Influence of Grape Pomace Intake on Nutritional Value, Lipid Oxidation and Volatile Profile of Poultry Meat
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Management and Sampling
2.2. Evaluation of pH and Color
2.3. Drip Loss, Cooking Loss, and Chemical Composition of Meat Samples
2.4. Fatty Acid Composition and Lipid Oxidation
2.5. Volatile Compounds Evaluation
2.6. High Performance Liquid Chromatography for the Analysis of Biogenic Amines
2.7. Statistical Analysis
3. Results
3.1. Physical and Chemical Characterization of Chicken Breast Meat
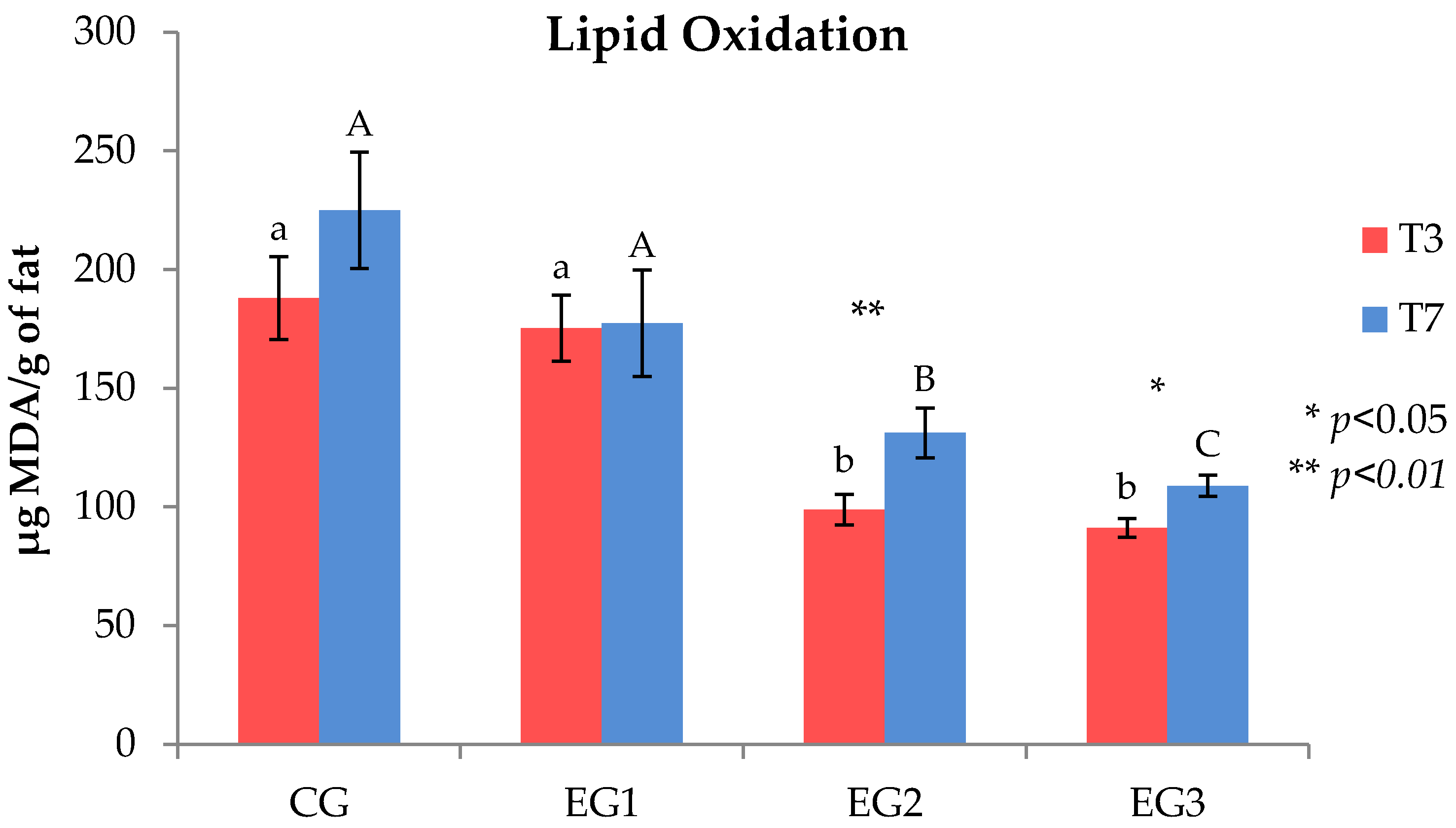
3.2. Fatty Acid Composition and Lipid Oxidative Stability
3.3. Identification of Volatile Compounds in Chicken Meat
3.4. Evaluation of Biogenic Amines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yu, J.; Ahmedna, M. Functional components of grape pomace: Their composition, biological properties and potential applications. Int. J. Food Sci. Technol. 2013, 48, 221–237. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Ianni, A.; Martino, G. Dietary Grape Pomace Supplementation in Dairy Cows: Effect on Nutritional Quality of Milk and Its Derived Dairy Products. Foods 2020, 9, 168. [Google Scholar] [CrossRef]
- Castellani, F.; Vitali, A.; Bernardi, N.; Marone, E.; Palazzo, F.; Grotta, L.; Martino, G. Dietary supplementation with dried olive pomace in dairy cows modifies the composition of fatty acids and the aromatic profile in milk and related cheese. J. Dairy Sci. 2017, 100, 8658–8669. [Google Scholar] [CrossRef]
- Iannaccone, M.; Ianni, A.; Ramazzotti, S.; Grotta, L.; Marone, E.; Cichelli, A.; Martino, G. Whole blood transcriptome analysis reveals positive effects of dried olive pomace-supplemented diet on inflammation and cholesterol in laying hens. Animals 2019, 9, 427. [Google Scholar] [CrossRef]
- Ianni, A.; Di Maio, G.; Pittia, P.; Grotta, L.; Perpetuini, G.; Tofalo, R.; Cichelli, A.; Martino, G. Chemical-nutritional quality and oxidative stability of milk and dairy products obtained from Friesian cows fed with a dietary supplementation of dried grape pomace. J. Sci. Food Agric. 2019, 99, 3635–3643. [Google Scholar] [CrossRef]
