef]
- Cheng, W.W.; Liu, G.Q.; Wang, L.Q.; Liu, Z.S. Glycidyl fatty acid esters in refined edible oils: A review on formation, occurrence, analysis, and elimination methods. Compr. Rev. Food Sci. Food Saf. 2017, 16, 263–281. [Google Scholar] [CrossRef]
- Hamlet, C.G.; Sadd, P.A.; Gray, D.A. Influence of composition, moisture, pH and temperature on the formation and decay kinetics of monochloropropanediols in wheat flour dough. Eur. Food Res. Technol. 2003, 216, 122–128. [Google Scholar] [CrossRef]
- Hamlet, C.G.; Sadd, P.A.; Gray, D.A. Generation of monochloropropanediols (MCPDs) in model dough systems. 1. Leavened doughs. J. Agric. Food Chem. 2004, 52, 2059–2066. [Google Scholar] [CrossRef]
- Shimizu, M.; Vosmann, K.; Matthäus, B. Generation of 3-monochloro-1,2-propanediol and related materials from tri-, di-, and monoolein at deodorization temperature. Eur. J. Lipid Sci. Technol. 2012, 114, 1268–1273. [Google Scholar] [CrossRef]
- Rahn, A.K.K.; Yaylayan, V.A. What do we know about the molecular mechanism of 3-MCPD ester formation? Eur. J. Lipid Sci. Technol. 2011, 113, 323–329. [Google Scholar] [CrossRef]
- Zhang, H.; Jin, P.; Zhang, M.; Cheong, L.Z.; Hu, P.; Zhao, Y.; Yu, L.; Wang, Y.; Jiang, Y.; Xu, X. Mitigation of 3-Monochloro-1,2-propanediol Ester Formation by Radical Scavengers. J. Agric. Food Chem. 2016, 64, 5887–5892. [Google Scholar] [CrossRef]
- Tabakaeva, O.V.; Piekoszewski, W.; Kalenik, T.K.; Maximova, S.N.; Tabakaev, A.V.; Poleshyk, D.V.; Proniewicz, L. Antiradical Activity of Hydrolysates and Extracts from Mollusk A. broughtonii and Practical Application to the Stabilization of Lipids. Foods 2020, 9, 304. [Google Scholar] [CrossRef]
- Frankel, E. Antioxidants in Food and Biology; The Oily Press LD.: Dundee, UK, 2007. [Google Scholar]
