Brewing Quality of Hop Varieties Cultivated in Central Italy Based on Multivolatile Fingerprinting and Bitter Acid Content
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Determination of α- and β-Acids
2.3. GC-MS Analysis of Volatile Components
2.4. Data Analysis
3. Results and Discussion
3.1. Precursors of Bitter Tasting Compounds of Beers
3.2. Aroma Components of Cones
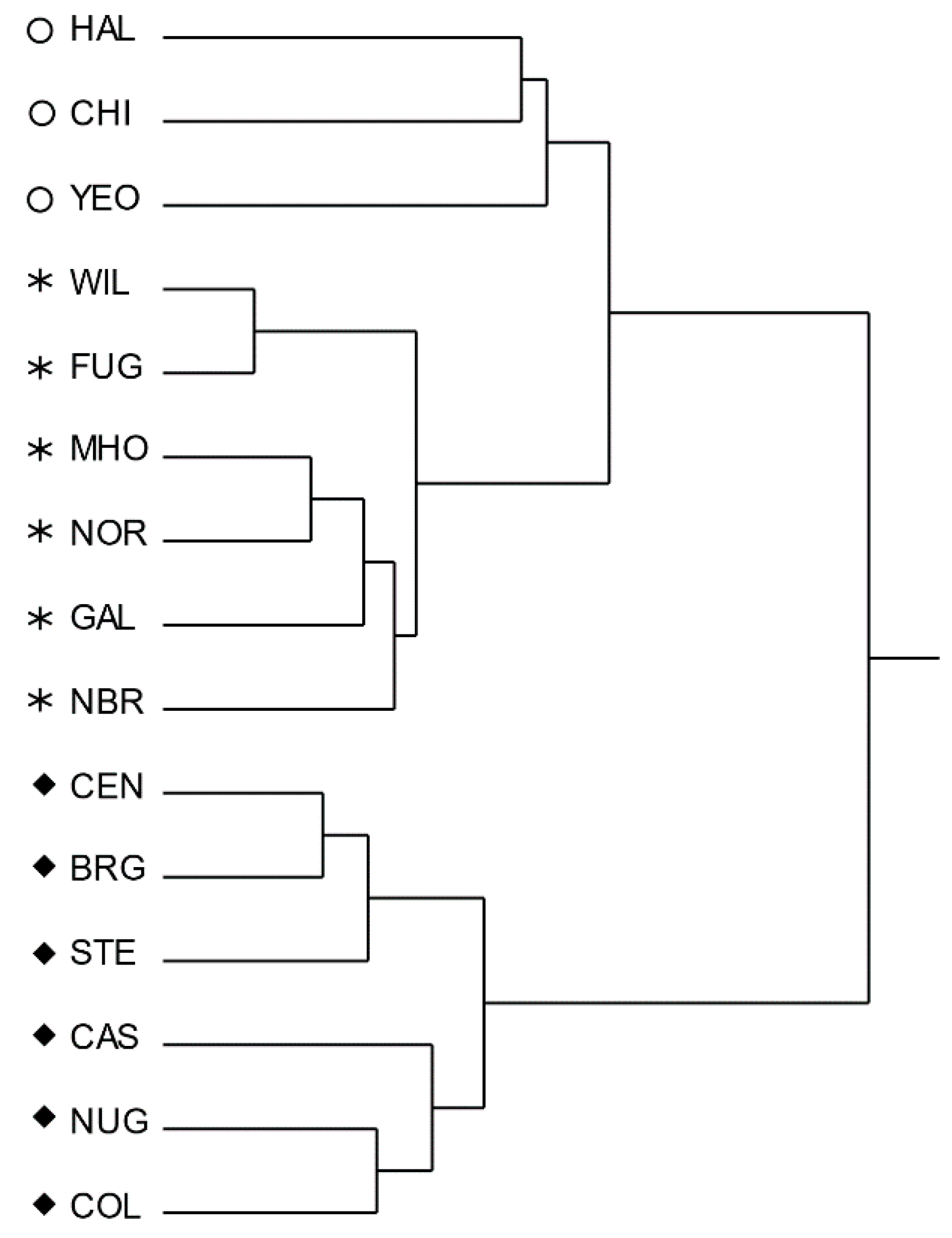
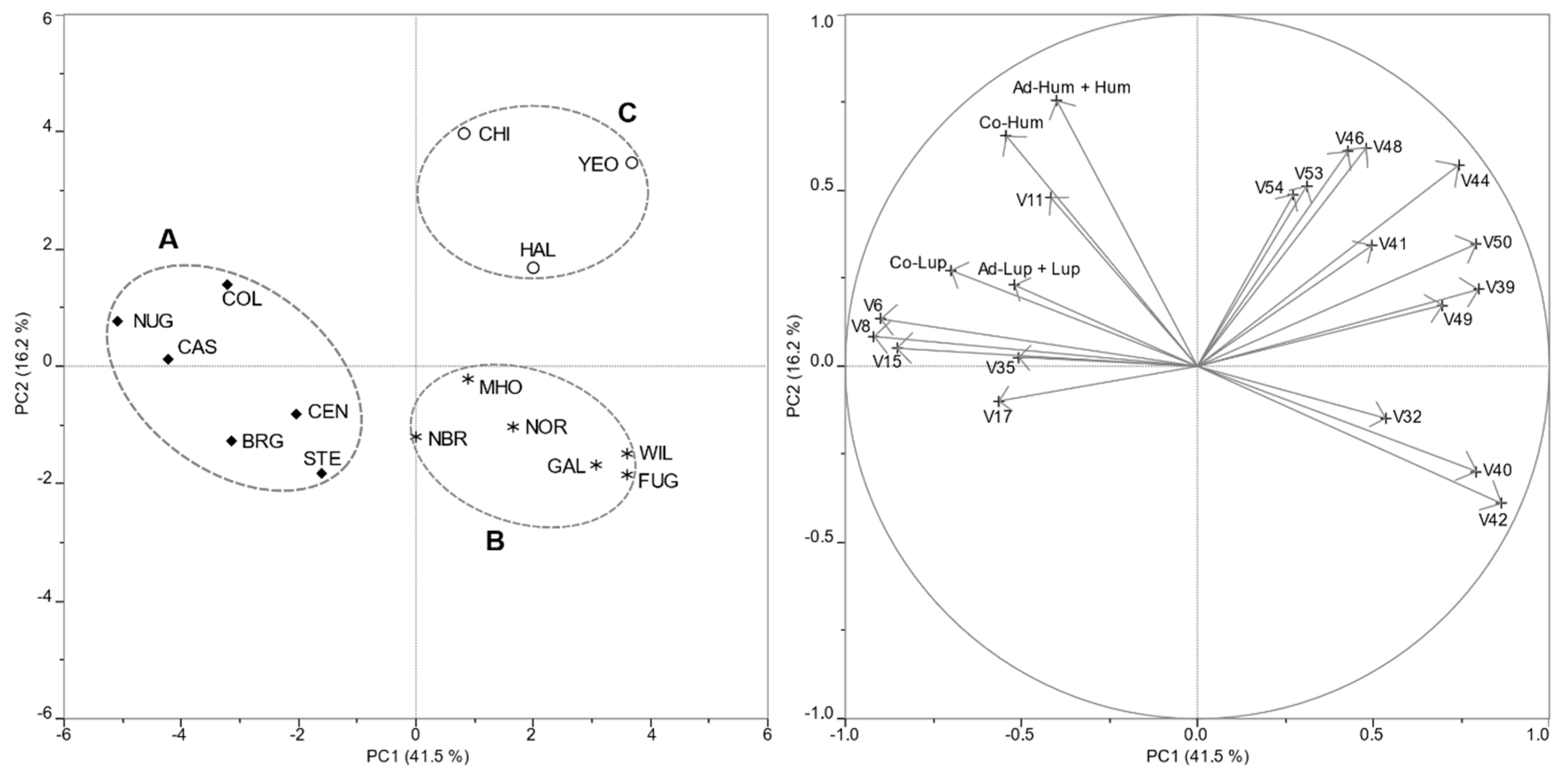
3.3. Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Almaguer, C.; Schönberger, C.; Gastl, M.; Arendt, E.K.; Becker, T. Humulus lupulus—A story that begs to be told. J. Inst. Brew. 2014, 120, 289–314. [Google Scholar] [CrossRef]
- Morales, P.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Molina, M.; Ferreira, I.C.F.R. Tocopherol composition and antioxidant activity of Spanish wild vegetables. Genet. Resour. Crop Evol. 2012, 59, 851–863. [Google Scholar] [CrossRef]
- Mozzon, M.; Pacetti, D.; Frega, N.G.; Lucci, P. Crude palm oil from interspecific hybrid Elaeis oleifera × E. guineensis: Alcoholic constituents of unsaponifiable matter. JAOCS 2015, 92, 717–724. [Google Scholar] [CrossRef]
- Van Cleemput, M.; Cattoor, K.; De Bosscher, K.; Haegeman, G.; De Keukeleire, D.; Heyerick, A. Hop (Humulus lupulus)-derived bitter acids as multipotent bioactive compounds. J. Nat. Prod. 2009, 72, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Inui, T.; Okumura, K.; Matsui, H.; Hosoya, T.; Kumazawa, S. Effect of harvest time on some in vitro functional properties of hop polyphenols. Food Chem. 2017, 225, 69–76. [Google Scholar] [CrossRef]
- Lafontaine, S.; Varnum, S.; Roland, A.; Delpech, S.; Dagan, L.; Vollmer, D.; Kishimoto, T.; Shellhammer, T. Impact of harvest maturity on the aroma characteristics and chemistry of Cascade hops used for dry-hopping. Food Chem. 2019, 278, 228–239. [Google Scholar] [CrossRef]
- Kao, T.H.; Wu, G.Y. Simultaneous determination of prenylflavonoid and hop bitter acid in beer lee by HPLC-DAD-MS. Food Chem. 2013, 14, 1218–1226. [Google Scholar] [CrossRef]
- Dresel, M.; Praet, T.; Van Opstaele, F.; Van Holle, A.; Naudts, D.; De Keukeleire, D.; De Cooman, L.; Aerts, G. Comparison of the analytical profiles of volatiles in single-hopped worts and beers as a function of the hop variety. Brew. Sci. 2015, 68, 8–28. [Google Scholar]
