An HS-GC-IMS Method for the Quality Classification of Virgin Olive Oils as Screening Support for the Panel Test
Abstract
1. Introduction
2. Materials and Methods
2.1. Virgin Olive Oil (VOO) Samples and Sensory Evaluation
2.2. Headspace Gas Chromatography-Ion Mobility Spectrometry (HS-GC-IMS): Instrumental Equipment
2.3. Selected Volatile Compounds
2.4. HS-GC-IMS Analysis of Volatile Compounds Mixtures
2.5. HS-GC-IMS Analysis of Virgin Olive Oil Samples
2.6. Performance of the Method
2.6.1. Linearity
2.6.2. Intra-Day and Inter-Day Repeatability
2.7. Data Analysis
2.8. Set-Up of Analytical Conditions
3. Results and Discussion
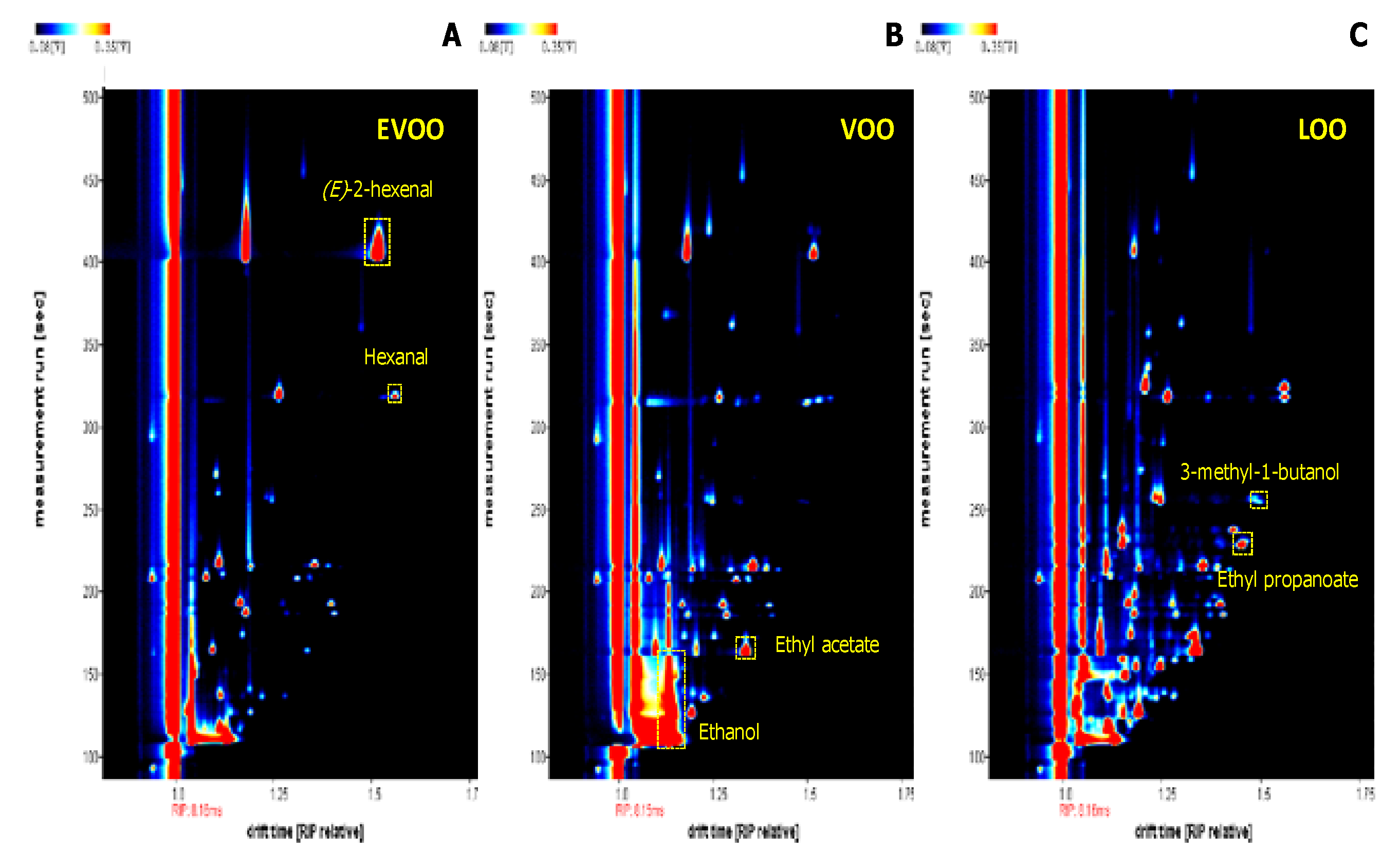
3.1. Selected Volatile Compounds
3.2. Performance of the Method
3.2.1. Linearity
3.2.2. Intra-Day and Inter-Day Repeatability
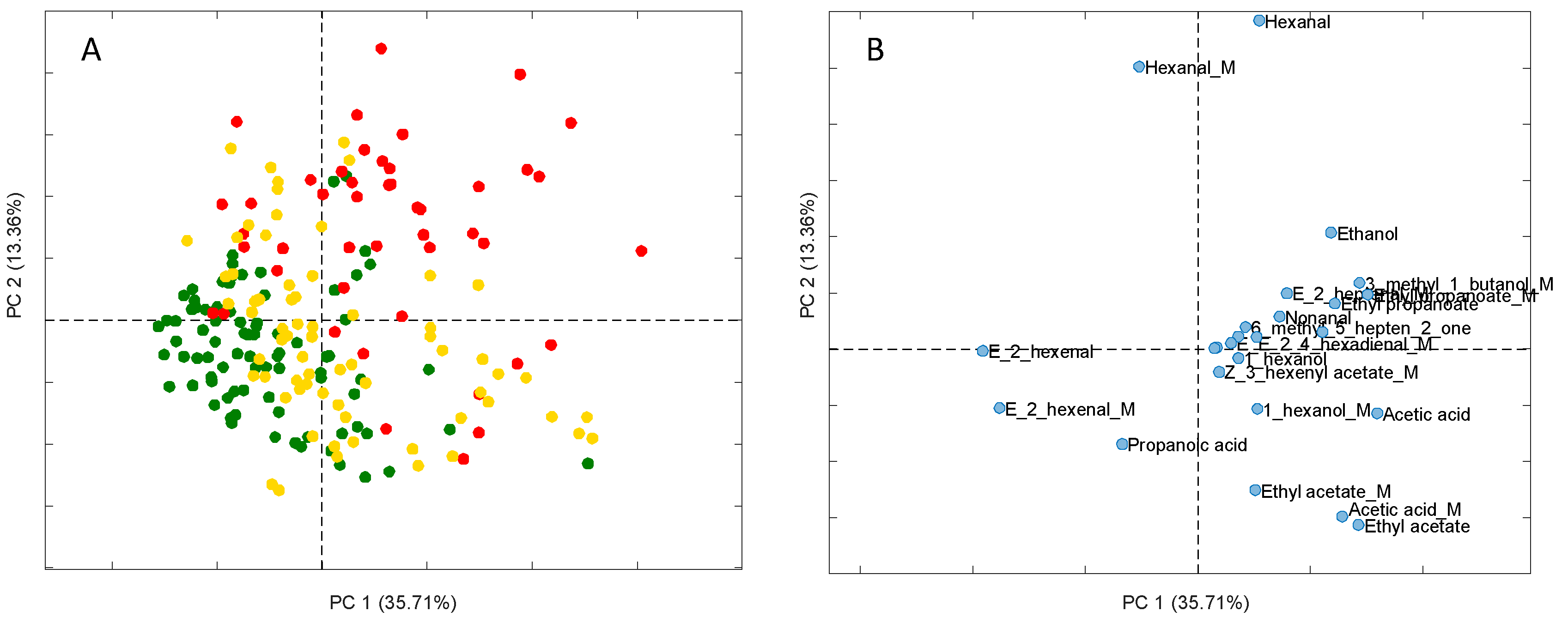
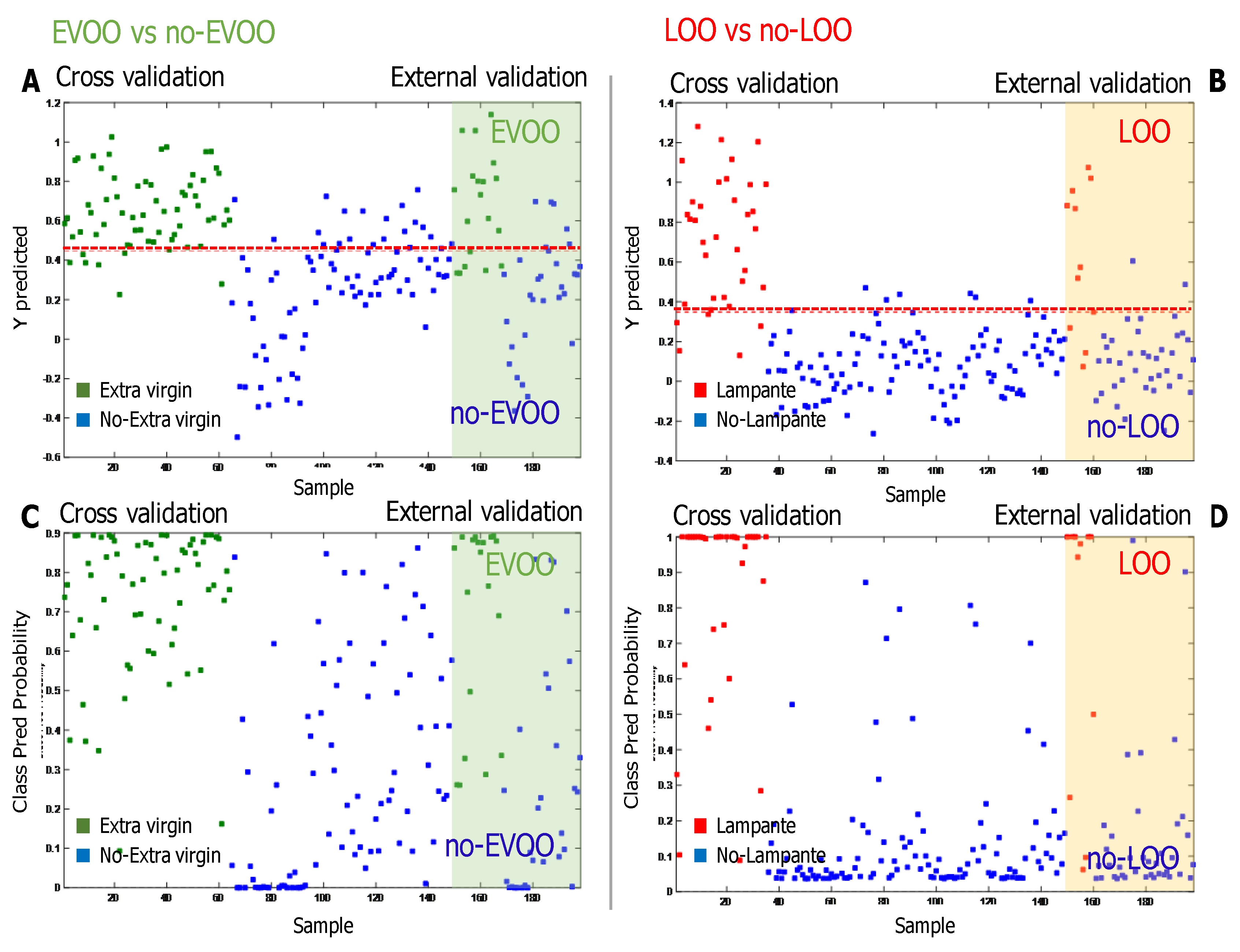
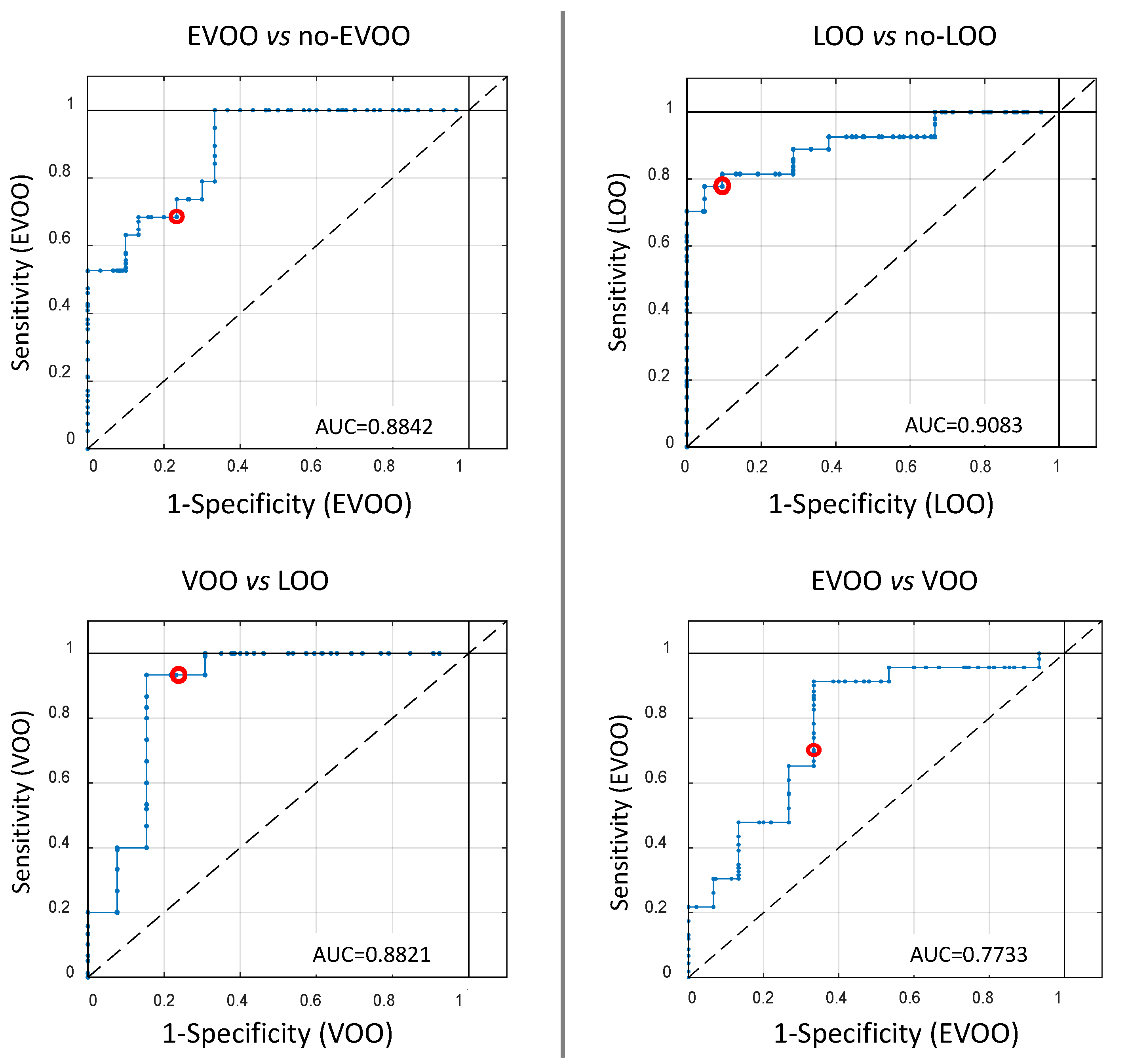
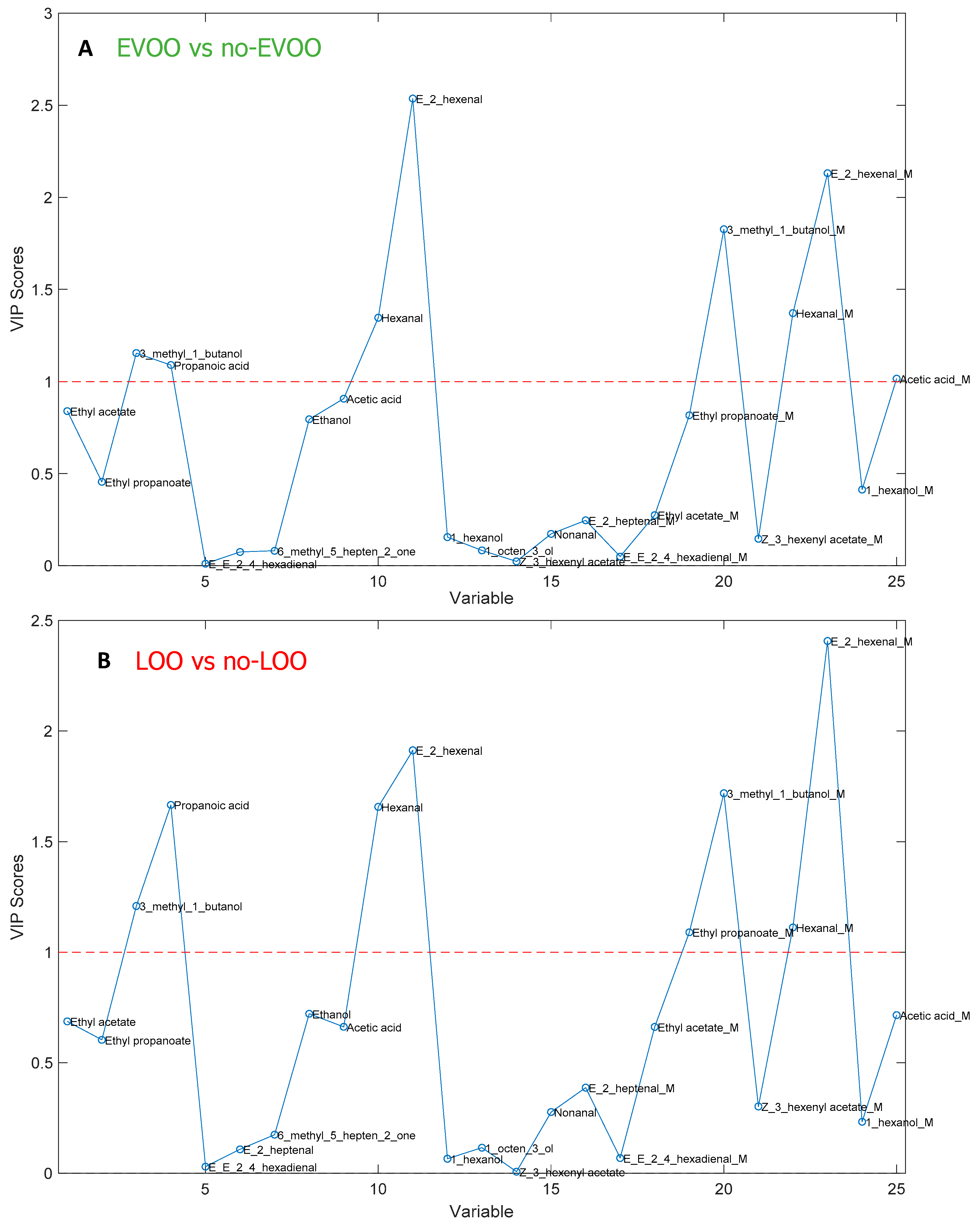
3.3. Results of the Semi-Targeted Chemometric Models for the Quality Grade Classification and on the Presence of the Defects
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Contreras, M.D.M.; Arroyo-Manzanares, N.; Arce, C.; Arce, L. HS-GC-IMS and chemometric data treatment for food authenticity assessment: Olive oil mapping and classification through two different devices as an example. Food Control 2019, 98, 82–93. [Google Scholar] [CrossRef]
