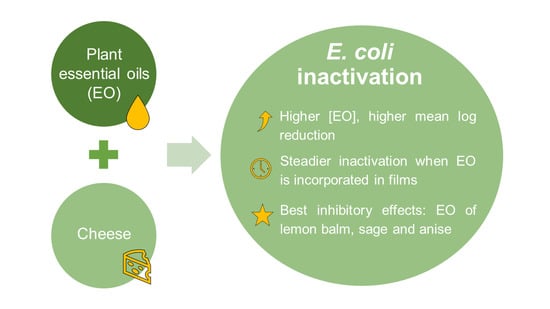
Effects of Essential Oils on Escherichia coli Inactivation in Cheese as Described by Meta-Regression Modelling
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Description of the Data Set
2.2. Meta-Regression Modelling
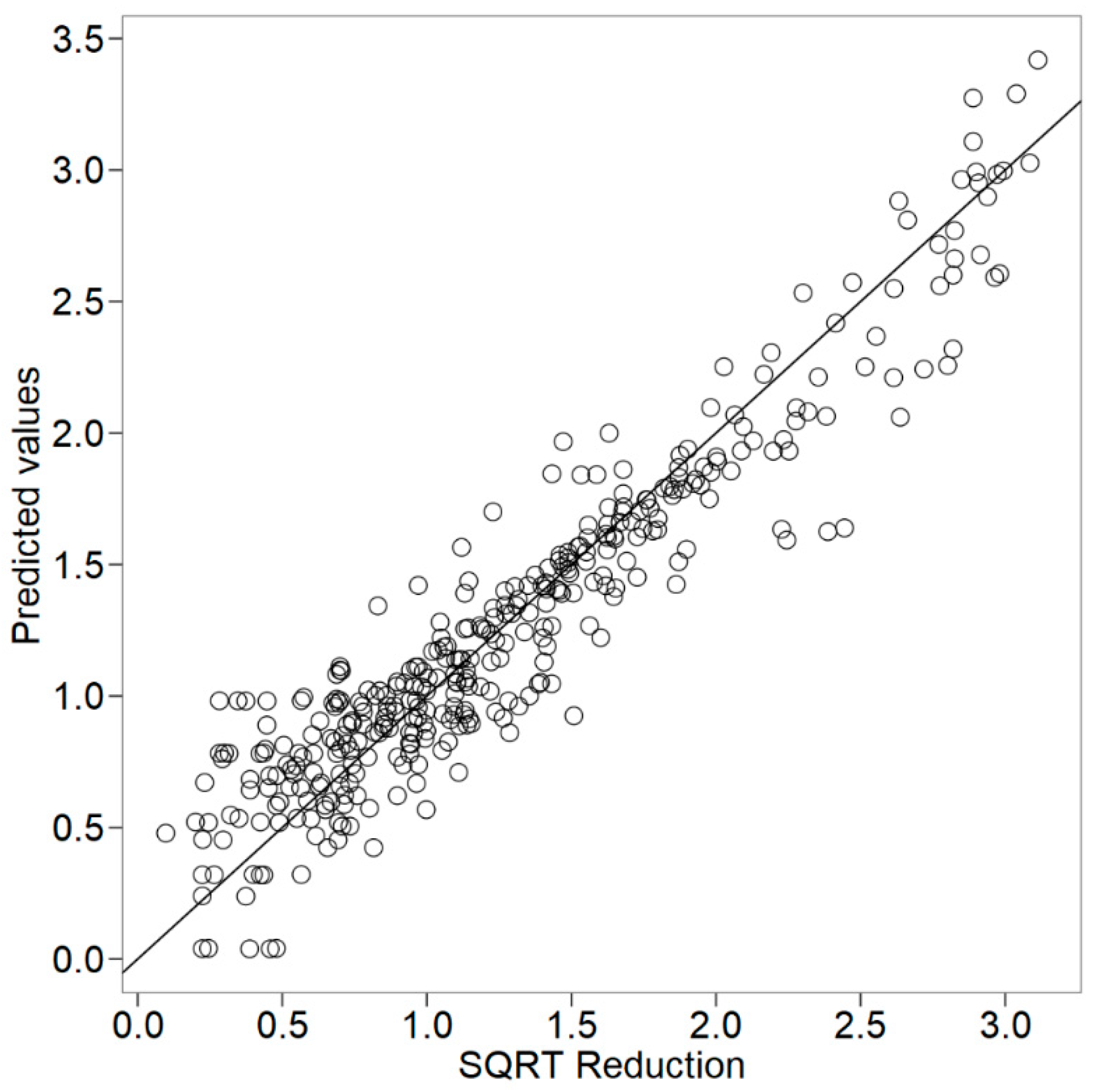
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kousta, M.; Mataragas, M.; Skandamis, P.; Drosinos, E.H. Prevalence and sources of cheese contamination with pathogens at farm and processing levels. Food Control 2010, 21, 805–815. [Google Scholar] [CrossRef]
- Choi, K.H.; Lee, H.; Lee, S.; Kim, S.; Yoon, Y. Cheese microbial risk assessments - A Review. Asian Australas. J. Anim. Sci. 2016, 29, 307–314. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention (ECDC). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, 233–239. [Google Scholar]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention (ECDC). The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, 201–204. [Google Scholar]
- Gonzales-Barron, U.; Gonçalves-Tenório, A.; Rodrigues, V.; Cadavez, V. Foodborne pathogens in raw milk and cheese of sheep and goat origin: A meta-analysis approach. Curr. Opin. Food Sci. 2017, 18, 7–13. [Google Scholar] [CrossRef]
- Mahgoub, S.A.; Sitohy, M.Z.; Osman, A.O. Counteracting recontamination of pasteurized milk by methylated soybean protein. Food Bioprocess Technol. 2013, 6, 101–109. [Google Scholar] [CrossRef]
- Mahgoub, S.; Osman, A.; Sitohy, M. Inhibition of growth of pathogenic bacteria in raw milk by legume protein esters. J. Food Prot. 2011, 74, 1475–1481. [Google Scholar] [CrossRef]
- Rosengren, Å.; Fabricius, A.; Guss, B.; Sylvén, S.; Lindqvist, R. Occurrence of foodborne pathogens and characterization of Staphylococcus aureus in cheese produced on farm-dairies. Int. J. Food Microbiol. 2010, 144, 263–269. [Google Scholar] [CrossRef]
- Öksüz, Ö.; Arici, M.; Kurultay, S.; Gümüs, T. Incidence of Escherichia coli O157 in raw milk and white pickled cheese manufactured from raw milk in Turkey. Food Control 2004, 15, 453–456. [Google Scholar] [CrossRef]
- Stephan, R.; Schumacher, S.; Corti, S.; Krause, G.; Danuser, J.; Beutin, L. Prevalence and characteristics of Shiga toxin-producing Escherichia coli in Swiss raw milk cheeses collected at producer level. J. Dairy Sci. 2008, 91, 2561–2565. [Google Scholar] [CrossRef]
