Volatile Organic Compounds Profiles to Determine Authenticity of Sweet Orange Juice Using Head Space Gas Chromatography Coupled with Multivariate Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Citrus Materials and Sample Preparation
2.2. Standards and Agents
2.3. Analysis of VOCs by HS-SPME–GC–MS
2.4. Determination of Detection Limit and Quantitative Limit
2.5. Identification of Volatile Organic Compounds
2.6. Statistical Analysis
3. Results and Discussion
3.1. VOCs Profiles of Different Varieties of Sweet Orange Juice and Mandarin Juice
3.1.1. Distribution Characteristics of VOCs in Sweet Orange and Mandarin Juices
3.1.2. Characteristic VOCs in Sweet Orange Juice and Mandarin Juices
3.1.3. Common VOCs in Both Sweet Orange Juice and Mandarin Juices
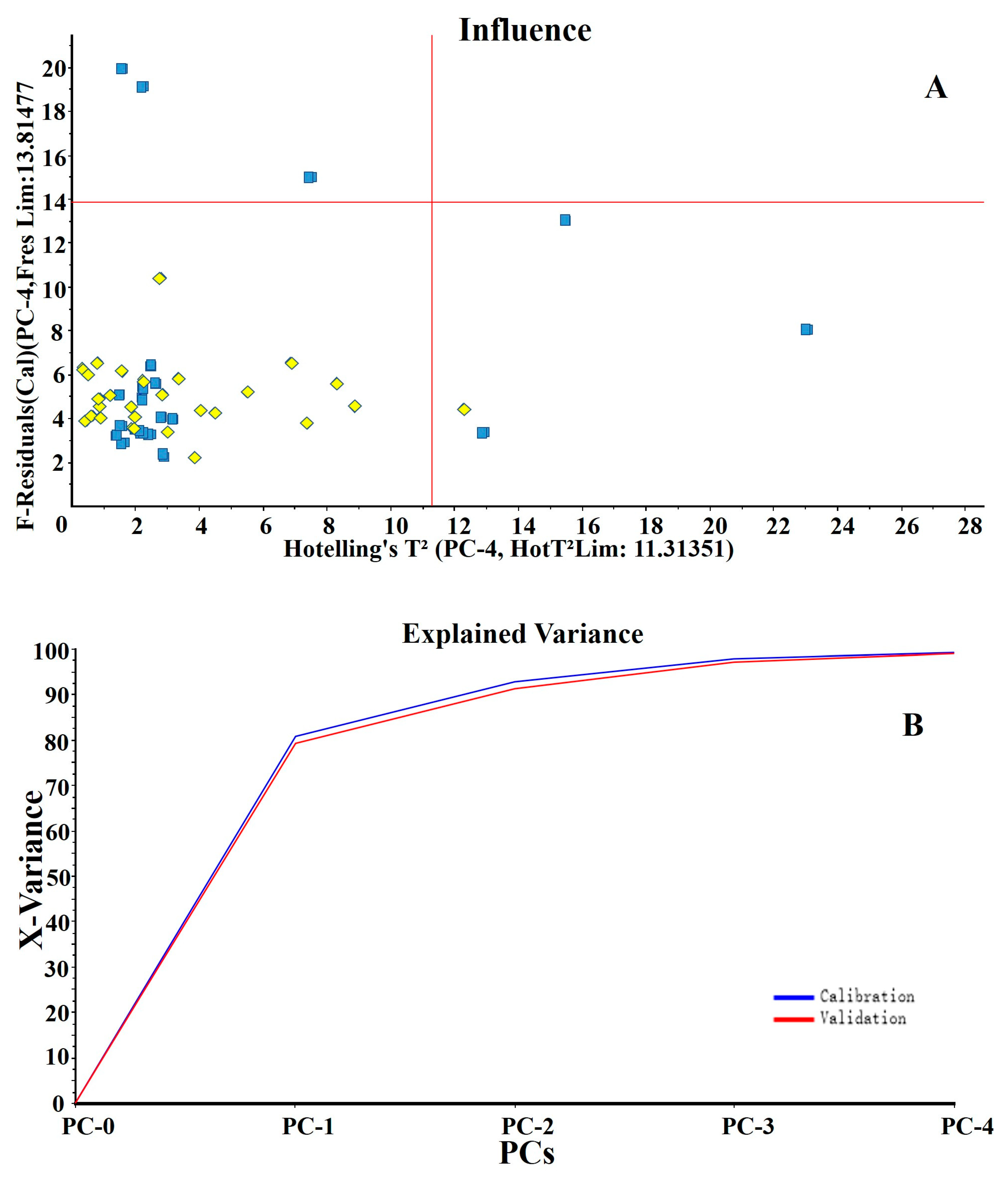
3.2. Principal Component Analysis of VOCs in Samples
3.3. Identification of Sweet Orange Juice Mixed With Mandarin Juice
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vervoort, L.; Grauwet, T.; Kebede, B.; Van Der Plancken, I.; Timmermans, R.; Hendrickx, M.; Van Loey, A. Headspace fingerprinting as an untargeted approach to compare novel and traditional processing technologies: A case-study on orange juice pasteurisation. Food Chem. 2012, 134, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Sellami, I.; Mall, V.; Schieberle, P. Changes in the Key Odorants and Aroma Profiles of Hamlin and Valencia Orange Juices Not from Concentrate (NFC) during Chilled Storage. J. Agric. Food Chem. 2018, 66, 7428–7440. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/statistics/zh/ (accessed on 3 February 2020).
- Cho, H.E.; Ahn, S.Y.; Kim, S.C.; Woo, M.H.; Hong, J.; Moon, D.C. Determination of flavonoid glycosides, polymethoxyflavones, and coumarins in herbal drugs of citrus and poncirus fruits by high performance liquid chromatography-electrospray ionization/tandem mass spectrometry. Anal. Lett. 2014, 47, 1299–1323. [Google Scholar] [CrossRef]
- Wang, Z.; Jablonski, J.E. Targeted and non-targeted detection of lemon juice adulteration by LC-MS and chemometrics. Food Addit. Contam. Part A 2016, 33, 560–573. [Google Scholar] [CrossRef]