- International Olive Council (IOC). Sensory Analysis of Olive Oil—Method for the Organoleptic Assessment of Virgin Olive Oil; International Olive Council (IOC): Madrid, Spain, 2018. [Google Scholar]
- Official Journal of the European Union. European Union, Commission Regulation 1348/2013. Amending Regulation (EEC) No 2568/91; Official Journal of the European Union: Brussels, Belgium, 2013; Volume L338, pp. 31–67. [Google Scholar]
- Angerosa, F.; Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.F. Volatile compounds in virgin olive oil: Occurrence and their relationship with the quality. J. Chromatogr. A 2004, 1054, 17–31. [Google Scholar] [CrossRef]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Aparicio, R.; Morales, M.T.; García-gonzález, D.L. Highlight Article Towards new analyses of aroma and volatiles to understand sensory perception of olive oil. Eur. J. Lipid Sci. Technol. 2012, 114, 1114–1125. [Google Scholar] [CrossRef]
- Cavalli, J.F.; Fernàndez, X.; Lizzani-Cuvelier, L.; Loiseau, A.M. Characterization of volatile compounds of french and spanish virgin olive oil by HS-SPME: Identification of quality-freshness markers. Food Chem. 2004, 88, 151–158. [Google Scholar] [CrossRef]
- Morales, M.T.; Luna, G.; Aparicio, R. Comparative study of virgin olive oil sensory defects. Food Chem. 2005, 91, 293–301. [Google Scholar] [CrossRef]