- Aladedunye, F.; Przybylski, R.; Matthaus, B. Performance of antioxidative compounds under frying conditions: A review. Crit. Rev. Food Sci. Nutr. 2015, 57, 1539–1561. [Google Scholar] [CrossRef]
- Wan Yahaya, W.A.; Abu Yazid, N.; Mohd Azman, N.A.; Almajano, M.P. Antioxidant Activities and Total Phenolic Content of Malaysian Herbs as Components of Active Packaging Film in Beef Patties. Antioxidants 2019, 8, 204. [Google Scholar] [CrossRef]
- Kim, H.W.; Choi, Y.S.; Choi, J.H.; Kim, H.Y.; Hwang, K.E.; Song, D.H.; Lee, S.Y.; Lee, M.A.; Kim, C.J. Antioxidant effects of soy sauce on color stability and lipid oxidation of raw beef patties during cold storage. Meat Sci. 2013, 95, 641–646. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Ferreira, S.S.; Bastos, R.; Ferreira, I.; Cruz, M.T.; Pinto, A.; Coelho, E.; Passos, C.P.; Coimbra, M.A.; Cardoso, S.M.; et al. Apple Pomace Extract as a Sustainable Food Ingredient. Antioxidants 2019, 8, 189. [Google Scholar] [CrossRef]
- Rico, D.; Penas, E.; Garcia, M.D.C.; Martinez-Villaluenga, C.; Rai, D.K.; Birsan, R.I.; Frias, J.; Martin-Diana, A.B. Sprouted Barley Flour as a Nutritious and Functional Ingredient. Foods 2020, 9, 296. [Google Scholar] [CrossRef]
- Wong, Y.H.; Goh, K.M.; Nyam, K.L.; Nehdi, I.A.; Sbihi, H.M.; Tan, C.P. Effects of natural and synthetic antioxidants on changes in 3-MCPD esters and glycidyl ester in palm olein during deep-fat frying. Food Control 2019, 96, 488–493. [Google Scholar] [CrossRef]
- Zhang, X.; Gao, B.; Qin, F.; Shi, H.; Jiang, Y.; Xu, X.; Yu, L.L. Free radical mediated formation of 3-monochloropropanediol (3-MCPD) fatty acid diesters. J. Agric. Food Chem. 2013, 61, 2548–2555. [Google Scholar] [CrossRef]