- Kovačevič, M.; Kač, M. Determination and verification of hop varieties by analysis of essential oils. Food Chem. 2002, 77, 489–494. [Google Scholar] [CrossRef]
- Yan, D.D.; Wong, Y.F.; Shellie, R.A.; Marriott, P.J.; Whittock, S.P.; Koutoulis, A. Assessment of the phytochemical profiles of novel hop (Humulus lupulus L.) cultivars: A potential route to beer crafting. Food Chem. 2019, 275, 15–23. [Google Scholar] [CrossRef]
- Van Opstaele, F.; De Causmaecker, B.; Aerts, G.; De Cooman, L. Characterization of novel varietal floral hop aromas by headspace solid phase microextraction and gas chromatography-mass spectrometry/olfactometry. J. Agric. Food Chem. 2012, 60, 12270–12281. [Google Scholar] [CrossRef]
- Assobirra. 2018. Available online: https://www.assobirra.it/ (accessed on 21 February 2020).
- LUPPOLO.IT. 2016. Available online: http://luppolo.crea.gov.it/ (accessed on 21 February 2020).
- Mongelli, A.; Rodolfi, M.; Ganino, T.; Marieschi, M.; Dall’Asta, C.; Bruni, R. Italian hop germplasm: Characterization of wild Humulus lupulus L. genotypes from Northern Italy by means of phytochemical, morphological traits and multivariate data analysis. Ind. Crop. Prod. 2015, 70, 16–27. [Google Scholar] [CrossRef]
- Hopslist. 2018. Available online: http://www.hopslist.com/hops/ (accessed on 14 January 2020).
- Stevens, J.F.; Taylor, A.W.; Deinzer, M.L. Quantitative analysis of xanthohumol and related prenylflavonoids in hops and beer by liquid chromatography—Tandem mass spectrometry. J. Chromatogr. A 1999, 832, 97–107. [Google Scholar] [CrossRef]
- Labor Veritas, A.G. A User’s Guide to the New International Calibration Standards for HPLC Analysis of Isomerized & Reduced Isomerized α-Acids. 2018. Available online: https://laborveritas.ch/de/produkte/ (accessed on 10 January 2020).
- Savini, S.; Loizzo, M.R.; Tundis, R.; Mozzon, M.; Foligni, R.; Longo, E.; Morozova, K.; Scampicchio, M.; Martin-Vertedor, D.; Boselli, E. Fresh refrigerated Tuber melanosporum truffle: Effect of the storage conditions on the antioxidant profile, antioxidant activity and volatile profile. Eur. Food Res. Technol. 2017, 243, 2255–2263. [Google Scholar] [CrossRef]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 5 March 2020).
