Pinot Blanc: Impact of the Winemaking Variables on the Evolution of the Phenolic, Volatile and Sensory Profiles
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling of Grapes
2.2. Winemaking Procedures and Sampling
2.2.1. Winemaking
2.2.2. Sampling during the Winemaking
2.3. Chemical Characterization
2.3.1. Materials
2.3.2. Determination of Enological Parameters
2.3.3. HPLC-DAD/FLD Profile
2.3.4. Identification of Phenolic Compounds with HPLC-MS
2.3.5. Profile of Volatile Compounds with HS-SPME-GC/MS
2.4. Sensory Analysis
2.5. Statistical Analysis
3. Results
3.1. Oenological Parameters
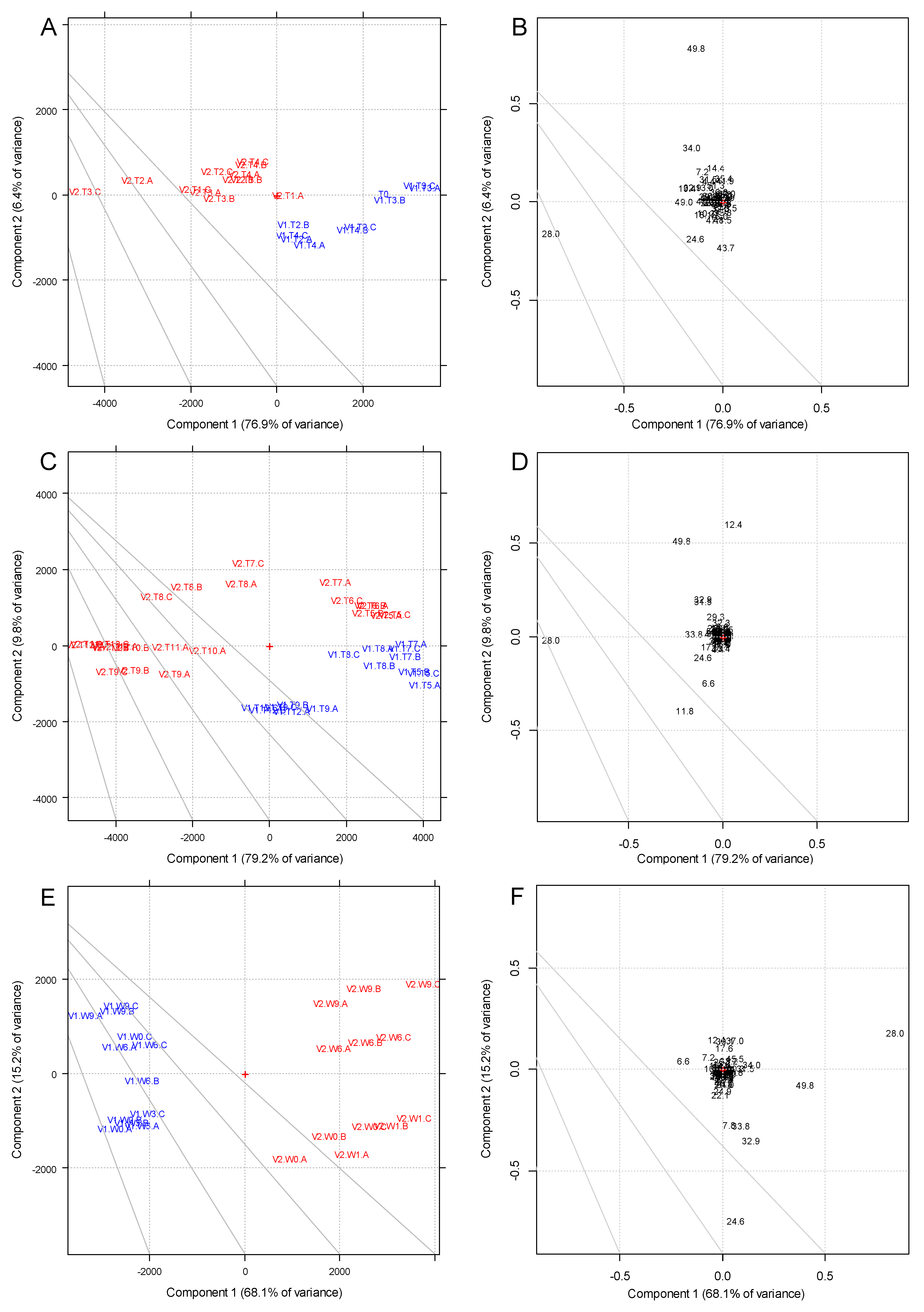
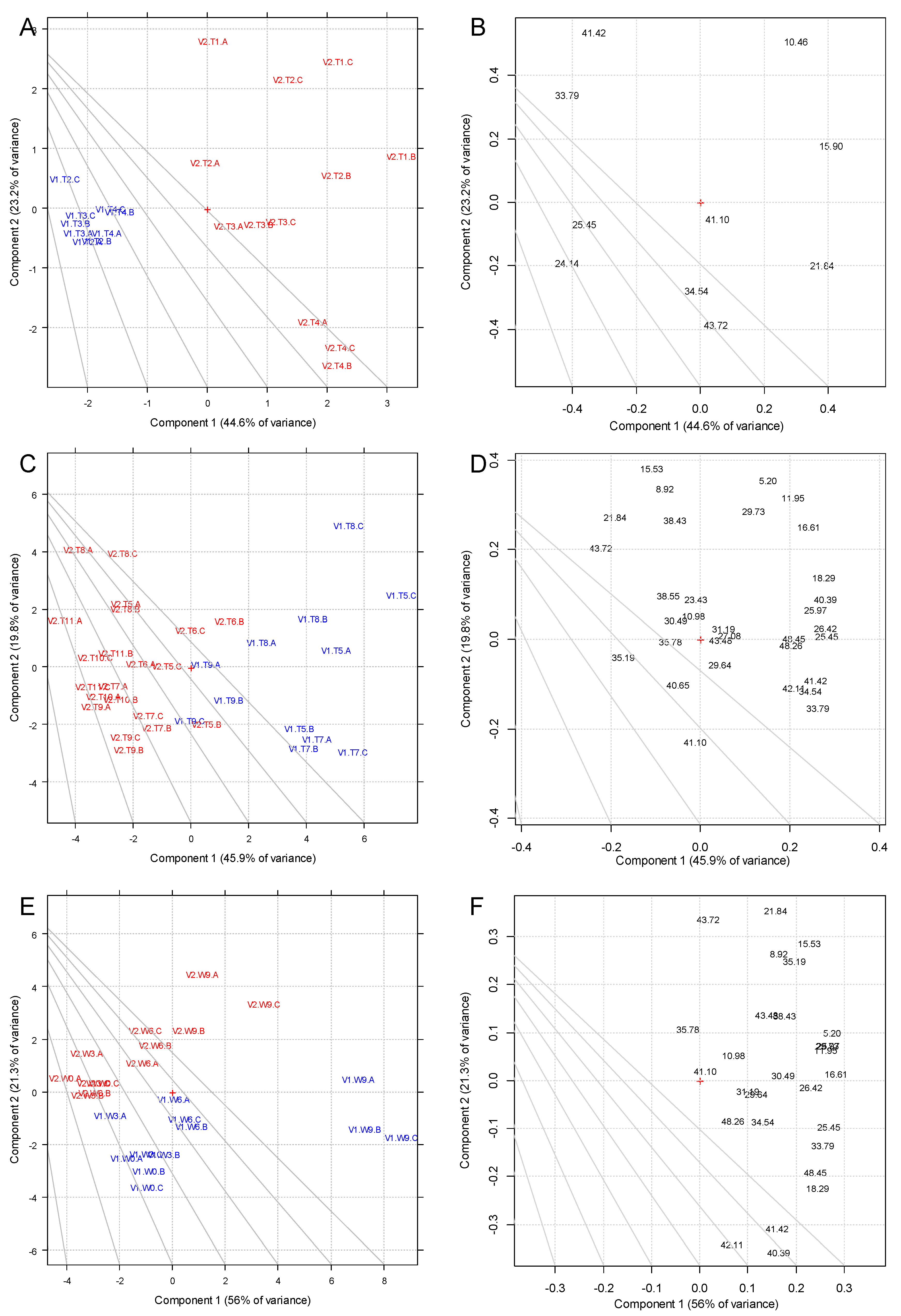
3.2. Phenolic Compounds
3.3. Volatile Compounds
3.4. Sensory Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent
Acknowledgments
Conflicts of Interest
References
- Robinson, J.; Harding, J.; Vouillamoz, J. Wine Grapes: A Complete Guide to 1368 Vine Varieties, Including Their Origins and Flavours; Penguin Books Ltd: London, UK, 2013; ISBN 9781846144462. [Google Scholar]
- Vezzulli, S.; Leonardelli, L.; Malossini, U.; Stefanini, M.; Velasco, R.; Moser, C. Pinot blanc and Pinot gris arose as independent somatic mutations of Pinot noir. J. Exp. Bot. 2012, 63, 6359–6369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Huglin, P. Nouveau mode d’évaluation des possibilites héliothermiques d’un milieu viticole in Proceedings of the Symposium International sur l’ecologie de la Vigne. Ministère de l’Agriculture et de l’Industrie Alimentaire, Contança. In Climate Change and Environmental Concerns: Breakthroughs in Research and Practice: Breakthroughs in Research and Practice; IGI Global: Hershey, PA, USA, 2018; pp. 89–98. [Google Scholar]