- Nikolaou, C.; Karabagias, I.K.; Gatzias, I.; Kontakos, S.; Badeka, A.; Kontominas, M.G. Differentiation of Fresh Greek Orange Juice of the Merlin Cultivar According to Geographical Origin Based on the Combination of Organic Acid and Sugar Content as well as Physicochemical Parameters Using Chemometrics. Food Anal. Methods 2017, 10, 2217–2228. [Google Scholar] [CrossRef]
- Rummel, S.; Hoelzl, S.; Horn, P.; Rossmann, A.; Schlicht, C. The combination of stable isotope abundance ratios of H, C, N and S with 87Sr/86Sr for geographical origin assignment of orange juices. Food Chem. 2010, 118, 890–900. [Google Scholar] [CrossRef]
- García, B.A.; Garmón-Lobato, S.; Sánchez-Ilárduya, M.B.; Berrueta, L.A.; Gallo, B.; Vicente, F.; Salces, R.M.A. Polyphenolic contents in Citrus fruit juices: Authenticity assessment. Eur. Food Res. Technol. 2014, 238, 803–818. [Google Scholar] [CrossRef]
- Jandric, Z.; Islam, M.; Singh, D.; Cannava, A. Authentication of Indian citrus fruit/fruit juices by untargeted and targeted metabolomics. Food Control. 2017, 72, 181–188. [Google Scholar] [CrossRef]
- Bocharova, O.; Reshta, S.; Eshtokin, V. Evaluation of orange juice authenticity in respect of added food flavors using dilution index. J. Food Process. Preserv. 2017, 41. [Google Scholar] [CrossRef]
- Cuevas, F.J.; Pereira-Caro, G.; Moreno, M.J.R.; Muñoz-Redondo, J.M.; Ruiz-Moreno, M.J. Assessment of premium organic orange juices authenticity using HPLC-HR-MS and HS-SPME-GC-MS combining data fusion and chemometrics. Food Control. 2017, 82, 203–211. [Google Scholar] [CrossRef]
- Shen, F.; Wu, Q.; Su, A.; Tang, P.; Shao, X.; Liu, B. Detection of adulteration in freshly squeezed orange juice by electronic nose and infrared spectroscopy. Czech J. Food Sci. 2016, 34, 224–232. [Google Scholar] [CrossRef]
- Le Gall, G.; Puaud, M.; Colquhoun, I.J. Discrimination between orange juice and pulp wash by (1)H Nuclear Magnetic Resonance spectroscopy: Identification of marker compounds. J. Agric. Food Chem. 2001, 49, 580–588. [Google Scholar] [CrossRef] [PubMed]
- Vigneau, E.; Thomas, F. Model calibration and feature selection for orange juice authentication by 1H NMR spectroscopy. Chemom. Intell. Lab. Syst. 2012, 117, 22–30. [Google Scholar] [CrossRef]