- Ianni, A.; Innosa, D.; Martino, C.; Bennato, F.; Martino, G. Compositional characteristics and aromatic profile of caciotta cheese obtained from Friesian cows fed with a dietary supplementation of dried grape pomace. J. Dairy Sci. 2019, 102, 1025–1032. [Google Scholar] [CrossRef]
- Iannaccone, M.; Elgendy, R.; Giantin, M.; Martino, C.; Giansante, D.; Ianni, A.; Dacasto, M.; Martino, G. RNA sequencing-based whole-transcriptome analysis of friesian cattle fed with grape pomace-supplemented diet. Animals 2018, 8, 188. [Google Scholar] [CrossRef]
- Gómez-Cortés, P.; Guerra-Rivas, C.; Gallardo, B.; Lavin, P.; Mantecón, A.; De La Fuente, M.; Manso, T.; Guerra, C. Grape pomace in ewes diet: Effects on meat quality and the fatty acid profile of their suckling lambs. Food Res. Int. 2018, 113, 36–42. [Google Scholar] [CrossRef]
- Ianni, A.; Di Luca, A.; Martino, C.; Bennato, F.; Marone, E.; Grotta, L.; Cichelli, A.; Martino, G. Dietary Supplementation of Dried Grape Pomace Increases the Amount of Linoleic Acid in Beef, Reduces the Lipid Oxidation and Modifies the Volatile Profile. Animals 2019, 9, 578. [Google Scholar] [CrossRef]
- Biscarini, F.; Palazzo, F.; Castellani, F.; Masetti, G.; Grotta, L.; Cichelli, A.; Martino, G. Rumen microbiome in dairy calves fed copper and grape-pomace dietary supplementations: Composition and predicted functional profile. PLoS ONE 2018, 13, e0205670. [Google Scholar] [CrossRef]
- Aditya, S.; Ohh, S.-J.; Ahammed, M.; Lohakare, J. Supplementation of grape pomace (Vitis vinifera) in broiler diets and its effect on growth performance, apparent total tract digestibility of nutrients, blood profile, and meat quality. Anim. Nutr. 2018, 4, 210–214. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirement of Poultry; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000.