- Balottia, A.; Tscholl, S.; Vigl, L.E. Linking viticultural climatic indices to grape phenology in the South Tyrolean Alps. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2018; Volume 50, p. 01031. [Google Scholar]
- Vini Alto Adige. Pinot Bianco. 2019. Available online: https://www.vinialtoadige.com/it/vitigni/pinot-bianco/22-12228.html (accessed on 31 December 2019).
- Ministero Delle Politiche Agricole 2010b. Decreto 6/8/2010: Modifica del Disciplinare di Produzione dei Vini a Denominazione di Origine Controllata «Alto Adige» o «dell’Alto Adige» in Lingua Tedesca «Sudtirol» o «Sudtiroler»; Gazzetta Ufficiale Della Repubblica Italiana: Roma, Italy, 2010; p. 197. [Google Scholar]
- Rapp, A. Volatile flavour of wine: Correlation between instrumental analysis and sensory perception. Food/Nahrung 1998, 42, 351–363. [Google Scholar] [CrossRef]
- Puckette, M.; Hammack, J. Wine Folly: The Essential Guide to Wine; Penguin Random House USA: New York, NY, USA, 2015. [Google Scholar]
- Pedri, U.; Pertoll, G. Influence of different locations on grape and wine quality with the grapevine variety ‘Pinot blanc’. Mitteilungen Klosterneuburg, Rebe und Wein. Obstbau und Früchteverwert. 2013, 63, 173–186. [Google Scholar]
- Philipp, C.; Phillip, E.; Brandes, W.; Regner, F.; Patzl-Fischerleitner, E.; Eder, R. Charakterisierung der Birnenaromatik von Österreichischem Weißburgunder (Pinot blanc) hinsichtlich Typizität und Qualität. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2017; Volume 9, p. 02033. [Google Scholar]
- Philipp, C.; Eder, P.; Brandes, W.; Patzl-Fischerleitner, E.; Eder, R. The Pear Aroma in the Austrian Pinot Blanc Wine Variety: Evaluation by Means of Sensorial-Analytical-Typograms with regard to Vintage, Wine Styles, and Origin of Wines. J. Food Qual. 2018. [Google Scholar] [CrossRef]
- Joslyn, M.A.; Dittmar, H.F.K. The proanthocyanidins of Pinot blanc grapes. Am. J. Enol. Vitic. 1967, 18, 1–10. [Google Scholar]