- Procida, G.; Giomo, A.; Cichelli, A.; Conte, L.S. Study of volatile compounds of defective virgin olive oils and sensory evaluation: A chemometric approach. J. Sci. Food Agric. 2005, 2183, 2175–2183. [Google Scholar] [CrossRef]
- Conte, L.; Bendini, A.; Valli, E.; Lucci, P.; Moret, S.; Maquet, A.; Gallina Toschi, T. Olive oil quality and authenticity: A review of current EU legislation, standards, relevant methods of analyses, their drawbacks and recommendations for the future. Trends Food Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Quintanilla-Casas, B.; Bustamante, J.; Guardiola, F.; García-González, D.L.; Barbieri, S.; Bendini, A.; Gallina Toschi, T.; Vichi, S.; Tres, A. Virgin olive oil volatile fingerprint and chemometrics: Towards an instrumental screening tool to grade the sensory quality. LWT-Food Sci. Technol. 2020, 121, 108936. [Google Scholar] [CrossRef]
- Piñero, M.Y.; Amo-Gonzalez, M.; Ballesteros, R.D.; Ruiz Pérez, L.; Fernandez De la Mora, G.; Arce, L. Chemical Fingerprinting of Olive Oils by Electrospray ionization-differential mobility analysis-mass spectrometry: A new alternative to food authenticity testing. J. Am. Soc. Mass Spectrom. 2020, 31, 527–537. [Google Scholar] [CrossRef]
- Buratti, S.; Malegori, C.; Benedetti, S.; Oliveri, P.; Giovanelli, G. E-nose, e-tongue and e-eye for edible olive oil characterization and shelf life assessment: A powerful data fusion approach. Talanta 2018, 182, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Delgado, R.; Dobao-Prieto, M.; Arce, L.; Valcárcel, M. Determination of volatile compounds by GC–IMS to assign the quality of virgin olive oil. Food Chem. 2015, 187, 72–579. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, N.; Birkenmeier, M.; Sanders, D.; Rohn, S. Resolution-optimized headspace gas chromatography-ion mobility spectrometry (HS-GC-IMS) for non-targeted olive oil profiling. Anal. Bioanal. Chem. 2017, 409, 3933–3942. [Google Scholar] [CrossRef] [PubMed]
- Schwolow, S.; Gerhardt, N.; Rohn, S.; Weller, P. Data fusion of GC-IMS data and FT-MIR spectra for the authentication of olive oils and honeys—Is it worth to go the extra mile? Anal. Bioanal. Chem. 2019, 411, 6005–6019. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Delgado, R.; Mercader-Trejo, F.; Sielemann, S.; Bruyn, W.; De Arce, L.; Valcárcel, M. Direct classification of olive oils by using two types of ion mobility spectrometers. Anal. Chim. Acta 2011, 696, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Delgado, R.; Arce, L.; Valcárcel, M. Multi-capillary column-ion mobility spectrometry: A potential screening system to differentiate virgin olive oils. Anal. Bioanal. Chem. 2012, 402, 489–498. [Google Scholar] [CrossRef]
- Contreras, M.D.M.; Jurado-Campos, N.; Arce, L. A robustness study of calibration models for olive oil classification: Targeted and non-targeted fingerprint approaches based on GC-IMS. Food Chem. 2019, 288, 315–324. [Google Scholar] [CrossRef]