- Zengin, A.; Badak, M.U.; Bilici, M.; Suludere, Z.; Aktas, N. Preparation of molecularly imprinted PDMS elastomer for selective detection of folic acid in orange juice. Appl. Surf. Sci. 2019, 471, 168–175. [Google Scholar] [CrossRef]
- Wang, H.; Sun, H. Potential use of electronic tongue coupled with chemometrics analysis for early detection of the spoilage of Zygosaccharomyces rouxii in apple juice. Food Chem. 2019, 290, 152–158. [Google Scholar] [CrossRef]
- Daikuzono, C.M.; Delaney, C.; Morrin, A.; Diamond, D.; Florea, L.; Oliveira, J.O.N.; Florea, L., Jr. Paper based electronic tongue—A low-cost solution for the distinction of sugar type and apple juice brand. Analyst 2019, 144, 2827–2832. [Google Scholar] [CrossRef]
- Ellis, D.I.; Ellis, J.; Muhamadali, H.; Xu, Y.; Horn, A.B.; Goodacre, R. Rapid, high-throughput, and quantitative determination of orange juice adulteration by Fourier-transform infrared spectroscopy. Anal. Methods 2016, 8, 5581–5586. [Google Scholar] [CrossRef]
- Rinaldi, M.; Gindro, R.; Barbeni, M.; Allegrone, G. Pattern recognition and genetic algorithms for discrimination of orange juices and reduction of significant components from headspace solid-phase microextraction. Phytochem. Anal. 2009, 20, 402–407. [Google Scholar] [CrossRef]
- Aldeguer, M.; López-Andreo, M.; Gabaldón, J.A.; Puyet, A. Detection of mandarin in orange juice by single-nucleotide polymorphism qPCR assay. Food Chem. 2014, 145, 1086–1091. [Google Scholar] [CrossRef]
- Mabood, F.; Hussain, J.; Jabeen, F.; Abbas, G.; Allaham, B.; Albroumi, M.; Alghawi, S.; Alameri, S.; Gilani, S.A.; Al-Harrasi, A.; et al. Applications of FT-NIRS combined with PLS multivariate methods for the detection & quantification of saccharin adulteration in commercial fruit juices. Food Addit. Contam. Part A 2018, 35, 1052–1060. [Google Scholar] [CrossRef]
- Różańska, A.K.; Dymerski, T.; Namieśnik, J. Novel analytical method for detection of orange juice adulteration based on ultra-fast gas chromatography. Monatshefte für Chemie Chemical Monthly 2018, 149, 1615–1621. [Google Scholar] [CrossRef]
- Bat, K.B.; Eler, K.; Mazej, D.; Vodopivec, B.M.; Mulič, I.; Kump, P.; Ogrinc, N. Isotopic and elemental characterisation of Slovenian apple juice according to geographical origin: Preliminary results. Food Chem. 2016, 203, 86–94. [Google Scholar] [CrossRef]