- Vrhovsek, U.; Wendelin, S. The effect of fermentation, storage and fining on the content of hydroxycinnamoyltartaric acids and on browning of Pinot blanc wines. Vitic. Enol. Sci. 1998, 53, 87–94. [Google Scholar]
- Tominaga, T.; Baltenweck-Guyot, R.; Des Gachons, C.P.; Dubourdieu, D. Contribution of volatile thiols to the aromas of white wines made from several Vitis vinifera grape varieties. Am. J. Enol. Vitic. 2000, 51, 178–181. [Google Scholar]
- Lenk, S.; Buschmann, C.; Pfündel, E.E. In vivo assessing flavonols in white grape berries (Vitis vinifera L. cv. Pinot Blanc) of different degrees of ripeness using chlorophyll fluorescence imaging. Funct. Plant Biol. 2007, 34, 1092–1104. [Google Scholar] [CrossRef]
- Longo, E.; Rossetti, F.; Jouin, A.; Teissedre, P.L.; Jourdes, M.; Boselli, E. Distribution of crown hexameric procyanidin and its tetrameric and pentameric congeners in red and white wines. Food Chem. 2019, 299, 125125. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.C.; de Revel, G. Development, validation and application of a specific method for the quantitative determination of wine esters by headspace-solid-phase microextraction-gas chromatography–mass spectrometry. Food Chem. 2010, 121, 1236–1245. [Google Scholar] [CrossRef]
- IOFI Working Group on Methods of Analysis. Guidelines for solid-phase micro-extraction (SPME) of volatile flavour compounds for gas-chromatographic analysis, from the Working Group on Methods of Analysis of the International Organization of the Flavor Industry (IOFI). Flavour Fragr. J. 2010, 25, 404–406. [Google Scholar] [CrossRef]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Leardi, R.; Melzi, C.; Polotti, G. CAT—Chemometric Agile Tool. 2019. Available online: http://gruppochemiometria.it/index.php/software (accessed on 1 February 2019).