- Morales, M.T.; Aparicio-Ruiz, R.; Aparicio, R. Chromatographic methodologies: Compounds for olive oil odor issues. In Handbook of Olive Oil: Analysis and Properties; Aparicio-Ruiz, R., Harwood, J., Eds.; Springer: Berlin, Germany, 2015; pp. 261–309. [Google Scholar]
- Barbieri, S.; Brkić Bubola, K.; Bendini, A.; Bučar-Miklavčič, M.; Lacoste, F.; Tibet, U.; Winkelmann, O.; García-González, D.L.; Gallina Toschi, T. Alignment and proficiency of virgin olive oil sensory panels: The OLEUM Approach. Foods 2020, 9, 355. [Google Scholar] [CrossRef]
- Valli, E.; Panni, F.; Casadei, E.; Barbieri, S.; Cevoli, C.; Bendini, A.; García-González, D.L.; Gallina Toschi, T.; OLEUM Project. Classification of Virgin Olive Oils Based on Quality Grades and Presence of Defects Using HS-GC-IMS as a Rapid Screening Tool; University of Bologna: Bologna, Italy, 2019. [Google Scholar] [CrossRef]
- Daszykowski, M.; Walczak, B.; Massart, D.L. Representative subset selection. Anal. Chim. Acta 2002, 468, 91–103. [Google Scholar] [CrossRef]
- Melucci, D.; Bendini, A.; Tesini, F.; Barbieri, S.; Zappi, A.; Vichi, S.; Conte, L.; Gallina Toschi, T. Rapid direct analysis to discriminate geographic origin of extra virgin olive oils by flash gas chromatography electronic nose and chemometrics. Food Chem. 2016, 204, 263–273. [Google Scholar] [CrossRef]