- Ombarak, R.A.; Hinenoya, A.; Awasthi, S.P.; Iguchi, A.; Shima, A.; Elbagory, A.-R.M.; Yamasaki, S. Prevalence and pathogenic potential of Escherichia coli isolates from raw milk and raw milk cheese in Egypt. Int. J. Food Microbiol. 2016, 221, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Currie, A.; Galanis, E.; Chacon, P.A.; Murray, R.; Wilcott, L.; Kirkby, P.; Honish, L.; Franklin, K.; Farber, J.; Parker, R.; et al. Outbreak of Escherichia coli O157:H7 infections linked to aged raw milk Gouda cheese, Canada, 2013. J. Food Prot. 2018, 81, 325–331. [Google Scholar] [CrossRef] [PubMed]
- McCollum, J.T.; Williams, N.J.; Beam, S.W.; Cosgrove, S.; Ettestad, P.J.; Ghosh, T.S.; Kimura, A.C.; Nguyen, L.; Stroika, S.G.; Vogt, R.L.; et al. Multistate outbreak of Escherichia coli O157:H7 infections associated with in-store sampling of an aged raw-milk Gouda cheese, 2010. J. Food Prot. 2012, 75, 1759–1765. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Lefèvre, S.; Donguy, M.-P.; Nisavanh, A.; Terpant, G.; Fougère, E.; Vaissière, E.; Guinard, A.; Mailles, A.; de Valk, H.; et al. Outbreak of Shiga toxin-producing Escherichia coli (STEC) O26 paediatric haemolytic uraemic syndrome (HUS) cases associated with the consumption of soft raw cow’s milk cheeses, France, March to May 2019. Euro Surveill. 2019, 24, 1900305. [Google Scholar] [CrossRef] [PubMed]
- Honish, L.; Predy, G.; Hislop, N.; Chui, L.; Kowalewska-Grochowska, K.; Trottier, L.; Kreplin, C.; Zazulak, I. An outbreak of E. coli O15:H7 hemorrhagic colitis associated with unpasteurized Gouda cheese. Can. J. Public Heal. 2005, 96, 182–184. [Google Scholar] [CrossRef]
- Espié, E.; Vaillant, V.; Mariani-Kurkdjian, P.; Grimont, F.; Martin-Schaller, R.; De Valk, H.; Vernozy-Rozand, C. Escherichia coli O157 outbreak associated with fresh unpasteurized goats’ cheese. Epidemiol. Infect. 2006, 134, 143–146. [Google Scholar] [CrossRef]
- Schmid-Hempel, P.; Frank, S.A. Pathogenesis, Virulence, and Infective Dose. PLoS Pathog. 2007, 3, 1372–1373. [Google Scholar] [CrossRef]
- Ehsani, A.; Hashemi, M.; Naghibi, S.S.; Mohammadi, S.; Sadaghiani, S.K. Properties of Bunium Persicum essential oil and its application in Iranian white cheese against Listeria monocytogenes and Escherichia coli O157:H7. J. Food Saf. 2016, 36, 563–570. [Google Scholar] [CrossRef]
- Ehsani, A.; Mahmoudi, R. Phytochemical properties and hygienic effects of Allium ascalonicum and Pimpinella anisum essential oils in Iranian white brined cheese. J. Essent. Oil Bear. Plants 2012, 15, 1013–1020. [Google Scholar] [CrossRef]
- Hamedo, H.A.; Abdelmigid, H.M. Use of antimicrobial and genotoxicity potentiality for evaluation of essential oils as food preservatives. Open Biotechnol. J. 2009, 3, 50–56. [Google Scholar] [CrossRef]