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of enology. In The Microbiology of Wine and Vinifications; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 1. [Google Scholar]
- Landrault, N.; Larronde, F.; Delaunay, J.C.; Castagnino, C.; Vercauteren, J.; Merillon, J.M.; Teissedre, P.L. Levels of stilbene oligomers and astilbin in French varietal wines and in grapes during noble rot development. J. Agric. Food Chem. 2002, 50, 2046–2052. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Zaya, J.; Trouslade, E.; Salgues, M. Caftaric acid in grapes and conversion to a reaction product during processing. Vitis 1984, 23, 113–120. [Google Scholar]
- Di Lecce, G.; Boselli, E.; D’Ignazi, G.; Frega, N.G. Evolution of phenolics and glutathione in Verdicchio wine obtained with maceration under reductive conditions. LWT-Food Sci. Tech. 2013, 53, 54–60. [Google Scholar] [CrossRef]
- Merkle, S.; Kleeberg, K.K.; Fritsche, J. Recent developments and applications of solid phase microextraction (SPME) in food and environmental analysis—A review. Chromatography 2015, 2, 293–381. [Google Scholar] [CrossRef]
- Setkova, L.; Risticevic, S.; Pawliszyn, J. Rapid headspace solid-phase microextraction-gas chromatographic–time-of-flight mass spectrometric method for qualitative profiling of ice wine volatile fraction: II: Classification of Canadian and Czech ice wines using statistical evaluation of the data. J. Chromatogr. A 2007, 1147, 224–240. [Google Scholar] [CrossRef]
- Pozo-Bayon, M.A.; Ruiz-Rodriguez, A.; Pernin, K.; Cayot, N. Influence of eggs on the aroma composition of a sponge cake and on the aroma release in model studies on flavored sponge cakes. J. Agric. Food Chem. 2007, 55, 1418–1426. [Google Scholar] [CrossRef]
- Vas, G.; Gal, L.; Harangi, J.; Dobo, A.; Vekey, K. Determination of volatile aroma compounds of Blaeufrankisch wines extracted by solid-phase microextraction. J. Chromatogr. Sci. 1998, 36, 505–510. [Google Scholar] [CrossRef][Green Version]
- Botelho, G.; Caldeira, I.; Mendes-Faia, A.; Clímaco, M.C. Evaluation of two quantitative gas chromatography-olfactometry methods for clonal red wines differentiation. Flavour Fragr. J. 2007, 22, 414–420. [Google Scholar] [CrossRef]
- Lopez, M.L.; Villatoro, C.; Fuentes, T.; Graell, J.; Lara, I.; Echeverria, G. Volatile compounds, quality parameters and consumer acceptance of ‘Pink Lady®’ apples stored in different conditions. Postharvest Biol. Technol. 2007, 43, 55–66. [Google Scholar] [CrossRef]
- Howard, K.L.; Mike, J.H.; Riesen, R. Validation of a Solid-Phase Microextraction Method for Headspace Analysis of Wine Aroma Components. Am. J. Enol. Vitic. 2005, 56, 37–45. [Google Scholar]
- De Llano, D.G.; Ramos, M.; Polo, C.; Sanz, J.; Martinez-Castro, I. Evolution of the volatile components of an artisanal blue cheese during ripening. J. Dairy Sci. 1990, 73, 1676–1683. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, J.-E.; Kim, H.-W.; Kim, S.-S.; Koh, K.-H. Development of Korean red wines using Vitis labrusca varieties: Instrumental and sensory characterization. Food Chem. 2006, 94, 385–393. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, Y.; Li, J.; Fan, W.; Jiang, W. Profile of volatile compounds in 11 brandies by headspace solid-phase microextraction followed by gas chromatography-mass spectrometry. J. Food. Sci. 2009, 74, c90–c99. [Google Scholar] [CrossRef]