- Eiceman, G.A.; Karpas, Z.; Hill, H.H., Jr. Introduction to ion mobility spectrometry. In Ion Mobility Spectrometry, 3rd ed.; Eiceman, G.A., Karpas, Z., Eds.; CRC Press: Oxford, UK, 2014; pp. 1–16. [Google Scholar]
- Gerhardt, N.; Schwolow, S.; Rohn, S.; Pérez-Cacho, P.R. Quality assessment of olive oils based on temperature ramped HS-GC-IMS and sensory evaluation: Comparison of different processing approaches by LDA, kNN, and SVM. Food Chem. 2019, 278, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, N.; Schwolow, S.; Rohn, S.; Pérez-Cacho, P.R. Corrigendum to “Quality assessment of olive oils based on temperature-ramped HS-GC-IMS and sensory evaluation: Comparison of different processing approaches by LDA, kNN, and SVM.”. Food Chem. 2019, 286, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.D.M.; Aparicio, L.; Arce, L. Usefulness of GC-IMS for rapid quantitative analysis without sample treatment: Focus on ethanol, one of the potential classification markers of olive oils. LWT-Food Sci. Technol. 2020, 120, 108897. [Google Scholar] [CrossRef]
| Volatile Compounds | Rt a (s) | Dt b (ms) | Calibration Curve Equation | Linearity Range (mg kg−1) | (R2) c |
|---|---|---|---|---|---|
| 1. Ethyl acetate | 170 | 10.908 | y = 672.5x + 70.5 | 0.05–0.5 | 0.980 |
| 2. Ethyl propanoate | 230 | 11.844 | y = 549.7x + 9.6 | 0.05–0.5 | 0.978 |