- Włodarska, K.; Khmelinskii, I.; Sikorska, E. Authentication of apple juice categories based on multivariate analysis of the synchronous fluorescence spectra. Food Control 2018, 86, 42–49. [Google Scholar] [CrossRef]
- Dugo, P.; Piperno, A.; Romeo, R.; Cambria, M.; Russo, M.; Carnovale, C.; Mondello, L. Determination of Oxygen Heterocyclic Components in Citrus Products by HPLC with UV Detection. J. Agric. Food Chem. 2009, 57, 6543–6551. [Google Scholar] [CrossRef]
- Bontempo, L.; Caruso, R.; Fiorillo, M.; Gambino, G.L.; Perini, M.; Simoni, M.; Traulo, P.; Wehrens, R.; Gagliano, G.; Camin, F. Stable isotope ratios of H, C, N and O in Italian citrus juices. J. Mass Spectrom. 2014, 49, 785–791. [Google Scholar] [CrossRef]
- Bononi, M.; Quaglia, G.; Tateo, F. Preliminary LC-IRMS Characterization of Italian Pure Lemon Juices and Evaluation of Commercial Juices Distributed in the Italian Market. Food Anal. Methods 2016, 9, 2824–2831. [Google Scholar] [CrossRef]
- Feng, S.; Suh, J.H.; Gmitter, F.G.; Wang, Y. Differentiation between Flavors of Sweet Orange (Citrus sinensis) and Mandarin (Citrus reticulata). J. Agric. Food Chem. 2017, 66, 203–211. [Google Scholar] [CrossRef]
- Peleg, H.; Naim, M.; Zehavi, U.; Rouseff, R.L.; Nagy, S. Pathways of 4-vinylguaiacol formation from ferulic acid in model solutions of orange juice. J. Agric. Food Chem. 1992, 40, 764–767. [Google Scholar] [CrossRef]
- Shui, M.; Feng, T.; Tong, Y.; Zhuang, H.; Lo, C.; Sun, H.; Chen, L.; Song, S. Characterization of Key Aroma Compounds and Construction of Flavor Base Module of Chinese Sweet Oranges. Molecule 2019, 24, 2384. [Google Scholar] [CrossRef]
- Geneva, N. Flavor Nnet and Human Odor Space. Available online: http://www.flavornet.org/flavornet.html (accessed on 3 February 2020).
- Zhang, M.; Li, L.; Wu, Z.; Wang, Y.; Zang, Y.; Liu, G. Volatile Composition in Two Pummelo Cultivars (Citrus grandis L. Osbeck) from Different Cultivation Regions in China. Molcules 2017, 22, 716. [Google Scholar] [CrossRef]
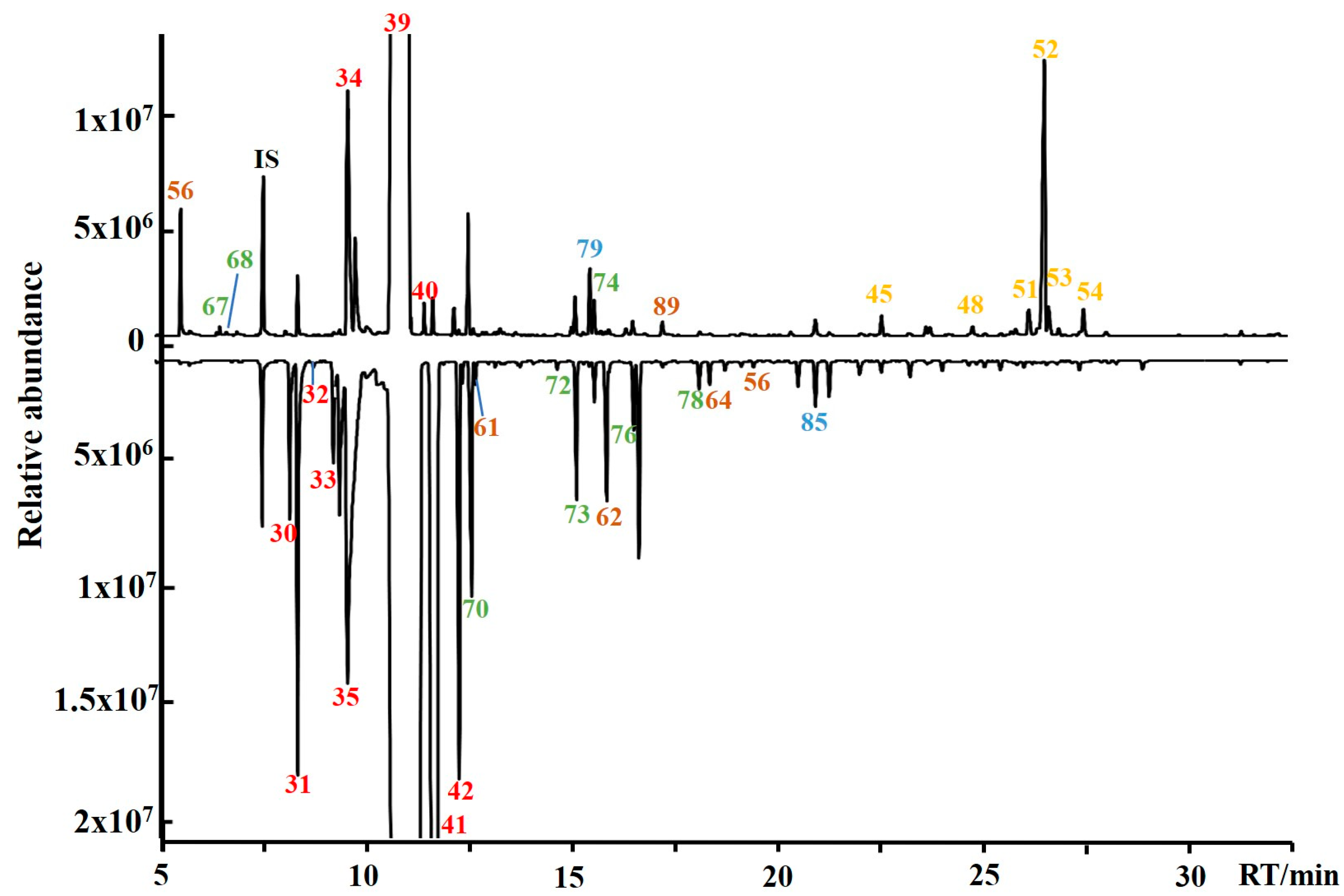


| Juice Type | Compound Category | No. | Calculated RI | Reference RI | Compound Name | CAS Number | Identification |
|---|---|---|---|---|---|---|---|
| Orange juice | Sesquiterpenes | 1 | 1431 | 1432 | cis-β-Copaene | 18252-44-3 | MS, RI |