- Gurbuz, O.; Rouseff, J.M.; Rouseff, R.L. Comparison of aroma volatiles in commercial Merlot and Cabernet Sauvignon wines using gas chromatography—Olfactometry and gas chromatography—Mass spectrometry. J. Agric. Food Chem. 2006, 54, 3990–3996. [Google Scholar] [CrossRef]
- Sumitani, H.; Suekane, S.; Nakatani, A.; Tatsuka, K. Changes in composition of volatile compounds in high pressure treated peach. J. Agric. Food Chem. 1994, 42, 785–790. [Google Scholar] [CrossRef]
- Tatsuka, K.; Suekane, S.; Sakai, Y.; Sumitani, H. Volatile constituents of kiwi fruit flowers: Simultaneous distillation and extraction versus headspace sampling. J. Agric. Food Chem. 1990, 38, 2176–2180. [Google Scholar] [CrossRef]
- Ledauphin, J.; Saint-Clair, J.-F.; Lablanquie, O.; Guichard, H.; Founier, N.; Guichard, E.; Barillier, D. Identification of trace volatile compounds in freshly distilled calvados and cognac using preparative separations coupled with gas chromatography-mass spectrometry. J. Agric. Food Chem. 2004, 52, 5124–5134. [Google Scholar] [CrossRef]
- Pena, R.M.; Barciela, J.; Herrero, C.; Garcia-Martin, S. Optimization of solid-phase microextraction methods for GC-MS determination of terpenes in wine. J. Sci. Food Agric. 2005, 85, 1227–1234. [Google Scholar] [CrossRef]
- Boti, J.B.; Muselli, A.; Tomi, F.; Koukoua, G.; N’Guessan, T.Y.; Costa, J.; Casanova, J. Combined analysis of Cymbopogon giganteus Chiov. leaf oil from Ivory Coast by GC/RI, GC/MS and 13C-NMR. C. R. Chim. 2006, 9, 164–168. [Google Scholar] [CrossRef]
- Flamini, G.; Tebano, M.; Cioni, P.L.; Bagci, Y.; Dural, H.; Ertugrul, K.; Uysal, T.; Savran, A. A multivariate statistical approach to Centaurea classification using essential oil composition data of some species from Turkey. Plant Syst. Evol. 2006, 261, 217–228. [Google Scholar] [CrossRef]
- Chua, J.; Lu, Y.; Liu, S. Biotransformation of soy whey into soy alcoholic beverage by four commercial strains of Saccharomyces cerevisiae. Int. J. Food Microbiol. 2017, 262, 14–22. [Google Scholar] [CrossRef]
- Wu, S.; Zorn, H.; Krings, U.; Berger, R.G. Characteristic Volatiles from Young and Aged Fruiting Bodies of Wild Polyporus sulfureus (Bull.:Fr.) Fr. J. Agric. Food Chem. 2005, 53, 4524–4528. [Google Scholar] [CrossRef]
- Kumazawa, K.; Sakai, N.; Amma, H.; Sakamoto, S.; Kodama, M.; Wada, Y.; Nishimura, O. Identification and formation of volatile components responsible for the characteristic aroma of Mat Rush (Igusa). Biosci. Biotechnol. Biochem. 2010, 74, 1231–1236. [Google Scholar] [CrossRef]
- Krammer, G.; Winterhalter, P.; Schwab, M.; Schreier, P. Glycosidically bound aroma compounds in the fruits of Prunus species: Apricot (P. armeniaca, L.) peach (P. persica, L.) yellow plum (P. domestica, L. ssp. Syriaca). J. Agric. Food Chem. 1991, 39, 778–781. [Google Scholar] [CrossRef]