- Bonwick, G.A.; Birch, C.S. European Regulation of Process Contaminants in Food; Royal Society of Chemistry: London, UK, 2019. [Google Scholar]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Update of the risk assessment on 3-monochloropropane diol and its fatty acid esters. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Arifin, N.; Cheong, L.-Z.; Koh, S.-P.; Long, K.; Tan, C.-P.; Yusoff, M.S.A.; Nor Aini, I.; Lo, S.-K.; Lai, O.-M. Physicochemical Properties and Sensory Attributes of Medium- and Long-Chain Triacylglycerols (MLCT)-Enriched Bakery Shortening. Food Bioprocess Technol. 2009, 4, 587–596. [Google Scholar] [CrossRef]
- Chen, C.W.; Chong, C.L.; Ghazali, H.M.; Lai, O.M. Interpretation of triacylglycerol profiles of palm oil, palm kernel oil and their binary blends. Food Chem. 2007, 100, 178–191. [Google Scholar] [CrossRef]
- AOCS. Official Method Ca 5a-40: Free Fatty Acids; The American Oil Chemists’ Society: Champaign, IL, USA, 2012. [Google Scholar]
- Goh, K.M.; Wong, Y.H.; Ang, M.Y.; Yeo, S.C.M.; Abas, F.; Lai, O.M.; Tan, C.P. Comparison assessment between SIM and MRM mode in the analysis of 3-MCPD ester, 2-MCPD ester and glycidyl ester. Food Res. Int. 2019, 121, 553–560. [Google Scholar] [CrossRef]
- Wong, Y.H.; Muhamad, H.; Abas, F.; Lai, O.M.; Nyam, K.L.; Tan, C.P. Effects of temperature and NaCl on the formation of 3-MCPD esters and glycidyl esters in refined, bleached and deodorized palm olein during deep-fat frying of potato chips. Food Chem. 2017, 219, 126–130. [Google Scholar] [CrossRef]
- Abd Razak, R.A.; Ahmad Tarmizi, A.H.; Kuntom, A. Survey on Commercial Palm Olein and Oil Extracted from Snack Products in Selected Asian Countries—Part 2: Quantification of 3-monochloropropane-1, 2-diol Esters (3-MCPDE). Palm Oil Dev. 2017, 66, 14–17. [Google Scholar]