| 3. Propanoic acid | 218 | 9.102 | y = 15.3x + 68.4 | 0.05–10 | 0.932 |
| 4. 3-methyl-1-butanol | 259 | 12.203 | y = 279.9x + 43.6 | 0.05–1.5 | 0.986 |
| 5. (E,E)-2,4-hexadienal | 522 | 11.827 | y = 87.3x + 27.8 | 1.5–10 | 0.982 |
| 6. (E)-2-heptenal | 639 | 13.71 | y = 18.4x + 175.6 | 1.5–10 | 0.969 |
| 7. 6-methyl-5-hepten-2-one | 749 | 9.588 | y = 72.2x + 162.5 | 0.05–10 | 0.994 |
| 8. Ethanol | 121 | 9.255 | y = 345.4x + 150.4 | 0.05–0.5 | 0.980 |
| 9. Acetic acid | 149 | 9.434 | y = 14.5x + 42.7 | 0.10–25 | 0.982 |
| 10. Hexanal | 317 | 12.723 | y = 198.3x + 23.3 | 0.05–1.5 | 0.991 |
| 11. (E)-2-hexenal | 404 | 12.358 | y = 47.3x + 7.3 | 0.10–10 | 0.989 |
| 12. 1-hexanol | 450 | 13.415 | y = 32.9x + 83.8 | 0.05–25 | 0.988 |
| 13. 1-octen-3-ol | 733 | 9.451 | y = 33.0x + 176.2 | 0.05–20 | 0.996 |
| 14. (Z)-3-hexenyl acetate | 846 | 14.908 | y = 6.9x + 281.7 | 5.0–25 | 0.989 |
| 15. Nonanal | 1554 | 12.128 | y = 5.1x + 138.0 | 0.05–15 | 0.990 |
| Category | Calibration | Cross Validation | External Validation |
|---|---|---|---|
| EVOO | 91% | 89% | 74% |
| no-EVOO | 84% | 75% | 77% |
| LOO | 89% | 86% | 73% |
| no-LOO | 94% | 94% | 95% |
| VOO | 92% | 91% | 87% |
| LOO | 83% | 76% | 77% |
| EVOO | 74% | 73% | 70% |
| VOO | 80% | 80% | 67% |
| Defects | Calibration | Cross Validation | External Validation |
|---|---|---|---|
| Musty | 71% | 63% | 60% |
| No-musty | 81% | 80% | 80% |