| 2 | 1449 | 1453 | α-Guaiene | 3691-12-1 | MS, RI | ||
| 3 | 1476 | 1477 | γ-Gurjunene | 22567-17-5 | MS, RI | ||
| 4 | 1499 | 1499 | α-Muurolene | 10208-80-7 | MS, RI | ||
| Aldehydes | 5 | 1059 | 1060 | 2-Octenal | 2363-89-5 | MS, RI | |
| 6 | 1159 | 1160 | trans-2-Nonenal | 18829-56-6 | MS, RI | ||
| 7 | 1408 | 1408 | Decanyl acetate | 112-17-4 | MS, RI | ||
| Alcohols | 8 | 1138 | 1138 | cis-2,8-p-Menthadien-1-ol | 3886-78-0 | MS, RI | |
| 9 | 1150 | 1144 | β-Terpineol | 138-87-4 | MS, RI | ||
| 10 | 1248 | 1252 | β-Geraniol | 106-24-1 | MS, RI | ||
| 11 | 1292 | 1294 | p-Mentha-1(7),8(10)-dien-9-ol | 29548-13-8 | MS, RI | ||
| Esters | 12 | 998 | 998 | Caproic acid ethyl ester | 123-66-0 | MS, RI | |
| 13 | 1097 | 1100 | Enanthylic ether | 106-30-9 | MS, RI | ||
| 14 | 1127 | 1126 | Ethyl 3-hydroxyhexanoate | 2305-25-1 | MS, RI | ||
| 15 | 1190 | 1185 | Hexyl butanoate | 2639-63-6 | MS, RI | ||
| 16 | 1308 | 1312 | Nonanol acetate | 143-13-5 | MS, RI | ||
| 17 | 1408 | 1408 | Decanyl acetate | 112-17-4 | MS, RI | ||
| Ketones | 18 | 682 | 680 | Ethyl vinyl ketone | 1629-58-9 | MS, RI | |
| 19 | 1421 | 1422 | α-Ionone | 127-41-3 | MS, RI | ||
| 20 | 1807 | 1814 | Nootkanone | 4674-50-4 | MS, RI | ||
| Mandarin juice | Sesquiterpenes | 21 | 1336 | 1340 | δ-Elemene | 20307-84-0 | MS, RI |
| 22 | 1430 | 1425 | γ-Elemene | 29873-99-2 | MS, RI | ||
| 23 | 1537 | 1538 | α-Cadinene | 24406-05-1 | MS, RI | ||
| 24 | 1560 | 1562 | Germacrene B | 15423-57-1 | MS, RI | ||
| Aldehydes | 25 | 1197 | 1197 | trans-4-Decen-1-al | 65405-70-1 | MS, RI | |
| Alcohols | 26 | 1153 | 1150 | trans-Isoperitenol | 89-79-2 | MS, RI | |
| 27 | 1289 | 1290 | Thyme camphor | 89-83-8 | MS, RI | ||
| Ketones | 28 | 984 | 982 | Methylheptenone | 110-93-0 | MS, RI | |
| 29 | 1256 | 1253 | 3-Carvomenthenone | 89-81-6 | MS, RI |
| Compound Category | No. | Calculated RI | Reference RI | Name | CAS Number | Identification |
|---|---|---|---|---|---|---|
| Monoterpenes | 30 | 927 | 928 | α-Thujene | 2867-05-2 | MS, RI |
| 31 | 936 | 936 | α-Pinene | 80-56-8 | MS, RI | |
| 32 | 953 | 953 | D-Camphene | 79-92-5 | MS, RI | |
| 33 | 975 | 976 | Sabenene | 3387-41-5 | MS, RI | |
| 34 | 981 | 981 | β-Pinene | 127-91-3 | MS, RI | |