- Folch, J.; Lees, M.; Stanley, G.H.S. A simple method for the isolation and purification of total lipids from animal tissues. J. Boil. Chem. 1957, 226, 497–509. [Google Scholar]
- Grotta, L.; Castellani, F.; Palazzo, F.; Haouet, M.N.; Martino, G. Treatment optimization and sample preparation for the evaluation of lipid oxidation in various meats through TBARs assays before analysis. Food Anal. Methods 2017, 10, 1870–1880. [Google Scholar] [CrossRef]
- Ianni, A.; Iannaccone, M.; Martino, C.; Innosa, D.; Grotta, L.; Bennato, F.; Martino, G. Zinc supplementation of dairy cows: Effects on chemical composition, nutritional quality and volatile profile of Giuncata cheese. Int. Dairy J. 2019, 94, 65–71. [Google Scholar] [CrossRef]
- Schirone, M.; Tofalo, R.; Fasoli, G.; Perpetuini, G.; Corsetti, A.; Manetta, A.C.; Ciarrocchi, A.; Suzzi, G. High content of biogenic amines in Pecorino cheeses. Food Microbiol. 2013, 34, 137–144. [Google Scholar] [CrossRef]
- Nelder, J.A.; Baker, R.J. Generalized Linear Models; John Wiley & Sons Inc.: New York, NY, USA, 1972. [Google Scholar]
- Lambert, I.H.; Nielsen, J.H.; Andersen, H.J.; Ørtenblad, N. Cellular model for induction of drip loss in meat. J. Agric. Food Chem. 2001, 49, 4876–4883. [Google Scholar] [CrossRef]
- Siedow, J.N. Plant lipoxygenase: Structure and function. Ann. Rev. Plant Biol. 1991, 42, 145–188. [Google Scholar] [CrossRef]
- Podolyan, A.; White, J.; Jordan, B.; Winefield, C. Identification of the lipoxygenase gene family from Vitis vinifera and biochemical characterisation of two 13-lipoxygenases expressed in grape berries of Sauvignon Blanc. Funct. Plant Biol. 2010, 37, 767–784. [Google Scholar] [CrossRef]
- Leifert, W.R.; Abeywardena, M.Y. Grape seed and red wine polyphenol extracts inhibit cellular cholesterol uptake, cell proliferation, and 5-lipoxygenase activity. Nutr. Res. 2008, 28, 842–850. [Google Scholar] [CrossRef]
- Kasapidou, E.; Sossidou, E.; Zdragas, A.; Papadaki, C.; Vafeas, G.; Mitlianga, P. Effect of grape pomace supplementation on broiler meat quality characteristics. Eur. Poult. Sci. 2016, 80, 135–142. [Google Scholar] [CrossRef]
- Lindahl, G.; Lundström, K.; Tornberg, E. Contribution of pigment content, myoglobin forms and internal reflectance to the colour of pork loin and ham from pure breed pigs. Meat Sci. 2001, 59, 141–151. [Google Scholar] [CrossRef]
- Francis, F.J.; Markakis, P.C. Food colorants: Anthocyanins. Crit. Rev. Food Sci. Nutr. 1989, 28, 273–314. [Google Scholar] [CrossRef] [PubMed]
- Sáyago-Ayerdi, S.G.; Brenes, A.; Viveros, A.; Goñi, I. Antioxidative effect of dietary grape pomace concentrate on lipid oxidation of chilled and long-term frozen stored chicken patties. Meat Sci. 2009, 83, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Moote, P.E.; Church, J.S.; Schwartzkopf-Genswein, K.S.; Van Hamme, J.D. Effect of Fermented Winery By-Product Supplemented Rations on the Temperament and Meat Quality of Angus-Hereford X Steers During Feeding in a British Columbia Feedlot. J. Food Res. 2014, 3, 124. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Sheard, P.R.; Richardson, R.I.; Hughes, S.I.; Whittington, F.M. Fat deposition, fatty acid composition and meat quality: A review. Meat Sci. 2008, 78, 343–358. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M. Factors influencing fatty acids in meat and the role of antioxidants in improving meat quality. Br. J. Nutr. 1997, 78, S49–S60. [Google Scholar] [CrossRef]
- Bennato, F.; Ianni, A.; Innosa, D.; Grotta, L.; D’Onofrio, A.; Martino, G. Chemical-nutritional characteristics and aromatic profile of milk and related dairy products obtained from goats fed with extruded linseed. Asian Australas. J. Anim. Sci. 2020, 33, 148. [Google Scholar] [CrossRef]
- Manso, T.; Gallardo, B.; Salvá, A.; Guerra-Rivas, C.; Mantecón, A.R.; Lavín, P.; De la Fuente, M.A. Influence of dietary grape pomace combined with linseed oil on fatty acid profile and milk composition. J. Dairy Sci. 2016, 99, 1111–1120. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Zervas, G. The effect of dietary inclusion of olive tree leaves and grape marc on the content of conjugated linoleic acid and vaccenic acid in the milk of dairy sheep and goats. J. Dairy Res. 2008, 75, 270–278. [Google Scholar] [CrossRef]
- Martino, G.; Mugnai, C.; Compagnone, D.; Grotta, L.; Del Carlo, M.; Sarti, F. Comparison of performance, meat lipids and oxidative status of pigs from commercial breed and organic crossbreed. Animals 2014, 4, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Brenes, A.; Centeno, C.; Viveros, A.; Saura-Calixto, F.; Rebolé, A.; Arija, I.; Estevez, R. Effect of Dietary Grape Pomace and Vitamin E on Growth Performance, Nutrient Digestibility, and Susceptibility to Meat Lipid Oxidation in Chickens. Poult. Sci. 2007, 86, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Palade, L.M.; Marin, D.E.; Pelmus, R.S.; Habeanu, M.; Rotar, M.C.; Gras, M.A.; Pistol, G.C.; Taranu, I. Intestinal absorption and antioxidant activity of grape pomace polyphenols. Nutrients 2018, 10, 588. [Google Scholar] [CrossRef] [PubMed]
- Insausti, K.; Goni, V.; Petri, E.; Gorraiz, C.; Beriain, M.J. Effect of weight at slaughter on the volatile compounds of cooked beef from Spanish cattle breeds. Meat Sci. 2005, 70, 83–90. [Google Scholar] [CrossRef]
- Mottram, D.S. Flavour formation in meat and meat products: A review. Food Chem. 1998, 62, 415–424. [Google Scholar] [CrossRef]
- Ianni, A.; Bennato, F.; Martino, C.; Grotta, L.; Martino, G. Volatile Flavor Compounds in Cheese as Affected by Ruminant Diet. Molecules 2020, 25, 461. [Google Scholar] [CrossRef]
- Bennato, F.; Ianni, A.; Martino, C.; Di Luca, A.; Innosa, D.; Fusco, A.M.; Pomilio, F.; Martino, G. Dietary supplementation of Saanen goats with dried licorice root modifies chemical and textural properties of dairy products. J. Dairy Sci. 2020, 103, 52–62. [Google Scholar] [CrossRef]