- Carunchia Whetstine, M.E.; Croissant, A.E.; Drake, M.A. Characterization of Dried Whey Protein Concentrate and Isolate Flavor. J. Dairy Sci. 2005, 88, 3826–3839. [Google Scholar] [CrossRef]
- Cho, I.H.; Namgung, H.-J.; Choi, H.-K.; Kim, Y.-S. Volatiles and key odorants in the pileus and stipe of pine-mushroom (Tricholoma matsutake Sing.). Food Chem. 2008, 106, 71–76. [Google Scholar] [CrossRef]
- Quijano, C.E.; Linares, D.; Pino, J.A. Changes in volatile compounds of fermented cereza agria [Phyllanthus acidus (L.) Skeels] fruit. Flavour Fragr. J. 2007, 22, 392–394. [Google Scholar] [CrossRef]
- Cho, I.H.; Choi, H.-K.; Kim, Y.-S. Difference in the volatile composition of pine-mushrooms (Tricholoma matsutake Sing.) according to their grades. J. Agric. Food Chem. 2006, 54, 4820–4825. [Google Scholar] [CrossRef] [PubMed]
- Soria, A.C.; Sanz, J.; Martinez-Castro, I. SPME followed by GC-MS: A powerful technique for qualitative analysis of honey volatiles. Eur. Food Res. Technol. 2008, 1–12. [Google Scholar] [CrossRef]
- Boti, J.B.; Koukoua, G.; N’Guessan, T.Y.; Casanova, J. Chemical variability of Conyza sumatrensis and Microglossa pyrifolia from Côte d’Ivoire. Flavour Fragr. J. 2007, 22, 27–31. [Google Scholar] [CrossRef]
- Fernandez-Segovia, I.; Escriche, I.; Gomez-Sintes, M.; Fuentes, A.; Serra, J.A. Influence of different preservation treatments on the volatile fraction of desalted cod. Food Chem. 2006, 98, 473–482. [Google Scholar] [CrossRef]
- Feng, T.; Zhuang, H.; Ye, R.; Jin, Z.; Xu, X.; Xie, Z. Analysis of volatile compounds of Mesona Blumes gum/rice extrudates via GC-MS and electronic nose. Sens. Actuators B Chem. 2011, 160, 964–973. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape Wine R. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Mozzon, M.; Savini, S.; Boselli, E.; Thorngate, J.H. The herbaceous character of wines. Ital. J. Food Sci. 2016, 28, 190–207. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology. In The Chemistry of Wine-Stabilization and Treatments; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 2. [Google Scholar]
- Herraiz, T.; Herraiz, M.; Reglero, G.; Martin-Alvarez, P.J.; Cabezudo, M.D. Changes in the composition of alcohols and aldehydes of C6 chain length during the alcoholic fermentation of grape must. J. Agric. Food Chem. 1990, 38, 969–972. [Google Scholar] [CrossRef]
- Darias-Martín, J.; Díaz-González, D.; Díaz-Romero, C. Influence of two pressing processes on the quality of must in white wine production. J. Food Eng. 2004, 63, 335–340. [Google Scholar] [CrossRef]
- Darias-Martín, J.J.; Rodríguez, O.; Díaz, E.; Lamuela-Raventós, R.M. Effect of skin contact on the antioxidant phenolics in white wine. Food Chem. 2000, 71, 483–487. [Google Scholar] [CrossRef]
- Lukić, I.; Jedrejčić, N.; Kovačević Ganić, K.; Staver, M.; Peršurić, Đ. Phenolic and aroma composition of white wines produced by prolonged maceration and maturation in wooden barrels. Food Tech. Biotech. 2015, 53, 407–418. [Google Scholar] [CrossRef]