- Mahgoub, S.A.; Ramadan, M.F.; El-Zahar, K.M. Cold pressed Nigella sativa oil inhibits the growth of foodborne pathogens and improves the quality of Domiati cheese. J. Food Saf. 2013, 33, 470–480. [Google Scholar] [CrossRef]
- Ribeiro, D.S.; Siqueira, F.G.; Velozo, S.; Guimarães, A.G. Evaluation rosemary essential oil in the control of multidrug-resistant Escherichia coli in Coalho cheese. J. Biotec. Biodivers. 2013, 4, 1–9. [Google Scholar]
- Xavier, C.; Gonzales-Barron, U.; Paula, V.; Estevinho, L.; Cadavez, V. Meta-analysis of the incidence of foodborne pathogens in Portuguese meats and their products. Food Res. Int. 2014, 55, 311–323. [Google Scholar] [CrossRef]
- Mehdizadeh, T.; Narimani, R.; Langroodi, A.M.; Kia, M.E.; Neyriz-Naghadehi, M. Antimicrobial effects of Zataria multiflora essential oil and Lactobacillus acidophilus on Escherichia coli O157 stability in the Iranian probiotic white-brined cheese. J. Food Saf. 2018, 38, e12476. [Google Scholar] [CrossRef]
- Hassanien, M.F.R.; Mahgoub, S.A.; El-Zahar, K.M. Soft cheese supplemented with black cumin oil: Impact on food borne pathogens and quality during storage. Saudi J. Biol. Sci. 2014, 21, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Selim, S. Antimicrobial activity of essential oils against Vancomycin-Resistant Enterococci (VRE) and Escherichia coli O157:H7 in feta soft cheese and minced beef meat. Braz. J. Microbiol. 2011, 42, 187–196. [Google Scholar] [CrossRef]
- Requena, R.; Vargas, M.; Chiralt, A. Eugenol and carvacrol migration from PHBV films and antibacterial action in different food matrices. Food Chemistry 2019, 277, 38–45. [Google Scholar] [CrossRef]
- Otero, V.; Becerril, R.; Santos, J.A.; Rodríguez-Calleja, J.M.; Nerín, C.; García-López, M.L. Evaluation of two antimicrobial packaging films against Escherichia coli O157: H7 strains in vitro and during storage of a Spanish ripened sheep cheese (Zamorano). Food Control 2014, 42, 296–302. [Google Scholar] [CrossRef]
- Kavas, G.; Kavas, N. Use of egg white protein powder based films fortified with sage and lemon balm essential oils in the storage of lor cheese. Mljekarstvo 2016, 66, 99–111. [Google Scholar]
- Raeisi, M.; Tajik, H.; Razavi, R.S.M.; Maham, M.; Moradi, M.; Hajimohammadi, B.; Naghili, H.; Hashemi, M.; Mehdizadeh, T. Essential oil of tarragon (Artemisia dracunculus) antibacterial activity on Staphylococcus aureus and Escherichia coli in culture media and Iranian white cheese. Iran. J. Microbiol. 2012, 4, 30–34. [Google Scholar]
- Torlak, E.; Nizamlioglu, M. Antimicrobial effectiveness of chitosan-essential oil coated plastic films against foodborne pathogens. J. Plast. Film Sheeting 2011, 27, 235–248. [Google Scholar] [CrossRef]