- Lucero, M.; Estell, R.; Tellez, M.; Fredrickson, E. A retention index calculator simplifies identification of plant volatile organic compounds. Phytochem. Anal. 2009, 20, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Pearson, B.J.; Smith, R.M.; Chen, J. Growth, strobile yield, and quality of four Humulus lupulus varieties cultivated in a protected open-sided greenhouse structure. HortScience 2016, 51, 838–842. [Google Scholar] [CrossRef]
- Drexler, G.; Bailey, B.; Schönberger, C.; Gahr, A.; Newmann, R.; Pöschl, M.; Eberhard, G. The influence of hop harvest date on flavor stability in dry-hopped beers. Tech. Q. Master Brew. Assoc. Am. 2010, 47, 1–4. [Google Scholar] [CrossRef]
- Eri, S.; Khoo, B.K.; Lech, J.; Hartman, T.G. Direct thermal desorption–gas chromatography and gas chromatography–mass spectrometry profiling of hop (Humulus lupulus L.) essential oils in support of varietal characterization. J. Agric. Food Chem. 2000, 48, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Menary, R.C.; Williams, E.A.; Nickerson, G.B. Effect of myrtenol on the rate of oxidation of alpha- and beta-acids in hops. Acta Hortic. 1986, 188, 149–156. [Google Scholar] [CrossRef]
- Peacock, V.E. The value of linalool in modeling hop aroma in beer. Tech. Q. Master Brew. Assoc. Am. 2010, 47, 29–32. [Google Scholar] [CrossRef]
- Rossini, F.; Loreti, P.; Provenzano, M.E.; De Santis, D.; Ruggeri, R. Agronomic performance and beer quality assessment of twenty hop cultivars grown in Central Italy. Ital. J. Agron. 2016, 11, 180–187. [Google Scholar] [CrossRef]
| Sample ID | Name | Brewing Use | Seasonal Maturity 1 | Origin | α-Acids% 2 | β-Acids% 2 | Cohumulone% | Myrcene% | Humulene% | Caryophyllene% |
|---|---|---|---|---|---|---|---|---|---|---|
| HAL | Hallertau | Aroma | E to M | Germany | 3.0–3.5 | 3.5–4.5 | 20–26 | 35–44 | 30–55 | 10–15 |
| WIL | Willamette | Aroma | E to M | USA | 4–6 | 3–4 | 30–35 | 30–55 | 20–30 | 7–8 |
| YEO | Yeoman | Dual purpose | E | UK | 12–16 | 4–5 | 25 | 48 | 20 | 10 |
| CEN | Centennial | Dual purpose | M | USA | 9.5–11.5 | 3.5–4.5 | 28–30 | 45–55 | 10–18 | 5–8 |
| FUG | Fuggle | Aroma | E to M | UK | 2.4–6.1 | 2.1–2.8 | 25–29 | 43.4 | 26.6 | 9.1 |
| MHO | Mount Hood | Aroma | E to M | USA | 4–8 | 5–8 | 21–23 | 30–40 | 12–38 | 7–16 |
| NBR | Northern Brewer | Dual purpose | E to M | Germany | 7–10 | 3.5–5 | 27–33 | 25–45 | 35–50 | 10–20 |
| GAL | Galena | Bittering | M | USA | 12 | 7.5 | 39 | 55–60 | 10–15 | 3–6 |
| BRG | Brewer’s Gold | Bittering | L | UK | 7.1–11.3 | 3.3–6.1 | 41 | 66.7 | 11.6 | 6.5 |
| STE | Sterling | Dual purpose | M | USA | 4.5–9 | 4–6 | 21–28 | 44–48 | 19–23 | 5–8 |
| CAS | Cascade | Dual purpose | M | USA | 4.5–8.9 | 3.6–7.5 | 33–40 | 45–60 | 8–16 | 4–6 |
| NUG | Nugget | Bittering | M | USA | 9.5–14 | 4.2–5.8 | 22–30 | 48–59 | 12–22 | 7–10 |
| COL | Columbus | Dual purpose | M to L | USA | 14–18 | 4.5–6 | 28–35 | 25–55 | 9–25 | 6–12 |
| NOR | Northdown | Dual purpose | M | UK | 7–10 | 4–5.5 | 24–32 | 23–29 | 37–45 | 13–17 |
| CHI | Chinook | Dual purpose | M to L | USA | 12–14 | 3–4 | 29–34 | 35–40 | 18–25 | 9–11 |
| Sample ID | CoH | AdH + Hum | CoL | AdL + Lup | Total α-Acids | Total β-Acids |
|---|---|---|---|---|---|---|
| HAL | 0.34 ± 0.05 g | 1.24 ± 0.20 efg | 0.76 ± 0.13 efg | 1.23 ± 0.20 cdef | 1.58 ± 0.24 ef | 1.99 ± 0.33 de |
| WIL | 0.31 ± 0.03 g | 0.34 ± 0.00 g | 1.25 ± 0.01 def | 1.09 ± 0.01 def | 0.65 ± 0.03 f | 2.34 ± 0.02 de |
| YEO | 1.86 ± 0.00 cd | 5.94 ± 0.03 b | 1.34 ± 0.01 cde | 1.66 ± 0.00 bcd | 7.81 ± 0.03 b | 3.00 ± 0.01 bcd |
| CEN | 0.72 ± 0.08 fg | 1.80 ± 0.22d ef | 1.08 ± 0.14 defg | 1.30 ± 0.18 cdef | 2.51 ± 0.29 de | 2.37 ± 0.32 de |
| FUG | 0.40 ± 0.03 g | 0.83 ± 0.10f g | 0.46 ± 0.06 fg | 0.42 ± 0.07 f | 1.23 ± 0.13 ef | 0.88 ± 0.13 e |
| MHO | 0.77 ± 0.04 fg | 2.51 ± 0.11 cd | 1.77 ± 0.08 bcd | 2.49 ± 0.09 b | 3.28 ± 0.15 cd | 4.26 ± 0.17 bc |
| NBR | 1.08 ± 0.13 ef | 2.36 ± 0.30 cde | 1.21 ± 0.12 defg | 1.14 ± 0.10 def | 3.44 ± 0.42 cd | 2.35 ± 0.22 de |
| GAL | 0.29 ± 0.11 g | 0.70 ± 0.26 fg | 0.41 ± 0.17 g | 0.53 ± 0.21 ef | 0.99 ± 0.37 ef | 0.95 ± 0.38 e |
| BRG | 0.78 ± 0.06 fg | 1.35 ± 0.11d efg | 1.06 ± 0.10 defg | 0.74 ± 0.07 def | 2.13 ± 0.17 def | 1.79 ± 0.17 de |