- Yu, L.L.; Cheng, Z. Application of electron spin resonance (ESR) spectrometry in nutraceutical and food research. Mol. Nutr. Food Res. 2008, 52, 62–78. [Google Scholar] [CrossRef]
- Bhat, R.; Sridhar, K.R.; Bhushan, B. Free radicals in velvet bean seeds (Mucuna pruriens L. DC.) and their status after γ-irradiation and conventional processing. LWT—Food Sci. Technol. 2007, 40, 1570–1577. [Google Scholar] [CrossRef]
- Bhushan, B.; Bhat, R.; Sharma, A. Status of free radicals in indian monsooned coffee beans γ-irradiated for disinfestation. J. Agric. Food Chem. 2003, 51, 4960–4964. [Google Scholar] [CrossRef]
- Qi, J.F.; Wang, X.Y.; Shin, J.A.; Lee, Y.H.; Jang, Y.S.; Lee, J.H.; Hong, S.T.; Lee, K.T. Relative oxidative stability of diacylglycerol and triacylglycerol oils. J. Food Sci. 2015, 80, C510–C514. [Google Scholar] [CrossRef]
- Wang, S.; Meckling, K.A.; Marcone, M.F.; Kakuda, Y.; Tsao, R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J. Agric. Food Chem. 2011, 59, 960–968. [Google Scholar] [CrossRef]
- Judde, A.; Villeneuve, P.; Rossignol-Castera, A.; Le Guillou, A. Antioxidant effect of soy lecithins on vegetable oil stability and their synergism with tocopherols. J. Am. Oil Chem. Soc. 2003, 80, 1209–1215. [Google Scholar] [CrossRef]
- Marinova, E.; Toneva, A.; Yanishlieva, N. Synergistic antioxidant effect of α-tocopherol and myricetin on the autoxidation of triacylglycerols of sunflower oil. Food Chem. 2008, 106, 628–633. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, B.; Zhang, X.; Jiang, Y.; Xu, X.; Yu, L.L. Formation of 3-monochloro-1,2-propanediol (3-MCPD) di- and monoesters from tristearoylglycerol (TSG) and the potential catalytic effect of Fe(2)(+) and Fe(3)(+). J. Agric. Food Chem. 2015, 63, 1839–1848. [Google Scholar] [CrossRef]