- Bennato, F.; Ianni, A.; Innosa, D.; Martino, C.; Grotta, L.; Pomilio, F.; Verna, M.; Martino, G. Influence of Licorice Root Feeding on Chemical-Nutritional Quality of Cow Milk and Stracciata Cheese, an Italian Traditional Fresh Dairy Product. Animals 2019, 9, 1153. [Google Scholar] [CrossRef]
- Ford, A.L.; Park, R.J.; Ratcliff, D. Effect of a protected lipid supplement on flavor properties of beef. J. Food Sci. 1976, 41, 94–96. [Google Scholar] [CrossRef]
- Shahidi, F.; Pegg, R.B. Hexanal as an indicator of meat flavor deterioration. J. Food Lipids 1994, 1, 177–186. [Google Scholar] [CrossRef]
- Van Ba, H.; Amna, T.; Hwang, I. Significant influence of particular unsaturated fatty acids and pH on the volatile compounds in meat-like model systems. Meat Sci. 2013, 94, 480–488. [Google Scholar] [CrossRef]
- Halász, A.; Barath, A.; Simon-Sarkadi, L.; Holzapfel, W. Biogenic amines and their production by microorganisms in food. Trends Food Sci. Technol. 1994, 5, 42–49. [Google Scholar] [CrossRef]
- Galgano, F.; Favati, F.; Bonadio, M.; Lorusso, V.; Romano, P. Role of biogenic amines as index of freshness in beef meat packed with different biopolymeric materials. Food Res. Int. 2009, 42, 1147–1152. [Google Scholar] [CrossRef]
- Alberto, M.R.; Arena, M.E.; De Nadra, M.C.M. Putrescine production from agmatine by Lactobacillus hilgardii: Effect of phenolic compounds. Food Control 2007, 18, 898–903. [Google Scholar] [CrossRef]
| Trait | CG | EG1 | EG2 | EG3 | |
|---|---|---|---|---|---|
| pH48 | 5.93 ± 0.10 | 5.88 ± 0.15 | 6.04 ± 0.17 | 5.95 ± 0.12 | |
| Drip loss, % | 2.30 ± 0.24 a | 2.35 ± 0.26 a | 2.60 ± 0.27 b | 2.89 ± 0.24 b | |
| Cooking loss, % | 12.4 ± 1.41 | 10.4 ± 1.73 | 11.4 ± 1.92 | 11.7 ± 1.52 | |
| Chromaticity coordinates | L* | 55.9 ± 3.22 | 54.6 ± 2.75 | 56.2 ± 2.29 | 54.2 ± 1.12 |
| a* | −2.37 ± 0.65 a | −0.88 ± 0.16 b | −1.07 ± 0.19 b | −0.74 ± 0.11 b | |
| b* | 7.88 ± 0.89 a | 11.3 ± 1.46 b | 11.9 ± 1.27 b | 11.9 ± 1.81 b | |
| Chemical composition, % | |||||
| Moisture | 74.4 ± 1.56 | 73.5 ± 1.10 | 73.5 ± 1.83 | 73.1 ± 1.17 | |
| Dry matter † (DM) | 25.6 ± 1.56 | 26.5 ± 1.10 | 26.5 ± 1.83 | 26.9 ± 1.17 | |
| Total lipids † | 1.14 ± 0.14 | 1.16 ± 0.07 | 1.22 ± 0.17 | 1.25 ± 0.10 | |
| Proteins † | 23.4 ± 0.74 | 24.2 ± 1.04 | 23.8 ± 0.77 | 24.3 ± 1.13 | |
| Ash † | 1.08 ± 0.06 | 1.08 ± 0.05 | 1.02 ± 0.05 | 1.09 ± 0.02 | |
| Fatty Acid † | CG | EG1 | EG2 | EG3 |
|---|---|---|---|---|
| C14:0 | 0.80 ± 0.10 | 0.66 ± 0.07 | 0.69 ± 0.09 | 0.72 ± 0.09 |
| C14:1 | 0.07 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 | 0.07 ± 0.01 |
| C15:0 | 0.14 ± 0.02 | 0.11 ± 0.01 | 0.11 ± 0.02 | 0.16 ± 0.03 |
| C16:0 | 26.6 ± 2.27 | 25.5 ± 1.16 | 24.1 ± 1.03 | 24.9 ± 1.89 |
| C16:1 | 1.99 ± 0.24 | 2.02 ± 0.21 | 1.72 ± 0.18 | 2.15 ± 0.21 |
| C17:0 | 0.36 ± 0.05 | 0.38 ± 0.03 | 0.36 ± 0.05 | 0.37 ± 0.06 |
| C18:0 | 12.5 ± 0.74 | 12.9 ± 0.36 | 10.6 ± 0.52 | 10.9 ± 1.65 |
| C18:1 cis 9 | 28.8 ± 2.36 | 28.8 ± 1.49 | 30.6 ± 2.22 | 29.5 ± 1.16 |