- Govaris, A.; Botsoglou, E.; Sergelidis, D.; Chatzopoulou, P.S. Antibacterial activity of oregano and thyme essential oils against Listeria monocytogenes and Escherichia coli O157:H7 in feta cheese packaged under modified atmosphere. LWT-Food Sci. Technol. 2011, 44, 1240–1244. [Google Scholar] [CrossRef]
- Ghasemi, G.; Javadi, N.H.S.; Morandi, M.; Khosravi-Darani, K. Application of zein antimicrobial edible film incorporating Zataria multiflora boiss essential oil for preservation of Iranian ultrafiltered Feta cheese. Afr. J. Biotechnol. 2015, 14, 2014–2021. [Google Scholar]
- Georgescu, M.; Tăpăloagă, P.R.; Tăpăloagă, D.A.N.A.; Furnaris, F.; Ginghină, O.C.T.A.V.; Negrei, C.; Giuglea, C.; Bălălău, C.R.I.S.T.I.A.N.; Ștefănescu, E.M.I.L.; Popescu, I.A.; et al. Evaluation of antimicrobial potential of Nigella sativa oil in a model food matrix. Farmacia 2018, 66, 1028–1036. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2019. Available online: https://www.R-project.org/ (accessed on 21 March 2019).
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Sheng, L.; Rasco, B.; Zhu, M.-J. Cinnamon oil inhibits Shiga toxin type 2 phage induction and Shiga toxin type 2 production in Escherichia coli O157:H7. Appl. Environ. Microbiol. 2016, 82, 6531–6540. [Google Scholar] [CrossRef]
- Kim, Y.-G.; Lee, J.-H.; Gwon, G.; Kim, S.-I.; Park, J.G.; Lee, J. Essential oils and eugenols inhibit biofilm formation and the virulence of Escherichia coli O157:H7. Sci. Rep. 2016. [Google Scholar] [CrossRef]
- International Organization for Standardization (ISO). ISO 20976-1:2019 - Microbiology of the Food Chain—Requirements and Guidelines for Conducting Challenge Tests of Food and Feed Products—Part 1: Challenge Tests to Study Growth Potential, Lag Time and Maximum Growth Rate. Available online: https://www.iso.org/standard/69673.html (accessed on 20 April 2020).
- Xu, T.; Gao, C.; Yang, Y.; Shen, X.; Huang, M.; Liu, S.; Tang, X. Retention and release properties of cinnamon essential oil in antimicrobial films based on chitosan and gum arabic. Food Hydrocoll. 2018, 84, 84–92. [Google Scholar] [CrossRef]
- Perricone, M.; Arace, E.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Bioactivity of essential oils: A review on their interaction with food components. Front. Microbiol. 2015, 6, 76. [Google Scholar] [CrossRef]
- Nunes Silva, B.; Cadavez, V.; Teixeira, J.A.; Gonzales-Barron, U. Meta-regression models describing the effects of essential oils and added lactic acid bacteria on pathogen inactivation in cheese. Microb. Risk Anal. under review.