- Bosmans, G.M.; Peene, L.J.; Van Haesendonck, I.; Brijs, K.; Delcour, J.A. Impact of chlorine treatment on properties of wheat flour and its components in the presence of sucrose. Food Chem. 2019, 274, 434–443. [Google Scholar] [CrossRef]
- Tiong, S.H.; Saparin, N.; Teh, H.F.; Ng, T.L.M.; Md Zain, M.Z.B.; Neoh, B.K.; Md Noor, A.; Tan, C.P.; Lai, O.M.; Appleton, D.R. Natural Organochlorines as Precursors of 3-Monochloropropanediol Esters in Vegetable Oils. J. Agric. Food Chem. 2018, 66, 999–1007. [Google Scholar] [CrossRef]
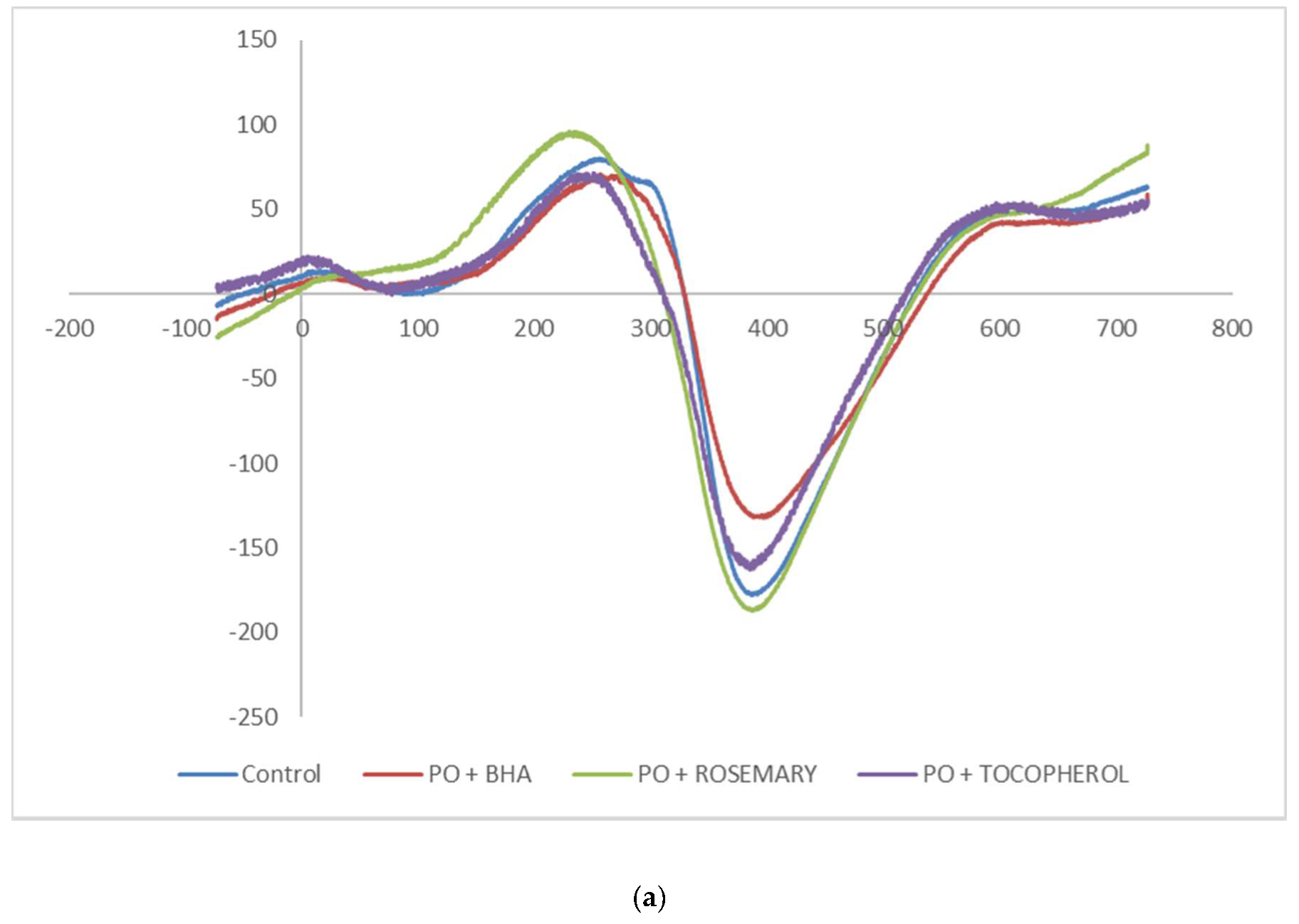
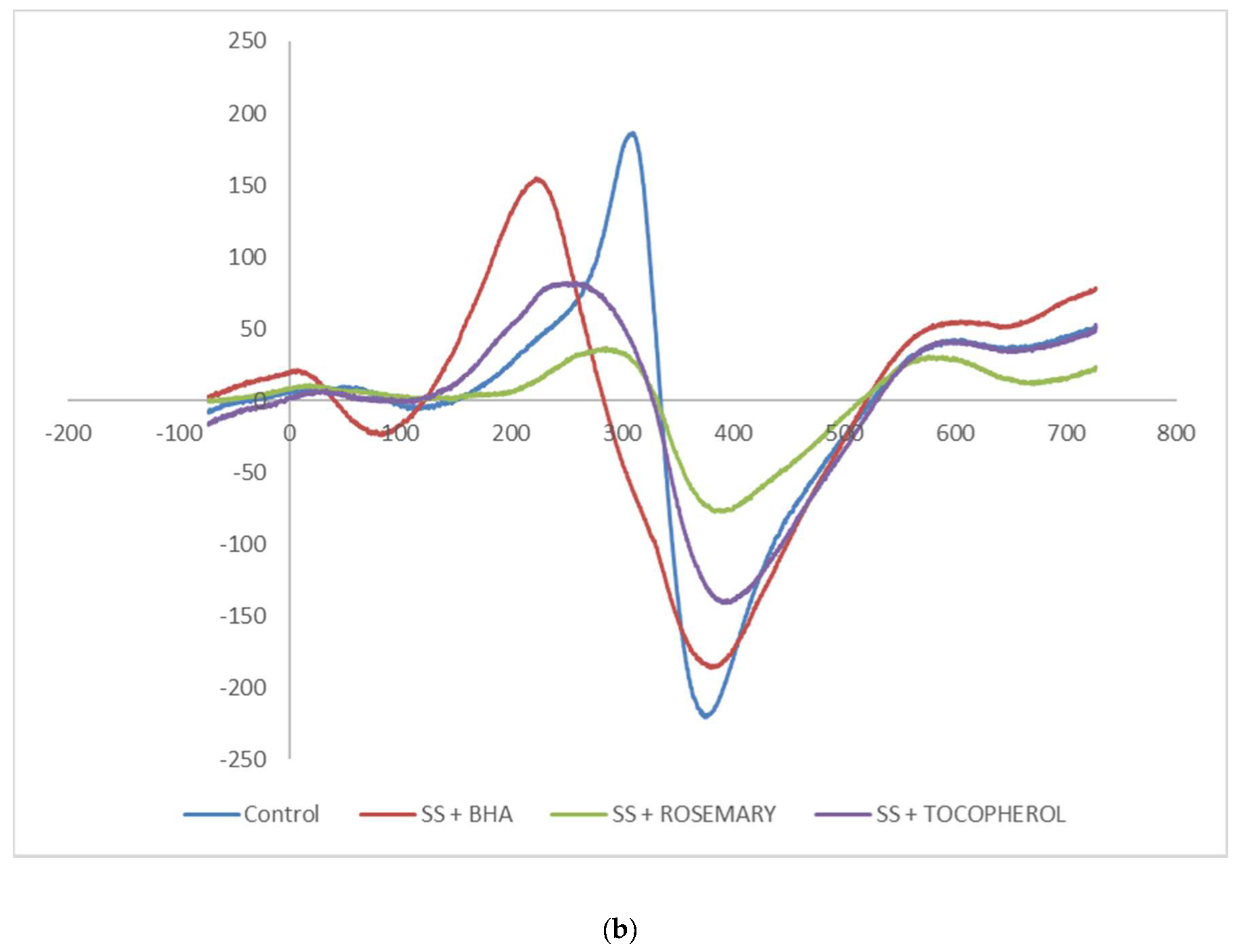
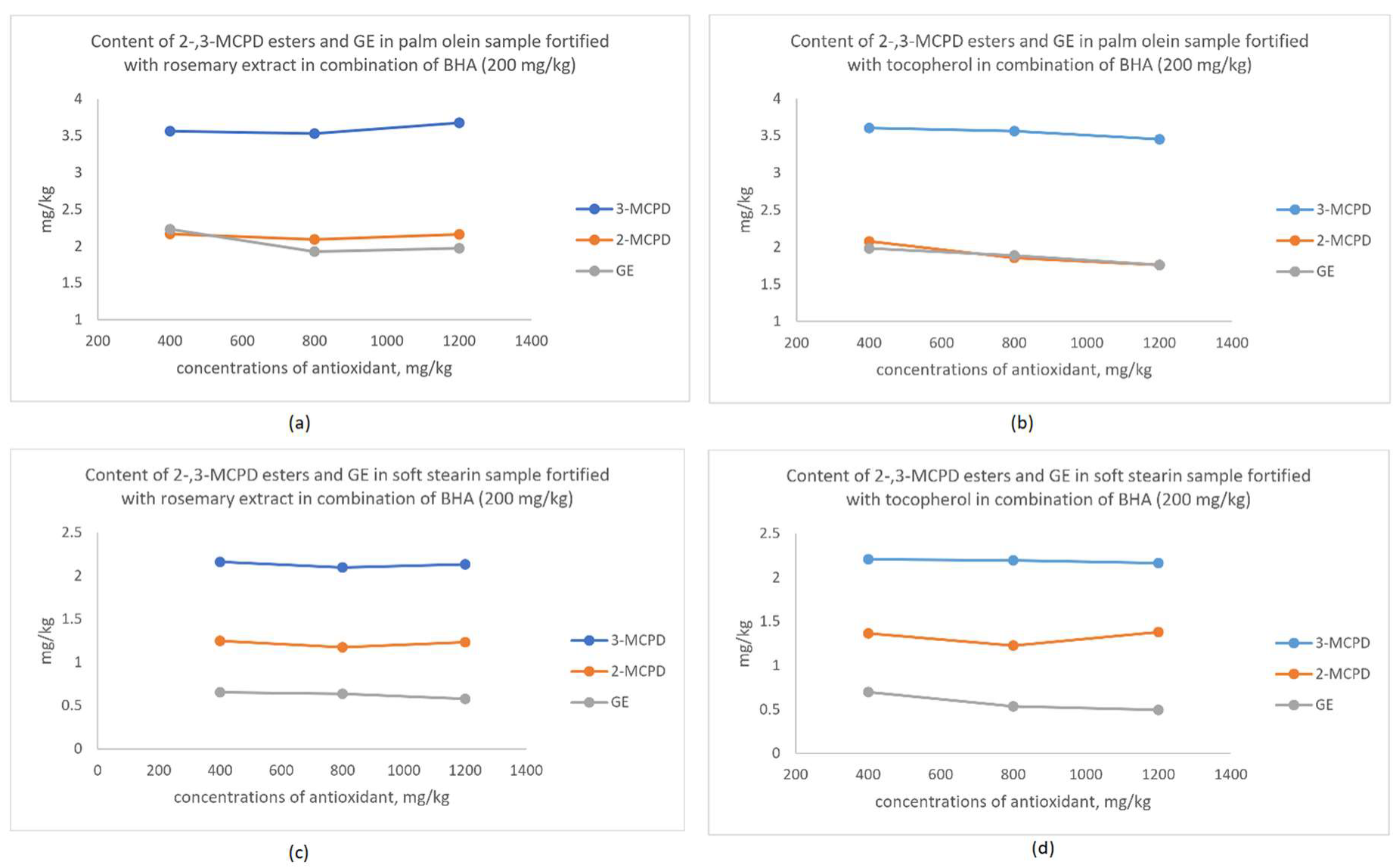
| Sample | Antioxidant | 3-MCPD, mg/kg | 2-MCPD, mg/kg | GE, mg/kg |
|---|---|---|---|---|
| BHA | 3.439 ± 0.029 a | 2.334 ± 0.021 a | 1.987 ± 0.039 a | |
| Palm Olein | Rosemary | 3.431 ± 0.065 a | 2.051 ± 0.010 b | 1.979 ± 0.029 a |
| Tocopherol | 3.527 ± 0.189 a | 2.143 ± 0.105 b | 1.985 ± 0.047 a | |
| BHA | 2.222 ± 0.028 a | 1.534 ± 0.114 a | 0.665 ± 0.001 a | |
| Soft Stearin | Rosemary | 2.176 ± 0.032 a | 1.489 ± 0.040 a | 0.607 ± 0.032 b |
| Tocopherol | 2.172 ± 0.023 a | 1.435 ± 0.012 a | 0.668 ± 0.010 a |
| Sample | Antioxidant | FFA, % | K268, CT | K232, CD | FFA/DAG | MAG/DAG | 1,3/1,2-DAG | G-Value | Total Chlorine, % |
|---|---|---|---|---|---|---|---|---|---|
| Palm Olein | BHA | 0.39 ± 0.14 b | 0.757 ± 0.072 a | 4.393 ± 0.505 b | 0.202 ± 0.006 b | 0.018 ± 0.002 a | 2.599 ± 0.017 a | 2.000 ± 0.004 b | 0.182 ± 0.010 a |
| Rosemary | 0.27 ± 0.05 ab | 0.753 ± 0.111 a | 4.149 ± 0.497 b | 0.172 ± 0.005 c | 0.018 ± 0.004 a | 2.583 ± 0.053 a | 2.120 ± 0.010 a | 0.181 ± 0.005 a | |
| Tocopherol | 0.49 ± 0.03 a | 0.832 ± 0.067 a | 5.076 ± 0.339 a | 0.236 ± 0.010 a | 0.017 ± 0.003 a | 2.499 ± 0.028 b | 2.133 ± 0.032 a | 0.203 ± 0.007 a | |