| Rancid | 81% | 78% | 62% |
| No-rancid | 69% | 64% | 64% |
| Fusty/muddy sediment | 82% | 79% | 67% |
| No-fusty/muddy sediment | 67% | 58% | 48% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valli, E.; Panni, F.; Casadei, E.; Barbieri, S.; Cevoli, C.; Bendini, A.; García-González, D.L.; Gallina Toschi, T. An HS-GC-IMS Method for the Quality Classification of Virgin Olive Oils as Screening Support for the Panel Test. Foods 2020, 9, 657. https://doi.org/10.3390/foods9050657
Valli E, Panni F, Casadei E, Barbieri S, Cevoli C, Bendini A, García-González DL, Gallina Toschi T. An HS-GC-IMS Method for the Quality Classification of Virgin Olive Oils as Screening Support for the Panel Test. Foods. 2020; 9(5):657. https://doi.org/10.3390/foods9050657
Chicago/Turabian StyleValli, Enrico, Filippo Panni, Enrico Casadei, Sara Barbieri, Chiara Cevoli, Alessandra Bendini, Diego L. García-González, and Tullia Gallina Toschi. 2020. "An HS-GC-IMS Method for the Quality Classification of Virgin Olive Oils as Screening Support for the Panel Test" Foods 9, no. 5: 657. https://doi.org/10.3390/foods9050657
APA StyleValli, E., Panni, F., Casadei, E., Barbieri, S., Cevoli, C., Bendini, A., García-González, D. L., & Gallina Toschi, T. (2020). An HS-GC-IMS Method for the Quality Classification of Virgin Olive Oils as Screening Support for the Panel Test. Foods, 9(5), 657. https://doi.org/10.3390/foods9050657