| STE | 0.79 ± 0.09 fg | 1.45 ± 0.02d efg | 1.36 ± 0.10 cde | 1.68 ± 0.11 bcd | 2.24 ± 0.11 def | 3.04 ± 0.21 bcd |
| CAS | 1.37 ± 0.24 de | 3.10 ± 0.65 c | 3.14 ± 0.61 a | 4.25 ± 0.81 a | 4.47 ± 0.89 c | 7.39 ± 1.42 a |
| NUG | 2.90 ± 0.05 a | 7.71 ± 0.15 a | 2.23 ± 0.06 b | 2.15 ± 0.06 bc | 10.61 ± 0.20 a | 4.38 ± 0.11 b |
| COL | 2.49 ± 0.35 ab | 4.92 ± 0.70 b | 2.10 ± 0.33 bc | 1.44 ± 0.23 cde | 7.41 ± 1.05 b | 3.54 ± 0.57 bcd |
| NOR | 0.63 ± 0.08 fg | 1.52 ± 0.19 defg | 0.87 ± 0.13 efg | 0.99 ± 0.14 def | 2.15 ± 0.28 def | 1.86 ± 0.26 de |
| CHI | 2.14 ± 0.10 bc | 5.84 ± 0.28 b | 1.29 ± 0.06 de | 1.28 ± 0.06 cdef | 7.98 ± 0.37 b | 2.56 ± 0.11 cde |
| Peak ID | RT (min) | RI | CI Parent and Base Ions | Name | CAS Number | Category |
|---|---|---|---|---|---|---|
| V1 | 6.616 | 919 | 145 [M + 1] | isobutyl isobutyrate | 97-85-8 | Ester |
| V2 | 6.773 | 926 | 137 [M + 1] | tricyclene | 508-32-7 | Monoterpene hydrocarbon |
| V3 | 6.947 | 934 | 137 [M + 1] | α-thujene | 2867-05-2 | Monoterpene hydrocarbon |
| V4 | 7.119 | 942 | 137 [M + 1] | α-pinene | 80-56-8 | Monoterpene hydrocarbon |
| V5 | 7.472 | 957 | 137 [M + 1] | camphene | 79-92-5 | Monoterpene hydrocarbon |
| V6 | 8.147 | 983 | 137 [M + 1] | β-pinene | 127-91-3 | Monoterpene hydrocarbon |
| V7 | 8.356 | 991 | 127 [M + 1]. 109 [M + 1 − H2O] | 6-methyl-5-heptene-2-one | 110-93-0 | Ketone |
| V8 | 8.510 | 996 | 137 [M + 1] | β-myrcene | 123-35-3 | Monoterpene hydrocarbon |
| V9 | 8.810 | 1009 | 137 [M + 1] | α-phellandrene | 99-83-2 | Monoterpene hydrocarbon |
| V10 | 8.957 | 1015 | 158 [M] | 2-methylbutyl isobutyrate | 2445-69-4 | Ester |
| V11 | 9.044 | 1019 | 158 [M] | isoamyl isobutyrate | 2050-01-3 | Ester |
| V12 | 9.105 | 1022 | 137 [M + 1] | α-terpinene | 99-86-5 | Monoterpene hydrocarbon |
| V13 | 9.251 | 1028 | 145 [M + 1] | methyl heptanoate | 106-73-0 | Ester |
| V14 | 9.332 | 1032 | 143 [M + 1] | heptenoic acid isomer. methyl ester | Ester | |
| V15 | 9.413 | 1035 | 137 [M + 1] | β-phellandrene | 555-10-2 | Monoterpene hydrocarbon |
| V16 | 9.594 | 1043 | 137 [M + 1] | (Z)-β-ocimene | 3338-55-4 | Monoterpene hydrocarbon |
| V17 | 9.859 | 1054 | 137 [M + 1] | (E)-β-ocimene | 3779-61-1 | Monoterpene hydrocarbon |
| V18 | 10.147 | 1065 | 137 [M + 1] | y-terpinene | 99-85-4 | Monoterpene hydrocarbon |
| V19 | 10.203 | 1067 | 159 [M + 1] | heptanoic acid. 2-methyl. methyl ester | 51209-78-0 | Ester |
| V20 | 10.808 | 1090 | 159 [M + 1] | heptanoic acid. 6-methyl. methyl ester | 2519-37-1 | Ester |
| V21 | 10.883 | 1092 | 137 [M + 1] | terpinolene | 586-62-9 | Monoterpene hydrocarbon |
| V22 | 10.931 | 1094 | 143 [M + 1] | 2-nonanone | 821-55-6 | Ketone |
| V23 | 11.152 | 1102 | 137 [M + 1 − H2O] | linalool | 78-70-6 | Oxygenated monoterpene |
| V24 | 11.726 | 1128 | 159 [M + 1] | methyl octanoate | 111-11-5 | Ester |
| V25 | 11.862 | 1134 | 137 [M + 1] | alloocimene | 7216-56-0 | Monoterpene hydrocarbon |
| V26 | 12.173 | 1147 | 137 [M + 1] | Unidentified | Monoterpene hydrocarbon | |
| V27 | 13.390 | 1195 | 157 [M + 1] | 2-decanone | 693-54-9 | Ketone |
| V28 | 13.549 | 1201 | 157 [M + 1 − CH2] | n-dodecane | 112-40-3 | n-alkane |
| V29 | 13.846 | 1215 | 171 [M + 1]. 139 [M + 1 − CH3OH] | methyl x-nonenoate | Ester | |
| V30 | 14.129 | 1227 | 173 [M + 1] | methyl nonanoate | 1731-84-6 | Ester |
| V31 | 14.638 | 1250 | 187 [M + 1] | heptyl isobutanoate | 2349-13-5 | Ester |
| V32 | 15.749 | 1295 | 171 [M + 1] | 2-undecanone | 112-12-9 | Ketone |
| V33 | 15.874 | 1300 | 171 [M + 1 − CH2] | n-tridecane | 629-50-5 | n-alkane |
| V34 | 16.092 | 1311 | 185 [M + 1]. 153 [M + 1 − CH3OH] | methyl 4-decenoate | 1191-02-2 | Ester |
| V35 | 16.443 | 1328 | 187 [M + 1] | methyl decanoate | 110-42-9 | Ester |
| V36 | 17.100 | 1358 | 205 [M + 1] | α-cubebene | 17699-14-8 | Sesquiterpene hydrocarbon |
| V37 | 17.208 | 1362 | 205 [M + 1] | sativene | 3650-28-0 | Sesquiterpene hydrocarbon |
| V38 | 17.610 | 1380 | 205 [M + 1] | ylangene | 14912-44-8 | Sesquiterpene hydrocarbon |
| V39 | 17.711 | 1384 | 205 [M + 1] | α-copaene | 3856-25-5 | Sesquiterpene hydrocarbon |
| V40 | 18.717 | 1432 | 205 [M + 1] | b-caryophillene | 87-44-5 | Sesquiterpene hydrocarbon |
| V41 | 18.879 | 1440 | 205 [M + 1] | β-cubebene | 13744-15-5 | Sesquiterpene hydrocarbon |