| Soft Stearin | BHA | 0.32 ± 0.04 a | 0.617 ± 0.012 b | 3.317 ± 0.067 b | 0.224 ± 0.009 b | 0.018 ± 0.002 b | 2.558 ± 0.052 a | 2.329 ± 0.022 a | 0.176 ± 0.017 a |
| Rosemary | 0.24 ± 0.03 b | 0.621 ± 0.001 b | 3.298 ± 0.004 b | 0.176 ± 0.016 c | 0.018 ± 0.001 b | 2.415 ± 0.078 b | 1.980 ± 0.002 b | 0.183 ± 0.001 a | |
| Tocopherol | 0.31 ± 0.02 a | 0.642 ± 0.018 a | 3.845 ± 0.099 a | 0.282 ± 0.018 a | 0.024 ± 0.003 a | 2.565 ± 0.067 a | 1.999 ± 0.003 b | 0.188 ± 0.004 a |
| Sample | Antioxidants | FFA, % | K268, CT | K232, CD | FFA/DAG | MAG/DAG | 1,3/1,2-DAG |
|---|---|---|---|---|---|---|---|
| Palm Olein | Rosemary, mg/kg | ||||||
| 400 | 0.34 ± 0.13 bA | 0.891 ± 0.029 bB | 3.453 ± 0.105 aB | 0.134 ± 0.023 aA | 0.015 ± 0.004 aA | 2.367 ± 0.079 bA | |
| 800 | 0.53 ± 0.04 aB | 0.886 ± 0.010 bB | 3.487 ± 0.073 aB | 0.123 ± 0.020 aB | 0.016 ± 0.004 aB | 2.564 ± 0.045 aA | |
| 1200 | 0.42 ± 0.08 abA | 0.903 ± 0.001 aB | 3.541 ± 0.054 aB | 0.108 ± 0.014 aB | 0.018 ± 0.005 aA | 2.416 ± 0.096 aA | |
| Tocopherol, mg/kg | |||||||
| 400 | 0.39 ± 0.04 bA | 1.008 ± 0.028 bA | 4.112 ± 0.087 cA | 0.148 ± 0.011 bA | 0.018 ± 0.003 aA | 2.346 ± 0.064 bA | |
| 800 | 0.63 ± 0.05 aA | 0.961 ± 0.001 cA | 4.536 ± 0.025 bA | 0.223 ± 0.001 aA | 0.024 ± 0.005 aA | 2.472 ± 0.063 aA | |
| 1200 | 0.53 ± 0.07 aA | 1.085 ± 0.002 aA | 4.811 ± 0.008 aA | 0.217 ± 0.006 aA | 0.019 ± 0.005 aA | 2.323 ± 0.050 bA | |
| Soft Stearin | Rosemary, mg/kg | ||||||
| 400 | 0.39 ± 0.06 bA | 0.728 ± 0.065 bB | 3.076 ± 0.045 bB | 0.160 ± 0.004 cB | 0.019 ± 0.004 aA | 2.477 ± 0.077 aA | |
| 800 | 0.55 ± 0.07 aA | 0.740 ± 0.008 bB | 2.899 ± 0.059 cB | 0.183 ± 0.005 bB | 0.028 ± 0.009 aA | 2.588 ± 0.034 aA | |