- Martillanes, S.; Rocha-Pimienta, J.; Cabrera-Bañegil, M.; Martín-Vertedor, D.; Delgado-Adámez, J. Application of Phenolic Compounds for Food Preservation: Food Additive and Active Packaging. In Phenolic Compounds - Biological Activity; Soto-Hernandez, M., Palma-Tenango, M., Garcia-Mateos, M., Eds.; InTechOpen: London, UK, 2017; pp. 39–58. [Google Scholar]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Brodes, C. Antibacterial properties of polyphenols: Characterization and QSAR (Quantitative structure-activity relationship) models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- World Flora Online. Available online: http://www.worldfloraonline.org/ (accessed on 20 April 2020).
- Pannek, J.; Gach, J.; Boratyński, F.; Olejniczak, T. Antimicrobial activity of extracts and phthalides occurring in Apiaceae plants. Phytother. Res. 2018, 32, 1459–1487. [Google Scholar] [CrossRef] [PubMed]
- Tzima, K.; Brunton, N.P.; Rai, D.K. Qualitative and quantitative analysis of polyphenols in Lamiaceae plants—A review. Plants 2018, 7, 25. [Google Scholar] [CrossRef]
- Mekinić, I.G.; Skroza, D.; Ljubenkov, I.; Šimat, V.; Možina, S.S.; Katalinić, V. In Vitro antioxidant and antibacterial activity of Lamiaceae phenolic extracts: A correlation study. Food Technol. Biotechnol. 2014, 52, 119–127. [Google Scholar]
- Guldiken, B.; Ozkan, G.; Catalkaya, G.; Ceylan, F.D.; Yalcinkaya, I.E.; Capanoglu, E. Phytochemicals of herbs and spices: Health versus toxicological effects. Food Chem. Toxicol. 2018, 119, 37–49. [Google Scholar] [CrossRef]