| V42 | 19.463 | 1468 | 205 [M + 1] | humulene | 6753-98-6 | Sesquiterpene hydrocarbon |
| V43 | 19.787 | 1483 | 205 [M + 1] | b-copaene | 18612-33-4 | Sesquiterpene hydrocarbon |
| V44 | 19.844 | 1486 | 205 [M + 1] | (E)-b-Famesene | 18794-84-8 | Sesquiterpene hydrocarbon |
| V45 | 19.916 | 1489 | 205 [M + 1] | α-Farnesene | 502-61-4 | Sesquiterpene hydrocarbon |
| V46 | 20.095 | 1497 | 205 [M + 1] | b-selinene | 17066-67-0 | Sesquiterpene hydrocarbon |
| V47 | 20.212 | 1503 | 205 [M + 1] | α-muurolene | 31983-22-9 | Sesquiterpene hydrocarbon |
| V48 | 20.276 | 1506 | 205 [M + 1] | α-selinene | 473-13-2 | Sesquiterpene hydrocarbon |
| V49 | 20.626 | 1524 | 205 [M + 1] | γ-muurolene | 30021-74-0 | Sesquiterpene hydrocarbon |
| V50 | 20.784 | 1532 | 205 [M + 1] | α-cadinene | 11044-40-9 | Sesquiterpene hydrocarbon |
| V51 | 20.844 | 1535 | 205 [M + 1] | b-cadinene | 523-47-7 | Sesquiterpene hydrocarbon |
| V52 | 20.986 | 1543 | 205 [M + 1] | cadina-1.4-diene | 16728-99-7 | Sesquiterpene hydrocarbon |
| V53 | 21.090 | 1548 | 205 [M + 1] | (E)-calamenene | 40772-39-2 | Sesquiterpene hydrocarbon |
| V54 | 21.210 | 1554 | 205 [M + 1] | α-calacorene | 21391-99-1 | Sesquiterpene hydrocarbon |
| V55 | 22.057 | 1595 | 203 [M + 1 − H2O] | caryophillene oxyde | 1139-30-6 | Oxygenated sesquiterpene |
| V56 | 22.356 | 203 [M + 1 − H2O] | Unidentified | Oxygenated sesquiterpene | ||
| V57 | 22.567 | 203 [M + 1 − H2O] | Unidentified | Oxygenated sesquiterpene |
| Peak ID | HAL | WIL | YEO | CEN | FUG | MHO | NBR | GAL | BRG | STE | CAS | NUG | COL | NOR | CHI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V1 | 0.03 ± 0.00 b | 0.03 ± 0.00 b | 0.28 ± 0.18 b | 0.08 ± 0.06 b | 0.05 ± 0.04 b | 0.05 ± 0.02 b | 0.09 ± 0.03 b | 0.08 ± 0.08 b | 0.35 ± 0.10 b | 0.04 ± 0.00 b | 0.11 ± 0.04 b | 0.44 ± 0.09 a | 0.90 ± 0.42 a | 0.07 ± 0.03 b | 0.30 ± 0.02 b |
| V2 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.07 ± 0.09 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| V3 | 0.02 ± 0.00 ab | 0.00 ± 0.00 b | 0.00 ± 0.00 b | 0.04 ± 0.03 ab | 0.14 ± 0.12 a | 0.01 ± 0.00 ab | 0.01 ± 0.00 ab | 0.00 ± 0.00 b | 0.02 ± 0.01 ab | 0.02 ± 0.00 ab | 0.03 ± 0.01 ab | 0.04 ± 0.02 ab | 0.05 ± 0.02 ab | 0.01 ± 0.00 b | 0.01 ± 0.00 ab |
| V4 | 0.08 ± 0.03 | 0.07 ± 0.03 | 0.15 ± 0.06 | 0.35 ± 0.35 | 0.09 ± 0.06 | 0.12 ± 0.06 | 0.18 ± 0.03 | 0.06 ± 0.04 | 0.22 ± 0.04 | 0.24 ± 0.07 | 0.25 ± 0.14 | 0.24 ± 0.04 | 0.31 ± 0.10 | 0.19 ± 0.12 | 0.22 ± 0.00 |
| V5 | 0.00 ± 0.00 c | 0.00 ± 0.00 bc | 0.02 ± 0.01 abc | 0.02 ± 0.02 abc | 0.01 ± 0.00 bc | 0.00 ± 0.00 c | 0.01 ± 0.00 bc | 0.01 ± 0.00 bc | 0.02 ± 0.01 abc | 0.01 ± 0.00 abc | 0.04 ± 0.03 abc | 0.06 ± 0.01 a | 0.06 ± 0.02 ab | 0.02 ± 0.01 abc | 0.02 ± 0.01 abc |
| V6 | 0.85 ± 0.21 | 0.44 ± 0.17 | 0.57 ± 0.24 | 2.49 ± 2.19 | 0.50 ± 0.35 | 1.03 ± 0.42 | 0.92 ± 0.17 | 0.49 ± 0.25 | 1.81 ± 0.20 | 1.67 ± 0.18 | 1.93 ± 0.86 | 2.00 ± 0.33 | 2.26 ± 0.74 | 1.01 ± 0.48 | 1.60 ± 0.11 |
| V7 | 0.01 ± 0.01 ab | 0.02 ± 0.01 ab | 0.11 ± 0.09 ab | 0.00 ± 0.00 b | 0.06 ± 0.03 ab | 0.01 ± 0.00 ab | 0.05 ± 0.03 ab | 0.04 ± 0.00 ab | 0.01 ± 0.01 ab | 0.02 ± 0.00 ab | 0.01 ± 0.01 ab | 0.04 ± 0.01 ab | 0.12 ± 0.02 a | 0.04 ± 0.01 ab | 0.00 ± 0.00 b |
| V8 | 41.44 ± 1.94 bcd | 14.14 ± 0.04 e | 14.95 ± 6.07 e | 49.45 ± 4.05 abc | 14.08 ± 2.47 e | 40.82 ± 7.23 bcd | 34.01 ± 2.05 cd | 26.33 ± 3.53 b | 54.05 ± 5.40 | 53.69 ± 5.85 ab | 67.10 ± 5.66 a | 64.45 ± 1.34 a | 45.20 ± 4.84 bc | 33.72 ± 4.43 cd | 45.35 ± 5.24 bc |
| V9 | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.06 ± 0.05 ab | 0.01 ± 0.01 b | 0.05 ± 0.04 b | 0.02 ± 0.01 b | 0.03 ± 0.00 b | 0.03 ± 0.02 b | 0.04 ± 0.01 b | 0.02 ± 0.00 b | 0.01 ± 0.01 b | 0.03 ± 0.00 b | 0.12 ± 0.01 a | 0.04 ± 0.01 b | 0.04 ± 0.00 b |
| V10 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.37 ± 0.22 | 0.05 ± 0.04 | 0.07 ± 0.05 | 0.02 ± 0.01 | 0.10 ± 0.03 | 0.12 ± 0.12 | 0.04 ± 0.03 | 0.01 ± 0.00 | 0.08 ± 0.08 | 0.21 ± 0.26 | 0.10 ± 0.12 | 0.09 ± 0.04 | 0.33 ± 0.02 |
| V11 | 0.03 ± 0.03 d | 0.15 ± 0.04 d | 1.16 ± 0.62 bcd | 0.28 ± 0.12 cd | 0.18 ± 0.18 d | 0.11 ± 0.03 d | 0.28 ± 0.11 cd | 0.60 ± 0.84 bcd | 2.16 ± 0.52 bc | 0.03 ± 0.03 d | 0.20 ± 0.14 d | 1.85 ± 0.01 bcd | 4.32 ± 1.41 a | 0.30 ± 0.08 cd | 2.36 ± 0.07 b |