| C18:2 | 22.6 ± 1.37 a | 23.7 ± 1.14 a | 25.7 ± 1.31 b | 25.8 ± 1.53 b |
| C18:3 | 1.56 ± 0.17 | 1.58 ± 0.15 | 1.53 ± 0.24 | 1.66 ± 0.33 |
| SFA | 40.4 ± 1.82 a | 38.6 ± 1.20 ab | 35.8 ± 1.07 b | 37.1 ± 1.57 b |
| MUFA | 30.9 ± 2.65 | 30.9 ± 1.75 | 32.4 ± 2.06 | 31.8 ± 1.61 |
| PUFA | 24.2 ± 2.09 a | 26.3 ± 1.22 ab | 27.2 ± 1.48 b | 27.4 ± 1.28 b |
| PUFA/SFA | 0.60 ± 0.07 a | 0.68 ± 0.08 a | 0.76 ± 0.09 b | 0.74 ± 0.08 a,b |
| VOC † | CG | EG1 | EG2 | EG3 |
|---|---|---|---|---|
| Pentanal | 1.67 ± 0.29 | 1.93 ± 0.31 | 2.28 ± 0.36 | 2.16 ± 0.31 |
| Hexanal | 65.1 ± 5.01 a | 56.8 ± 4.15 b | 54.0 ± 4.18 b | 56.9 ± 4.49 b |
| Heptanal | 3.64 ± 0.47 | 3.22 ± 0.41 | 3.86 ± 0.58 | 2.93 ± 0.42 |
| Octanal | 3.20 ± 0.43 a | 4.70 ± 0.52 a | 6.47 ± 0.72 b | 5.83 ± 0.62 b |
| Nonanal | 5.95 ± 0.63 | 6.64 ± 0.66 | 6.61 ± 0.60 | 6.74 ± 0.63 |
| 2-heptenal | 0.77 ± 0.09 | 0.90 ± 0.15 | 0.62 ± 0.18 | 0.77 ± 0.11 |
| 2-octenal | 0.61 ± 0.8 | 0.69 ± 0.07 | 0.63 ± 0.07 | 0.88 ± 0.11 |
| 1-pentanol | 0.54 ± 0.08 a | 0.86 ± 0.10 b | 0.90 ± 0.12 b | 0.96 ± 0.12 b |
| 1-heptanol | 0.26 ± 0.05 a | 1.56 ± 0.19 b | 1.75 ± 0.21 b | 1.62 ± 0.14 b |
| 1-octanol | 0.49 ± 0.06 a | 1.13 ± 0.16 b | 1.59 ± 0.21 c | 1.53 ± 0.18 c |
| 1-octen-3-ol | 9.24 ± 1.61 | 12.2 ± 1.95 | 12.6 ± 1.62 | 11.7 ± 1.38 |
| 2-octen-1-ol. (Z)- | 0.69 ± 0.11 | 0.71 ± 0.09 | 0.67 ± 0.08 | 0.53 ± 0.07 |
| 2,5-octanedione | 7.45 ± 0.77 | 8.14 ± 0.92 | 7.43 ± 0.58 | 7.03 ± 0.95 |
| Benzaldehyde | 0.39 ± 0.05 | 0.48 ± 0.07 | 0.56 ± 0.07 | 0.49 ± 0.08 |
| Biogenic Amine † | CG | EG1 | EG2 | EG3 |
| Putrescine | 0.27 ± 0.04 a | 0.20 ± 0.03 b | 0.17 ± 0.02 b | 0.15 ± 0.01 b |
| Cadaverine | 0.24 ± 0.04 | nd | nd | nd |
| Tyramine | 0.18 ± 0.03 | 0.13 ± 0.05 | 0.20 ± 0.06 | 0.13 ± 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bennato, F.; Di Luca, A.; Martino, C.; Ianni, A.; Marone, E.; Grotta, L.; Ramazzotti, S.; Cichelli, A.; Martino, G. Influence of Grape Pomace Intake on Nutritional Value, Lipid Oxidation and Volatile Profile of Poultry Meat. Foods 2020, 9, 508. https://doi.org/10.3390/foods9040508
Bennato F, Di Luca A, Martino C, Ianni A, Marone E, Grotta L, Ramazzotti S, Cichelli A, Martino G. Influence of Grape Pomace Intake on Nutritional Value, Lipid Oxidation and Volatile Profile of Poultry Meat. Foods. 2020; 9(4):508. https://doi.org/10.3390/foods9040508
Chicago/Turabian StyleBennato, Francesca, Alessio Di Luca, Camillo Martino, Andrea Ianni, Elettra Marone, Lisa Grotta, Solange Ramazzotti, Angelo Cichelli, and Giuseppe Martino. 2020. "Influence of Grape Pomace Intake on Nutritional Value, Lipid Oxidation and Volatile Profile of Poultry Meat" Foods 9, no. 4: 508. https://doi.org/10.3390/foods9040508
APA StyleBennato, F., Di Luca, A., Martino, C., Ianni, A., Marone, E., Grotta, L., Ramazzotti, S., Cichelli, A., & Martino, G. (2020). Influence of Grape Pomace Intake on Nutritional Value, Lipid Oxidation and Volatile Profile of Poultry Meat. Foods, 9(4), 508. https://doi.org/10.3390/foods9040508