| 35 | 990 | 991 | β-Myrcene | 123-35-3 | MS, RI | |
| 36 | 1009 | 1008 | α-Phellandrene | 99-83-2 | MS, RI | |
| 37 | 1011 | 1011 | δ-3-Carene | 13466-78-9 | MS, RI | |
| 38 | 1018 | 1018 | α-Terpinene | 99-86-5 | MS, RI | |
| 39 | 1034 | 1033 | d-Limonene | 138-86-3 | MS, RI | |
| 40 | 1045 | 1043 | β-trans-Ocimene | 3779-61-1 | MS, RI | |
| 41 | 1060 | 1060 | γ-Terpinene | 99-85-4 | MS, RI | |
| 42 | 1090 | 1090 | α-Terpinolene | 586-62-9 | MS, RI | |
| 43 | 1139 | 1134 | allo-Ocimene | 673-84-7 | MS, RI | |
| 44 | 1348 | 1345 | α-Cubebene | 17699-14-8 | MS, RI | |
| Sesquiterpenes | 45 | 1390 | 1390 | β-Elemen | 515-13-9 | MS, RI |
| 46 | 1440 | 1441 | (+)-Aromadendrene | 489-39-4 | MS, RI | |
| 47 | 1451 | 1457 | β-Farnesene | 18794-84-8 | MS, RI | |
| 48 | 1457 | 1455 | α-Caryophyllene | 6753-98-6 | MS, RI | |
| 49 | 1475 | 1475 | γ-Muurolene | 30021-74-0 | MS, RI | |
| 50 | 1482 | 1487 | Germacrene D | 23986-74-5 | MS, RI | |
| 51 | 1490 | 1490 | β-Selinene | 17066-67-0 | MS, RI | |
| 52 | 1493 | 1490 | Valencene | 4630-07-3 | MS, RI | |
| 53 | 1498 | 1498 | α-Selinene | 473-13-2 | MS, RI | |
| 54 | 1496 | 1497 | Viridiflorene | 21747-46-6 | MS, RI | |
| 55 | 1518 | 1519 | (+)-δ-Cadinene | 483-76-1 | MS, RI | |
| Aldehydes | 56 | 801 | 801 | Hexanal | 66-25-1 | MS, RI |
| 57 | 853 | 854 | trans-2-Hexenal | 6728-26-3 | MS, RI | |
| 58 | 902 | 903 | 1-Heptaldehyde | 111-71-7 | MS, RI | |
| 59 | 957 | 957 | trans-2-Heptenal | 18829-55-5 | MS, RI | |
| 60 | 1004 | 1002 | Octanal | 124-13-0 | MS, RI | |
| 61 | 1006 | 1004 | Nonanal | 124-19-6 | MS, RI | |
| 62 | 1206 | 1203 | Decanal | 112-31-2 | MS, RI | |
| 63 | 1268 | 1270 | α-Citral | 141-27-5 | MS, RI | |
| 64 | 1278 | 1279 | Perilla aldehyde | 2111-75-3 | MS, RI | |
| 65 | 1307 | 1306 | Undecanal | 112-44-7 | MS, RI | |
| 66 | 1409 | 1409 | Dodecanal | 112-54-9 | MS, RI | |
| Alcohols | 67 | 854 | 855 | Leaf alcohol | 928-96-1 | MS, RI |
| 68 | 867 | 865 | 1-Hexanol | 111-27-3 | MS, RI | |
| 69 | 1071 | 1072 | 1-Octanol | 111-87-5 | MS, RI | |
| 70 | 1101 | 1100 | Linalool | 78-70-6 | MS, RI | |
| 71 | 1124 | 1122 | cis-p-Menth-2,8-diene-1-ol | 7212-40-0 | MS, RI | |
| 72 | 1170 | 1171 | 1-Nonanol | 143-08-8 | MS, RI | |
| 73 | 1184 | 1182 | 4-Terpinenol | 562-74-3 | MS, RI | |