| V12 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.23 ± 0.26 | 0.04 ± 0.04 | 0.47 ± 0.50 | 0.02 ± 0.02 | 0.04 ± 0.01 | 0.01 ± 0.01 | 0.06 ± 0.00 | 0.03 ± 0.00 | 0.20 ± 0.23 | 0.02 ± 0.01 | 0.06 ± 0.01 | 0.03 ± 0.01 | 0.06 ± 0.01 |
| V13 | 0.14 ± 0.05 bc | 0.01 ± 0.01 c | 0.13 ± 0.06 bc | 0.12 ± 0.05 bc | 0.07 ± 0.04 bc | 0.23 ± 0.07 abc | 0.23 ± 0.19 abc | 0.13 ± 0.14 bc | 0.35 ± 0.09 ab | 0.13 ± 0.00 bc | 0.17 ± 0.02 bc | 0.50 ± 0.06 a | 0.23 ± 0.05 abc | 0.09 ± 0.05 bc | 0.27 ± 0.06 abc |
| V14 | 0.62 ± 0.07 | 0.07 ± 0.00 | 0.06 ± 0.03 | 0.20 ± 0.12 | 0.15 ± 0.05 | 0.20 ± 0.05 | 0.29 ± 0.22 | 0.16 ± 0.12 | 0.06 ± 0.07 | 0.16 ± 0.02 | 0.11 ± 0.03 | 0.25 ± 0.02 | 0.44 ± 0.46 | 0.19 ± 0.05 | 0.11 ± 0.02 |
| V15 | 0.68 ± 0.05 ab | 0.30 ± 0.02 b | 0.44 ± 0.18 ab | 1.46 ± 0.79 a | 0.41 ± 0.18 ab | 0.73 ± 0.26 ab | 0.87 ± 0.16 ab | 0.42 ± 0.10 ab | 1.22 ± 0.32 ab | 0.99 ± 0.01 ab | 1.08 ± 0.03 ab | 0.95 ± 0.02 ab | 1.16 ± 0.39 ab | 0.60 ± 0.19 bc | 0.92 ± 0.04 ab |
| V16 | 0.02 ± 0.00 b | 0.02 ± 0.00 b | 0.05 ± 0.03 b | 0.05 ± 0.05 b | 0.03 ± 0.00 b | 0.02 ± 0.01 b | 0.13 ± 0.02 a | 0.01 ± 0.01 b | 0.04 ± 0.01 b | 0.04 ± 0.01 b | 0.03 ± 0.01 b | 0.04 ± 0.00 b | 0.01 ± 0.01 b | 0.05 ± 0.01 b | 0.02 ± 0.00 b |
| V17 | 0.03 ± 0.00 b | 0.13 ± 0.05 b | 0.09 ± 0.06 b | 0.14 ± 0.10 b | 0.09 ± 0.03 b | 0.06 ± 0.02 b | 1.39 ± 0.21 a | 0.10 ± 0.08 b | 1.17 ± 0.41 a | 0.07 ± 0.01 b | 0.23 ± 0.02 b | 1.01 ± 0.09 a | 1.09 ± 0.27 a | 0.26 ± 0.01 b | 0.06 ± 0.01 b |
| V18 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.06 ± 0.02 | 0.04 ± 0.04 | 0.03 ± 0.01 | 0.14 ± 0.16 | 0.01 ± 0.00 | 0.04 ± 0.01 | 0.02 ± 0.00 | 0.04 ± 0.00 | 0.04 ± 0.01 | 0.10 ± 0.01 | 0.02 ± 0.01 | 0.04 ± 0.01 |
| V19 | 0.15 ± 0.20 | 0.12 ± 0.04 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.11 ± 0.06 | 0.01 ± 0.01 | 0.08 ± 0.07 | 0.04 ± 0.00 | 0.03 ± 0.02 | 0.11 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.02 | 0.10 ± 0.03 | 0.01 ± 0.00 |
| V20 | 0.19 ± 0.09 b | 0.04 ± 0.02 b | 0.03 ± 0.03 b | 0.12 ± 0.05 b | 0.06 ± 0.01 b | 0.10 ± 0.02 b | 0.09 ± 0.05 b | 0.25 ± 0.31 b | 0.15 ± 0.21 b | 0.04 ± 0.00 b | 0.16 ± 0.00 b | 0.35 ± 0.04 b | 0.45 ± 0.20 ab | 0.14 ± 0.04 b | 0.87 ± 0.03 a |
| V21 | 0.18 ± 0.18 | 0.02 ± 0.01 | 0.04 ± 0.03 | 0.07 ± 0.03 | 0.06 ± 0.06 | 0.03 ± 0.01 | 0.05 ± 0.01 | 0.03 ± 0.02 | 0.16 ± 0.12 | 0.04 ± 0.01 | 0.06 ± 0.00 | 0.05 ± 0.00 | 0.07 ± 0.05 | 0.05 ± 0.00 | 0.00 ± 0.00 |
| V22 | 0.28 ± 0.19 | 0.01 ± 0.00 | 0.34 ± 0.02 | 0.18 ± 0.06 | 0.21 ± 0.08 | 0.30 ± 0.09 | 0.17 ± 0.04 | 0.22 ± 0.17 | 0.10 ± 0.02 | 0.15 ± 0.01 | 0.16 ± 0.01 | 0.12 ± 0.03 | 0.31 ± 0.18 | 0.15 ± 0.07 | 0.02 ± 0.01 |
| V23 | 0.94 ± 0.12 a | 0.21 ± 0.10 d | 0.17 ± 0.07 d | 0.50 ± 0.20 abcd | 0.40 ± 0.07 bcd | 0.33 ± 0.33 cd | 0.40 ± 0.02 bcd | 0.42 ± 0.07 bcd | 0.45 ± 0.02 abcd | 0.83 ± 0.14 ab | 0.35 ± 0.08 bcd | 0.72 ± 0.06 abc | 0.47 ± 0.04 abcd | 0.63 ± 0.11 abcd | 0.30 ± 0.01 cd |
| V24 | 0.18 ± 0.14 bcde | 0.02 ± 0.01 e | 0.06 ± 0.03 de | 0.12 ± 0.05 cde | 0.03 ± 0.02 e | 0.05 ± 0.00 de | 0.06 ± 0.04 de | 0.10 ± 0.08 cde | 0.34 ± 0.01 abcd | 0.02 ± 0.00 e | 0.14 ± 0.01 cde | 0.48 ± 0.02 ab | 0.50 ± 0.02 a | 0.08 ± 0.03 cde | 0.37 ± 0.02 abc |
| V25 | 0.01 ± 0.00 c | 0.00 ± 0.00 c | 0.01 ± 0.01 bc | 0.04 ± 0.01 abc | 0.05 ± 0.03 a | 0.01 ± 0.00 bc | 0.05 ± 0.00 ab | 0.01 ± 0.01 bc | 0.02 ± 0.00 abc | 0.01 ± 0.01 bc | 0.05 ± 0.01 ab | 0.02 ± 0.01 abc | 0.01 ± 0.00 bc | 0.02 ± 0.01 bc | 0.01 ± 0.00 bc |
| V26 | 0.02 ± 0.01 bc | 0.01 ± 0.01 c | 0.01 ± 0.01 bc | 0.04 ± 0.02 bc | 0.04 ± 0.03 bc | 0.01 ± 0.00 bc | 0.12 ± 0.02 a | 0.01 ± 0.01 bc | 0.03 ± 0.01 bc | 0.02 ± 0.01 bc | 0.03 ± 0.00 bc | 0.04 ± 0.02 bc | 0.06 ± 0.01 b | 0.05 ± 0.00 bc | 0.01 ± 0.01 c |
| V27 | 0.35 ± 0.08 ab | 0.07 ± 0.05 b | 0.28 ± 0.01 ab | 0.07 ± 0.05 b | 0.42 ± 0.13 | 0.25 ± 0.06 ab | 0.36 ± 0.05 ab | 0.61 ± 0.33 a | 0.07 ± 0.00 b | 0.29 ± 0.01 ab | 0.06 ± 0.01 b | 0.09 ± 0.01 b | 0.07 ± 0.10 ab | 0.26 ± 0.03 ab | 0.57 ± 0.05 a |
| V28 | 0.03 ± 0.03 ab | 0.00 ± 0.00 b | 0.01 ± 0.01 ab | 0.01 ± 0.01 ab | 0.06 ± 0.01 ab | 0.01 ± 0.01 ab | 0.04 ± 0.03 ab | 0.08 ± 0.01 a | 0.00 ± 0.00 ab | 0.01 ± 0.01 ab | 0.02 ± 0.02 ab | 0.00 ± 0.00 b | 0.04 ± 0.05 ab | 0.04 ± 0.02 ab | 0.00 ± 0.01 ab |