- Nair, J.J.; Wilhelm, A.; Bonnet, S.L.; van Staden, J. Antibacterial constituents of the plant family Amaryllidaceae. Bioorg. Med. Chem. Lett. 2017, 27, 4943–4951. [Google Scholar] [CrossRef]
| Categories | Level | N |
|---|---|---|
| Milk treatment | Pasteurised | 147 |
| Raw | 27 | |
| Sterilised | 10 | |
| Not stated | 178 | |
| Milk species | Bovine | 74 |
| Caprine | 160 | |
| Ovine | 29 | |
| Not stated | 99 | |
| Type of cheese | Hard cheese | 29 |
| Semi-hard cheese | 10 | |
| Soft cheese | 171 | |
| Not stated | 152 | |
| Label | Coalho cheese | 7 |
| Domiati cheese | 24 | |
| Feta cheese | 160 | |
| Iranian white cheese | 62 | |
| Kashar cheese | 3 | |
| Lor cheese | 24 | |
| Zamorano cheese | 29 | |
| Undefined cheese | 53 | |
| Starters | Present | 78 |
| Absent | 112 | |
| Not stated | 172 | |
| Essay type | Inoculated | 350 |
| Non-inoculated | 12 | |
| Strain/Serotype | ATCC 8739 | 24 |
| CECT 101 | 1 | |
| EC 16 | 7 | |
| O157 | 15 | |
| O157:H7 | 70 | |
| O157:H7 ATCC 43895 | 24 | |
| O157:H7 CECT 5947 | 12 | |
| O157:H7 EDL-932 | 42 | |
| O157:H7 LH1 | 34 | |
| O157:H7 M364VO | 17 | |
| O157:H7 VT7 negative | 56 | |
| PTCC 1533 | 8 | |
| Not stated | 52 |
| Moderators | Level | N |
|---|---|---|
| Antimicrobial | Anise | 13 |
| Black cumin seed | 47 | |
| Lemon balm | 12 | |
| Oregano | 97 | |
| Rosemary | 27 | |
| Sage | 26 | |
| Shallot | 15 | |
| Tarragon | 8 | |
| Thyme | 46 | |
| Zataria multiflora Boiss. | 71 | |
| Application type | Cheese mixture | 68 |
| Film | 113 | |
| Milk | 98 | |
| Cheese surface | 83 | |
| Exposure time, t (days) | 0 ≤ t < 20 20 ≤ t < 4040 ≤ t ≤ 60 | 272 64 26 |