- Valero, E.; Millán, C.; Ortega, J.M. Influence of pre-fermentative treatment on the fatty acid content of Saccharomyces cerevisiae (M330-9) during alcoholic fermentation of grape must. J. Biosci. Bioeng. 2001, 91, 117–122. [Google Scholar] [CrossRef]
- Valdés, E.; Vilanova, M.; Sabio, E.; Benalte, M.J. Clarifying agents effect on the nitrogen composition in must and wine during fermentation. Food Chem. 2011, 25, 430–437. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Castro-Vázquez, L.; Hermosín-Gutiérrez, I.; Pérez-Coello, M.S. Combined effects of prefermentative skin maceration and oxygen addition of must on color-related phenolics, volatile composition, and sensory characteristics of Airén white wine. J. Agric. Food Chem. 2011, 59, 12171–12182. [Google Scholar] [CrossRef]
- Oliveira, J.M.; Faria, M.; Sá, F.; Barros, F.; Araújo, I.M. C6-alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2006, 563, 300–309. [Google Scholar] [CrossRef]

| Codes | Operation | Sampling Points | |
|---|---|---|---|
| V1 | V2 | ||
| T0 (M) | Before pressing | X | X |
| T1 | Prefermentative cold maceration with pectolytic enzyme | X | |
| T2 | Two pressing steps at 1 bar | X | X |
| T3 | Two pressing steps at 2 bar | X | X |
| T4 | Cold sedimentation | X | X |
| T5 | At half of fermentation | X | X |
| T6 | Addition of yeast autolysate | X | |
| T7 | End of fermentation | X | X |
| T8 | Cold stabilization | X | X |
| T9 | Before addition of bentonite | X | X |
| T10 | After bentonite clarification | X | |
| T11 | Pre-filtration | X | X |
| T12 | After-filtration | X | X |
| T13 | Bottling (W0) | X | X |
| T14 | 3 months into bottle (W3) | X | X |
| T15 | 6 months into bottle (W6) | X | X |
| T16 | 9 months into bottle (W9) | X | X |
| Assignment | R.t. (min) | Full MS (m/z) | MS/MS (m/z) | UV-Vis λmax (nm) | Identification | |
|---|---|---|---|---|---|---|
| 1 | gallic acid, hexoside (*) | 12.4 | 331 (ESI -) | 153 ← 331 | 267 | Assigned by DAD (λMAX) and MS2 analysis. |
| 2 | gallic acid | 17.1 | 169 (ESI -) | na | 267 | Standard injection. |
| 3 | (-)-gallocatechin | 24.0 | 305 (ESI -) | 125, 219 ← 305 | 279 | Fluorescence analysis. Standard injection; assigned by DAD (λMAX) and MS2 analysis. |
| 4 | glutathionylcaftaric acid (GRP) | 24.6 2 | 618 (ESI +) | 135, 179 ← 618 | 297, 327 | Assigned by DAD (λMAX) and MS2 analysis. |
| 5 | caftaric acid | 26.8 1, 28.0 2 | 311 (ESI -) | 135, 179 ← 311 | 297, 327 | Standard injection (trans isomer). |
| 6 | (-)-epigallocatechin | 31.4 | 305 (ESI -) | 125, 219 ← 305 | 279 | Fluorescence analysis. Standard injection; assigned by DAD (λMAX) and MS2 analysis. |
| 7 | (+)-catechin | 33.1 | 289 (ESI -) | na | 279 | Fluorescence analysis. Standard injection. |
| 8 | coutaric acid | 33.8 1, 34.5 2 | 295 (ESI -) | 163 ← 295 | 295, 308 | Standard injection; assigned by DAD (λMAX) and MS2 analysis. |
| 9 | trans-caffeic acid | 37.0 | 179 (ESI -) | na | 297, 327 | Standard injection. |
| 10 | (-)-epicatechin | 38.5 | 289 (ESI -) | na | 279 | Fluorescence analysis. Standard injection. |
| 11 | p-coumaric acid | 45.5 | 163 (ESI -) | na | 295, 308 | Standard injection. |