| V29 | 0.06 ± 0.02 ab | 0.01 ± 0.00 b | 0.04 ± 0.03 ab | 0.03 ± 0.03 ab | 0.06 ± 0.01 ab | 0.03 ± 0.00 ab | 0.07 ± 0.04 ab | 0.14 ± 0.14 ab | 0.04 ± 0.02 ab | 0.08 ± 0.01 ab | 0.08 ± 0.01 ab | 0.19 ± 0.04 a | 0.06 ± 0.00 ab | 0.07 ± 0.06 ab | 0.08 ± 0.02 ab |
| V30 | 0.07 ± 0.03 bcd | 0.02 ± 0.01 d | 0.02 ± 0.01 d | 0.03 ± 0.03 d | 0.06 ± 0.00 bcd | 0.05 ± 0.01 bcd | 0.07 ± 0.04 bcd | 0.18 ± 0.22 abcd | 0.24 ± 0.02 abcd | 0.03 ± 0.00 cd | 0.11 ± 0.01 bcd | 0.38 ± 0.02 a | 0.29 ± 0.06 ab | 0.10 ± 0.07 bcd | 0.29 ± 0.03 abc |
| V31 | 0.01 ± 0.01 bc | 0.01 ± 0.00 c | 0.05 ± 0.02 bc | 0.01 ± 0.00 bc | 0.11 ± 0.08 ab | 0.02 ± 0.01 bc | 0.08 ± 0.03 abc | 0.05 ± 0.02 bc | 0.04 ± 0.01 bc | 0.01 ± 0.00 bc | 0.02 ± 0.02 bc | 0.10 ± 0.01 abc | 0.17 ± 0.01 a | 0.10 ± 0.03 abc | 0.03 ± 0.01 bc |
| V32 | 0.81 ± 0.01 ab | 0.05 ± 0.05 b | 0.44 ± 0.04 ab | 0.15 ± 0.07 b | 0.35 ± 0.08 b | 0.32 ± 0.20 b | 0.37 ± 0.03 b | 1.22 ± 0.73 a | 0.01 ± 0.001 b | 0.44 ± 0.01 ab | 0.09 ± 0.05 b | 0.11 ± 0.02 b | 0.11 ± 0.05 b | 0.62 ± 0.28 ab | 0.12 ± 0.02 b |
| V33 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.04 ± 0.05 | 0.04 ± 0.00 | 0.12 ± 0.03 | 0.03 ± 0.03 | 0.20 ± 0.00 | 0.14 ± 0.16 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.06 ± 0.07 | 0.20 ± 0.27 | 0.07 ± 0.05 | 0.00 ± 0.00 |
| V34 | 0.64 ± 0.39 | 0.07 ± 0.05 | 0.16 ± 0.03 | 0.35 ± 0.41 | 0.18 ± 0.03 | 0.21 ± 0.10 | 0.20 ± 0.04 | 0.55 ± 0.68 | 0.76 ± 0.07 | 0.19 ± 0.04 | 0.31 ± 0.06 | 1.00 ± 0.07 | 0.95 ± 0.18 | 0.54 ± 0.03 | 0.88 ± 0.09 |
| V35 | 0.13 ± 0.07 | 0.09 ± 0.00 | 0.10 ± 0.06 | 0.96 ± 0.72 | 0.18 ± 0.09 | 0.11 ± 0.00 | 0.90 ± 1.00 | 0.31 ± 0.19 | 1.22 ± 0.30 | 0.06 ± 0.04 | 0.45 ± 0.15 | 0.28 ± 0.04 | 0.81 ± 0.08 | 0.15 ± 0.02 | 0.76 ± 0.04 |
| V36 | 0.18 ± 0.02 cd | 0.12 ± 0.03 def | 0.03 ± 0.00 f | 0.17 ± 0.02 cd | 0.07 ± 0.01 def | 0.28 ± 0.02 bc | 0.04 ± 0.00 ef | 0.15 ± 0.02 de | 0.12 ± 0.05 def | 0.08 ± 0.00 def | 0.10 ± 0.01 def | 0.11 ± 0.00 def | 0.31 ± 0.08 | 0.04 ± 0.01 ef | 0.44 ± 0.04 a |
| V37 | 0.01 ± 0.00 e | 0.05 ± 0.00 bc | 0.08 ± 0.01 a | 0.03 ± 0.01 cde | 0.04 ± 0.00 bcd | 0.04 ± 0.01 bcde | 0.04 ± 0.00 bcde | 0.03 ± 0.01 cde | 0.02 ± 0.00 e | 0.02 ± 0.00 de | 0.02 ± 0.00 de | 0.02 ± 0.00 e | 0.05 ± 0.01 ab | 0.04 ± 0.00 bcd | 0.03 ± 0.00 bcde |
| V38 | 0.36 ± 0.06 bcd | 0.47 ± 0.03 ab | 0.57 ± 0.05 a | 0.18 ± 0.01 fg | 0.46 ± 0.03 ab | 0.27 ± 0.05 cdef | 0.42 ± 0.00 abc | 0.24 ± 0.06 defg | 0.15 ± 0.05 fg | 0.20 ± 0.04 efg | 0.12 ± 0.01 fg | 0.10 ± 0.01 g | 0.25 ± 0.01 defg | 0.39 ± 0.06 bcd | 0.35 ± 0.04 bcde |
| V39 | 0.87 ± 0.02 efg | 1.50 ± 0.0 ab | 1.71 ± 0.14 a | 0.73 ± 0.03 fgh | 1.43 ± 0.02 ab | 0.94 ± 0.09 cdef | 1.36 ± 0.01 abc | 0.86 ± 0.16 efg | 0.51 ± 0.13 gh | 0.63 ± 0.12 fgh | 0.69 ± 0.12 fgh | 0.36 ± 0.07 | 0.90 ± 0.09 defg | 1.21 ± 0.13 bcde | 1.28 ± 0.14 bcd |
| V40 | 12.08 ± 0.22 efg | 25.54 ± 2.35 a | 20.84 ± 2.10 abc | 12.71 ± 1.78 efg | 23.14 ± 1.34 ab | 18.17 ± 0.38 bcd | 19.57 ± 0.05 bcd | 16.66 ± 0.24 cde | 10.44 ± 1.54 fg | 12.20 ± 1.78 efg | 7.44 ± 1.24 g | 7.64 ± 0.13 g | 14.20 ± 1.58 def | 18.94 ± 1.40 bcd | 9.75 ± 0.98 fg |
| V41 | 0.79 ± 0.10 bcd | 1.21 ± 0.02 b | 0.72 ± 0.05 bcd | 0.75 ± 0.15 bcd | 1.02 ± 0.02 bc | 1.00 ± 0.13 bc | 0.80 ± 0.10 bcd | 1.00 ± 0.23 bc | 0.63 ± 0.20 cd | 0.54 ± 0.07 cd | 0.44 ± 0.07 d | 0.49 ± 0.11 g | 0.87 ± 0.08 bcd | 0.77 ± 0.13 bcd | 1.91 ± 0.29 a |
| V42 | 21.72 ± 0.66 defg | 46.10 ± 0.16 a | 33.84 ± 2.81 bc | 20.85 ± 5.05 defg | 45.79 ± 2.37 a | 25.63 ± 3.83 cdef | 28.79 ± 2.07 bcde | 38.53 ± 1.28 ab | 17.81 ± 3.80 efg | 22.35 ± 3.13 defg | 12.01 ± 3.45 g | 11.58 ± 1.07 g | 15.72 ± 1.17 fg | 29.11 ± 4.09 bcd | 17.21 ± 1.71 fg |
| V43 | 0.35 ± 0.13 ab | 0.10 ± 0.13 abc | 0.16 ± 0.08 abc | 0.02 ± 0.00 c | 0.17 ± 0.02 abc | 0.24 ± 0.07 abc | 0.21 ± 0.04 abc | 0.11 ± 0.12 abc | 0.17 ± 0.04 abc | 0.08 ± 0.11 bc | 0.01 ± 0.01 c | 0.09 ± 0.00 abc | 0.26 ± 0.00 abc | 0.23 ± 0.05 abc | 0.38 ± 0.08 a |
| V44 | 1.55 ± 0.19 bcd | 1.60 ± 0.33 bcd | 2.92 ± 0.56 a | 1.11 ± 0.26 bcd | 1.32 ± 0.14 bcd | 1.69 ± 0.66 bc | 1.13 ± 0.23 bcd | 1.34 ± 0.18 bcd | 0.77 ± 0.10 cd | 0.75 ± 0.01 cd | 0.82 ± 0.13 cd | 0.47 ± 0.06 d | 0.99 ± 0.14 bcd | 1.39 ± 0.11 bcd | 2.03 ± 0.38 ab |