| 1200 | 0.41 ± 0.06 aA | 0.879 ± 0.006 aA | 4.171 ± 0.013 aA | 0.269 ± 0.011 aA | 0.019 ± 0.006 aA | 2.420 ± 0.121 aA | |
| Tocopherol, mg/kg | |||||||
| 400 | 0.39 ± 0.03 abA | 0.822 ± 0.009 aA | 3.712 ± 0.034 sA | 0.230 ± 0.032 bA | 0.020 ± 0.004 aA | 2.512 ± 0.121 aA | |
| 800 | 0.43 ± 0.04 aB | 0.781 ± 0.002 bA | 3.678 ± 0.002 aA | 0.282 ± 0.013 aA | 0.024 ± 0.005 aA | 2.523 ± 0.126 aA | |
| 1200 | 0.35 ± 0.03 bA | 0.757 ± 0.003 cB | 2.993 ± 0.018 aB | 0.280 ± 0.010 aA | 0.025 ± 0.001 aA | 2.403 ± 0.059 aA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goh, K.M.; Wong, Y.H.; Abas, F.; Lai, O.M.; Mat Yusoff, M.; Tan, T.B.; Wang, Y.; Nehdi, I.A.; Tan, C.P. Changes in 3-, 2-Monochloropropandiol and Glycidyl Esters during a Conventional Baking System with Addition of Antioxidants. Foods 2020, 9, 739. https://doi.org/10.3390/foods9060739
Goh KM, Wong YH, Abas F, Lai OM, Mat Yusoff M, Tan TB, Wang Y, Nehdi IA, Tan CP. Changes in 3-, 2-Monochloropropandiol and Glycidyl Esters during a Conventional Baking System with Addition of Antioxidants. Foods. 2020; 9(6):739. https://doi.org/10.3390/foods9060739
Chicago/Turabian StyleGoh, Kok Ming, Yu Hua Wong, Faridah Abas, Oi Ming Lai, Masni Mat Yusoff, Tai Boon Tan, Yonghua Wang, Imeddedine Arbi Nehdi, and Chin Ping Tan. 2020. "Changes in 3-, 2-Monochloropropandiol and Glycidyl Esters during a Conventional Baking System with Addition of Antioxidants" Foods 9, no. 6: 739. https://doi.org/10.3390/foods9060739
APA StyleGoh, K. M., Wong, Y. H., Abas, F., Lai, O. M., Mat Yusoff, M., Tan, T. B., Wang, Y., Nehdi, I. A., & Tan, C. P. (2020). Changes in 3-, 2-Monochloropropandiol and Glycidyl Esters during a Conventional Baking System with Addition of Antioxidants. Foods, 9(6), 739. https://doi.org/10.3390/foods9060739