| Storage temperature, T (°C) | 3 ≤ T < 1313 ≤ T < 2323 ≤ T ≤ 35 | 337 17 8 |
| Inoculum level, Inoc (log CFU/g or log CFU/mL) | 1.5 ≤ Inoc < 4.254.25 ≤ Inoc ≤ 7Non-inoculated | 248 102 12 |
| Antimicrobial concentration, Conc (%v/v or w/w) | 5 × 10−3 ≤ Conc < 7 7 ≤ Conc < 1414 ≤ Conc ≤ 20 | 343 12 7 |
| Fixed Effects | Num DF/Den DF | F-Value | Pr > F |
|---|---|---|---|
| Pathogenicity | 1/341 | 84.45 | <0.0001 |
| Application type | 3/341 | 41.77 | <0.0001 |
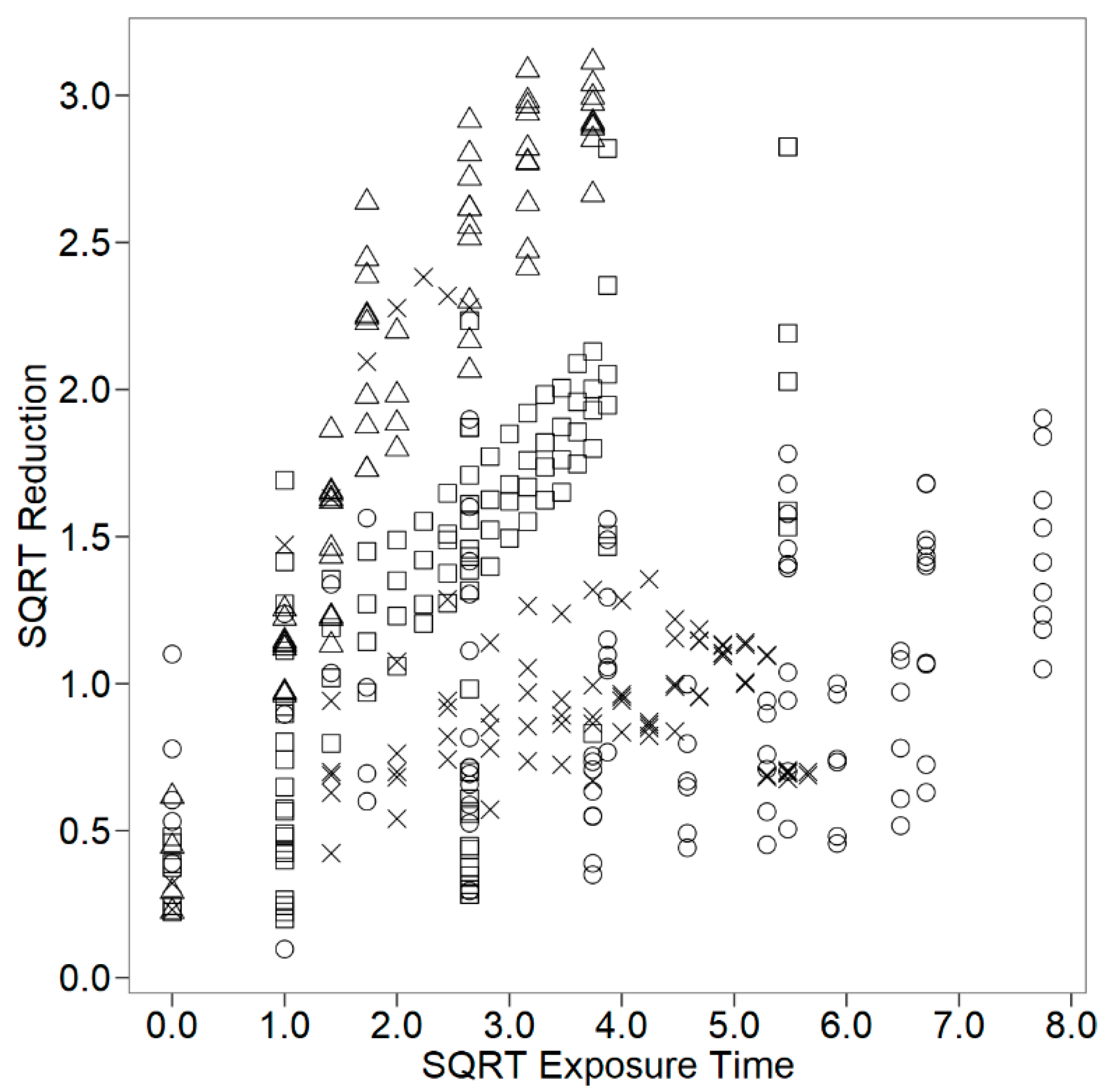
| App(√t) | 4/341 | 179.3 | <0.0001 |
| ConcUnit | 1/341 | 12.70 | 0.0004 |
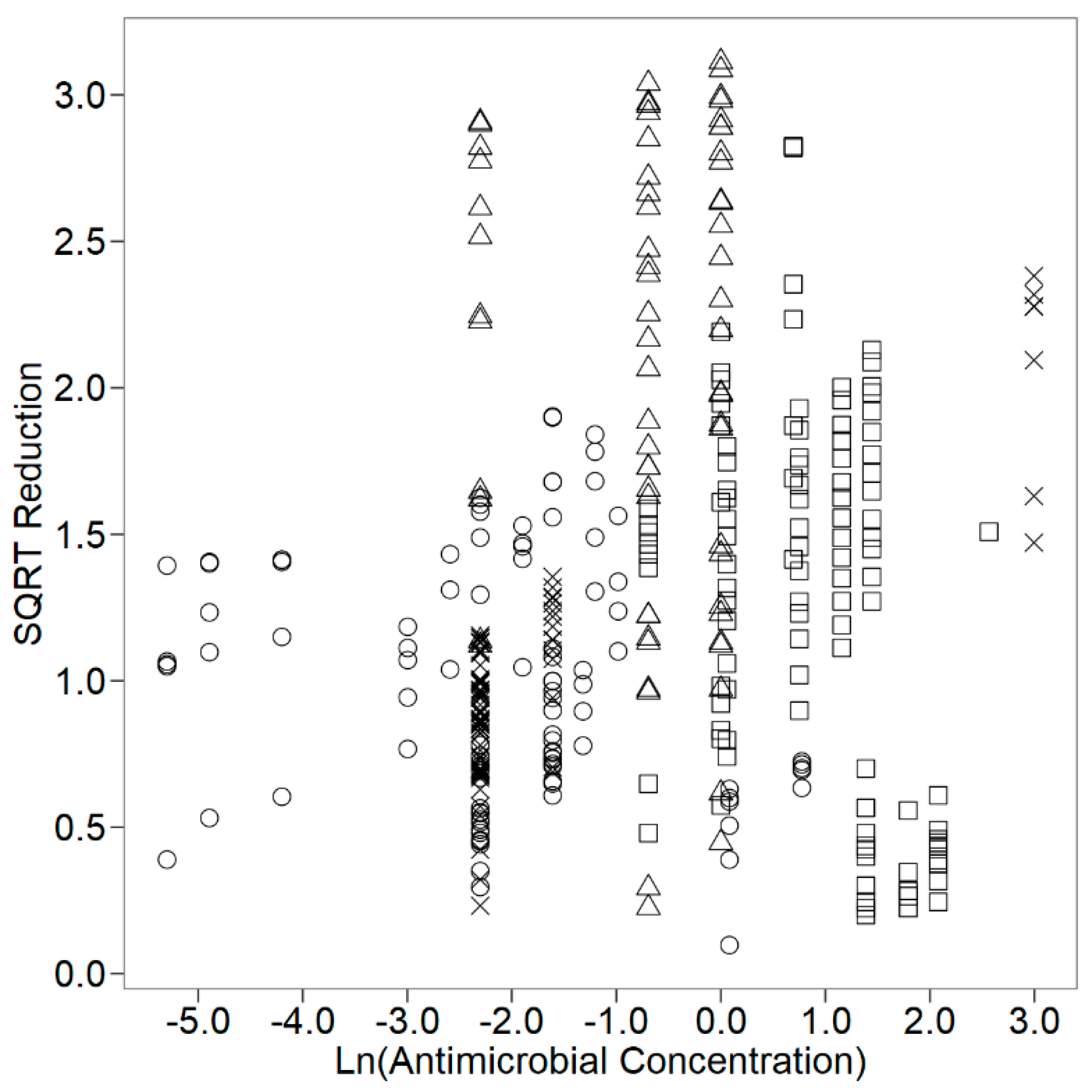
| ConcUnit(LnConc) | 2/341 | 6.291 | 0.0021 |
| Parameters | Mean | SE | Pr > |t| | Heterogeneity |
|---|---|---|---|---|
| Predictors of √Rijk | τ2res = 0.316 R2 > 95% | |||
| β0 (intercept) | 0.661 | 0.227 | 0.004 | |
| β1n (Pathogenicity) | ||||
| Non-pathogenic | 0 | - | - | |
| Pathogenic | −0.200 | 0.052 | 0.000 | |
| β2k (Application type) | ||||
| Application type: mixture | 0 | - | - | |
| Application type: film | 0.023 | 0.117 | 0.842 | |
| Application type: milk | 1.312 | 0.284 | 0.000 | |
| Application type: surface | 0.645 | 0.200 | 0.001 | |
| β3k (App(√t)) | ||||
| Application: mixture | 0.676 | 0.033 | 0.000 | |
| Application: film | 0.281 | 0.019 | 0.000 | |
| Application: milk | 0.075 | 0.012 | 0.000 | |
| Application: surface | 0.078 | 0.018 | 0.000 | |
| β4m (ConcUnit) | ||||
| Unit: %v/v | 0 | - | - | |
| Unit: %w/w | −0.248 | 0.112 | 0.028 | |
| β5m (ConcUnit(LnConc)) | ||||
| Unit: %v/v | 0.324 | 0.094 | 0.001 | |