| V45 | 0.06 ± 0.03 bc | 0.21 ± 0.02 ab | 0.25 ± 0.07 a | 0.09 ± 0.00 abc | 0.13 ± 0.04 abc | 0.07 ± 0.09 bc | 0.16 ± 0.02 abc | 0.12 ± 0.08 abc | 0.08 ± 0.02 bc | 0.10 ± 0.02 abc | 0.07 ± 0.00 bc | 0.04 ± 0.00 c | 0.15 ± 0.01 abc | 0.18 ± 0.03 abc | 0.22 ± 0.04 ab |
| V46 | 3.18 ± 0.51 b | 0.85 ± 0.26 c | 5.69 ± 0.58 a | 1.17 ± 0.50 c | 1.00 ± 0.18 c | 0.56 ± 0.18 c | 0.47 ± 0.24 c | 0.93 ± 0.32 c | 0.51 ± 0.03 c | 0.36 ± 0.02 c | 1.05 ± 0.30 c | 0.78 ± 0.09 c | 0.76 ± 0.03 c | 1.29 ± 0.31 c | 1.09 ± 0.20 c |
| V47 | 0.00 ± 0.00 c | 0.31 ± 0.05 ab | 0.16 ± 0.18 abc | 0.21 ± 0.06 abc | 0.31 ± 0.03 ab | 0.38 ± 0.19 ab | 0.34 ± 0.01 ab | 0.35 ± 0.02 ab | 0.14 ± 0.01 bc | 0.17 ± 0.04 abc | 0.11 ± 0.08 bc | 0.10 ± 0.00 bc | 0.27 ± 0.04 abc | 0.25 ± 0.05 abc | 0.46 ± 0.33 a |
| V48 | 5.19 ± 1.18 b | 1.57 ± 0.36 c | 8.41 ± 1.02 a | 1.69 ± 0.68 c | 1.53 ± 0.18 c | 1.05 ± 0.02 c | 1.31 ± 0.39 c | 1.76 ± 0.66 c | 1.06 ± 0.03 c | 0.74 ± 0.05 c | 1.55 ± 0.41 c | 0.98 ± 0.21 c | 1.38 ± 0.00 c | 2.56 ± 0.55 c | 2.28 ± 0.37 c |
| V49 | 0.61 ± 0.02 bc | 1.07 ± 0.28 abc | 0.96 ± 0.16 abc | 0.72 ± 0.26 abc | 1.04 ± 0.13 abc | 1.20 ± 0.63 abc | 0.85 ± 0.23 abc | 1.28 ± 0.25 ab | 0.59 ± 0.06 bc | 0.61 ± 0.05 bc | 0.46 ± 0.15 bc | 0.31 ± 0.06 c | 0.78 ± 0.04 abc | 0.91 ± 0.16 abc | 1.55 ± 0.26 a |
| V50 | 1.81 ± 0.25 ab | 2.09 ± 0.60 ab | 2.24 ± 0.32 ab | 1.36 ± 0.49 ab | 2.15 ± 0.50 ab | 2.39 ± 1.41 ab | 1.62 ± 0.41 ab | 2.13 ± 0.41 ab | 1.10 ± 0.07 ab | 1.06 ± 0.15 ab | 0.89 ±0.36 b | 0.56 ± 0.07 b | 1.50 ± 0.02 ab | 1.81 ± 0.30 ab | 3.03 ± 0.39 a |
| V51 | 0.04 ± 0.00 c | 0.26 ± 0.08 abc | 0.38 ± 0.03 a | 0.16 ± 0.00 abc | 0.18 ± 0.16 abc | 0.09 ± 0.09 bc | 0.31 ± 0.02 ab | 0.17 ± 0.01 abc | 0.14 ± 0.00 abc | 0.16 ± 0.03 abc | 0.10 ± 0.00 bc | 0.05 ± 0.02 c | 0.05 ± 0.07 c | 0.31 ± 0.01 ab | 0.11 ± 0.11 bc |
| V52 | 0.07 ± 0.01 b | 0.14 ± 0.04 ab | 0.16 ± 0.03 ab | 0.09 ± 0.04 ab | 0.13 ± 0.02 ab | 0.17 ± 0.09 ab | 0.13 ± 0.04 ab | 0.13 ± 0.03 ab | 0.09 ± 0.00 ab | 0.06 ± 0.00 b | 0.05 ± 0.03 b | 0.04 ± 0.00 b | 0.10 ± 0.01 ab | 0.13 ± 0.02 ab | 0.19 ± 0.01 a |
| V53 | 1.45 ± 0.38 a | 0.20 ± 0.10 c | 0.19 ± 0.03 c | 0.12 ± 0.06 c | 0.17 ± 0.03 c | 0.20 ± 0.13 c | 0.14 ± 0.04 c | 0.24 ± 0.09 c | 0.11 ± 0.01 c | 0.09 ± 0.01 c | 0.08 ± 0.03 c | 0.06 ± 0.01 c | 0.15 ± 0.01 c | 0.16 ± 0.02 c | 0.90 ± 0.13 b |
| V54 | 1.30 ± 0.37 a | 0.08 ± 0.05 c | 0.09 ± 0.02 c | 0.03 ± 0.02 c | 0.06 ± 0.02 c | 0.06 ± 0.04 c | 0.05 ± 0.02 c | 0.09 ± 0.05 c | 0.03 ± 0.00 c | 0.03 ± 0.00 c | 0.03 ± 0.01 c | 0.01 ± 0.00 c | 0.01 ± 0.01 c | 0.07 ± 0.01 c | 0.66 ± 0.12 b |
| V55 | 0.10 ± 0.03 | 0.09 ± 0.07 | 0.03 ± 0.00 | 0.05 ± 0.02 | 0.49 ± 0.61 | 0.06 ± 0.06 | 0.05 ± 0.03 | 0.10 ± 0.12 | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.06 ± 0.04 | 0.01 ± 0.00 | 0.04 ± 0.01 | 0.07 ± 0.03 | 0.03 ± 0.00 |
| V56 | 0.03 ± 0.00 | 0.05 ± 0.03 | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.05 ± 0.00 | 0.02 ± 0.02 | 0.02 ± 0.02 | 0.55 ± 0.76 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.03 ± 0.02 | 0.00 ± 0.00 | 0.01 ± 0.00 | 0.04 ± 0.02 | 0.01 ± 0.00 |
| V57 | 0.20 ± 0.02 | 0.19 ± 0.18 | 0.04 ± 0.01 | 0.14 ± 0.10 | 0.24 ± 0.00 | 0.16 ± 0.16 | 0.12 ± 0.08 | 0.31 ± 0.42 | 0.11 ± 0.06 | 0.15 ± 0.01 | 0.15 ± 0.10 | 0.02 ± 0.01 | 0.04 ± 0.01 | 0.20 ± 0.08 | 0.04 ± 0.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mozzon, M.; Foligni, R.; Mannozzi, C. Brewing Quality of Hop Varieties Cultivated in Central Italy Based on Multivolatile Fingerprinting and Bitter Acid Content. Foods 2020, 9, 541. https://doi.org/10.3390/foods9050541
Mozzon M, Foligni R, Mannozzi C. Brewing Quality of Hop Varieties Cultivated in Central Italy Based on Multivolatile Fingerprinting and Bitter Acid Content. Foods. 2020; 9(5):541. https://doi.org/10.3390/foods9050541
Chicago/Turabian StyleMozzon, Massimo, Roberta Foligni, and Cinzia Mannozzi. 2020. "Brewing Quality of Hop Varieties Cultivated in Central Italy Based on Multivolatile Fingerprinting and Bitter Acid Content" Foods 9, no. 5: 541. https://doi.org/10.3390/foods9050541
APA StyleMozzon, M., Foligni, R., & Mannozzi, C. (2020). Brewing Quality of Hop Varieties Cultivated in Central Italy Based on Multivolatile Fingerprinting and Bitter Acid Content. Foods, 9(5), 541. https://doi.org/10.3390/foods9050541