| 74 | 1198 | 1195 | α-Terpineol | 98-55-5 | MS, RI | |
| 75 | 1220 | 1217 | trans-Carveol | 1197-07-5 | MS, RI | |
| 76 | 1225 | 1224 | β-Citronellol | 106-22-9 | MS, RI | |
| 77 | 1233 | 1229 | cis-Carveol | 1197-06-4 | MS, RI | |
| 78 | 1270 | 1272 | 1-Decanol | 112-30-1 | MS, RI | |
| Esters | 79 | 1195 | 1193 | Caprylic acid ethyl ester | 106-32-1 | MS, RI |
| 80 | 1209 | 1208 | Acetic acid octanyl ester | 112-14-1 | MS, RI | |
| 81 | 1285 | 1285 | Bornyl acetic ester | 76-49-3 | MS, RI | |
| 82 | 1331 | 1337 | trans-Carvyl acetate | 1134-95-8 | MS, RI | |
| 83 | 1346 | 1350 | Terpinyl acetate | 80-26-2 | MS, RI | |
| 84 | 1347 | 1354 | Cephrol acetate | 150-84-5 | MS, RI | |
| 85 | 1356 | 1362 | Acetic acid neryl ester | 141-12-8 | MS, RI | |
| 86 | 1376 | 1382 | Geranyl acetate | 105-87-3 | MS, RI | |
| 87 | 1393 | 1394 | Capric acid ethyl ester | 110-38-3 | MS, RI | |
| Ketones | 88 | 1201 | 1201 | Dihydrocarvone | 5948-04-9 | MS, RI |
| 89 | 1245 | 1249 | L-Carvone | 6485-40-1 | MS, RI | |
| 90 | 1445 | 1448 | Geranylacetone | 3796-70-1 | MS, RI |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Q.; Li, G.; Ou-Yang, Z.; Yi, X.; Huang, L.; Wang, H. Volatile Organic Compounds Profiles to Determine Authenticity of Sweet Orange Juice Using Head Space Gas Chromatography Coupled with Multivariate Analysis. Foods 2020, 9, 505. https://doi.org/10.3390/foods9040505
Zhou Q, Li G, Ou-Yang Z, Yi X, Huang L, Wang H. Volatile Organic Compounds Profiles to Determine Authenticity of Sweet Orange Juice Using Head Space Gas Chromatography Coupled with Multivariate Analysis. Foods. 2020; 9(4):505. https://doi.org/10.3390/foods9040505
Chicago/Turabian StyleZhou, Qi, Guijie Li, Zhu Ou-Yang, Xin Yi, Linhua Huang, and Hua Wang. 2020. "Volatile Organic Compounds Profiles to Determine Authenticity of Sweet Orange Juice Using Head Space Gas Chromatography Coupled with Multivariate Analysis" Foods 9, no. 4: 505. https://doi.org/10.3390/foods9040505
APA StyleZhou, Q., Li, G., Ou-Yang, Z., Yi, X., Huang, L., & Wang, H. (2020). Volatile Organic Compounds Profiles to Determine Authenticity of Sweet Orange Juice Using Head Space Gas Chromatography Coupled with Multivariate Analysis. Foods, 9(4), 505. https://doi.org/10.3390/foods9040505