| Unit: %w/w | 0.302 | 0.093 | 0.001 | |
| Variances | ||||
| su | 0.562 | |||
| sv | 0.260 | |||
| ρ(susv) | 0.628 | |||
| s (residual) | 0.120 |
| Essential Oil | Intercept | Slope |
|---|---|---|
| Anise | 0.087 | 0.025 |
| Black cumin seed | −1.368 | −0.345 |
| Lemon balm | 0.229 | 0.269 |
| Oregano | −0.193 | −0.304 |
| Rosemary | 0.228 | −0.139 |
| Sage | 0.283 | 0.345 |
| Shallot | 0.615 | 0.182 |
| Tarragon | −0.127 | 0.194 |
| Thyme | 0.118 | −0.116 |
| Zataria multiflora Boiss. | 0.128 | −0.111 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes Silva, B.; Cadavez, V.; Teixeira, J.A.; Gonzales-Barron, U. Effects of Essential Oils on Escherichia coli Inactivation in Cheese as Described by Meta-Regression Modelling. Foods 2020, 9, 716. https://doi.org/10.3390/foods9060716
Nunes Silva B, Cadavez V, Teixeira JA, Gonzales-Barron U. Effects of Essential Oils on Escherichia coli Inactivation in Cheese as Described by Meta-Regression Modelling. Foods. 2020; 9(6):716. https://doi.org/10.3390/foods9060716
Chicago/Turabian StyleNunes Silva, Beatriz, Vasco Cadavez, José António Teixeira, and Ursula Gonzales-Barron. 2020. "Effects of Essential Oils on Escherichia coli Inactivation in Cheese as Described by Meta-Regression Modelling" Foods 9, no. 6: 716. https://doi.org/10.3390/foods9060716
APA StyleNunes Silva, B., Cadavez, V., Teixeira, J. A., & Gonzales-Barron, U. (2020). Effects of Essential Oils on Escherichia coli Inactivation in Cheese as Described by Meta-Regression Modelling. Foods, 9(6), 716. https://doi.org/10.3390/foods9060716