| 12 | astilbin | 49.8 | 449 (ESI -) | 125, 285, 303 ← 449 | 290, ~340 | Standard injection; assigned by DAD (λMAX) and MS2 analysis. |
| 13 | taxifolin (*) | 50.8 | 303 (ESI -) | 125, 285 ← 303 | 290, ~340 | Standard injection; assigned by DAD (λMAX) and MS2 analysis. |
| WINES | ||||
| Compound Name | Base Peak (*) (m/z) | Retention Time (**) (min) | Linear Retention Index (***) | Odor Descriptor |
| Ethyl Esters | ||||
| ethyl butanoate | 71;43 | 8.9 | 1033 (ref.: DB-Wax, 1035 [27]; PEG -{H2 carrier}, 1044 [28]) | Apple b |
| ethyl hexanoate | 88 | 16.6 | 1232 (ref.: DB-Wax, 1232 [29]; FFAP, 1243 [30]) | Apple peel, fruit a |
| ethyl octanoate | 88 | 25.4 | 1436 (ref.: DB-Wax, 1433 [29]; PEG {H2 carrier}, 1436 [28]) | Fruit, fat a |
| ethyl nonanoate | 88 | 29.6 | 1535 (ref.: Supelcowax-10, 1537, [31]) | Waxy c |
| ethyl decanoate | 88 | 33.8 | 1638 (ref.: SP-1000, 1644 [32]; Innovax, 1630, [33]) | Grape a |
| diethyl succinate | 101 | 35.2 | 1674 (ref.: DB-Wax, 1687 [33]) | Wine, fruit b |
| ethyl 9-decenoate | 88;55 | 35.8 | 1689 (ref.: DB-Wax, 1688 [34]) | Fruity b |
| ethyl dodecanoate | 88 | 41.4 | 1841 (ref.: DB-Wax, 1856 [35]) | Leaf b |
| ethyl tetradecanoate | 88 | 48.2 | 2050 (ref.: DB-Wax, 2070 [35]) | Ether b |
| Acetate esters | ||||
| ethyl acetate | 43 | 5.2 | 795 (ref.: DB-Wax, 890 [36]) | Pineapple, nail polish a |
| isoamyl acetate | 43 | 11.9 | 1119 (ref.: DB-Wax, 1126 [37]) | Banana a |
| hexyl acetate | 43 | 18.3 | 1271 (ref.: DB-Wax, 1279 [35]) | Fruit, herb b |
| octyl acetate | 43 | 27.1 | 1474 (ref.: DB-Wax, 1490 [35]) | Fruit b |
| phenylethyl acetate | 104 | 40.4 | 1812 (ref.: ZB-Wax, 1811 [38]) | Rose, honey, tobacco a |
| Other Esters | ||||
| methyl octanoate | 74 | 23.4 | 1388 (ref.: Innowax, 1386 [39]; ZB-Wax, 1386 [38]) | Orange b |
| isopentyl hexanoate | 70 | 26.4 | 1458 (ref.: DB-Wax, 1469 [35]; BP-20, 1450 [40]) | Fruity c |
| isobutyl octanoate | 57 | 30.3 | 1551 (ref.: ZB-Wax, 1550 [38]) | Fruity b |
| methyl decanoate | 74 | 32.0 | 1593 (ref.: HP-Wax, 1593 [41]; ZB-Wax, 1586 [38]) | Wine b |
| isoamyl octanoate | 70 | 34.5 | 1657 (ref.: ZB-Wax, 1658 [38]; DB-FFAP, 1647 [42]) | Fruity c |
| isopropyl dodecanoate | 43 | 41.1 | 1831 (ref.: ZB-Wax, 1832 [43]) | Green c |
| isoamyl decanoate | 70 | 42.1 | 1860 (ref.: ZB-Wax, 1859 [38]) | Waxy c |
| ethyl isoamyl succinate | 101 | 43.5 | 1898 (ref.: DB-Wax, 1907 [34]) | (not found) |
| Acids | ||||
| acetic acid | 43 | 26.0 | 1448 (ref.: DB-Wax, 1439 [44]) | Sour, pungent, vinegar a |
| octanoic acid | 60 | 48.4 | 2057 (ref.: DB-Wax, 2050 [45]) | Sweet, Cheese a |
| nonanoic acid | 60 | 50.7 | 2161 (ref.: DB-Wax, 2159 [46]) | Green, Fat b |
| C6 Alcohols | ||||
| n-hexanol | 56 | 21.8 | 1352 (ref.: DB-Wax, 1360 [47]) | Resin, flower, green a |
| Higher Alcohols | ||||
| isobutyl alcohol | 43 | 11.0 | 1094 (ref.: DB-Wax, 1093 [35]) | Ethereal, nail polish c |
| isoamyl alcohol | 55 | 15.5 | 1207 (ref.: HP-Innowax, 1206 [48]) | Whiskey, malt, burnt a |
| 2,3-butanediol | 45 | 29.7 | 1573 (ref.: DB-Wax, 1580 [35]) | Fruit, onion b |
| n-octanol | 41 | 30.5 | 1556 (ref.: DB-Wax, 1561 [49]) | Chemical, metal, burnt b |
| n-decanol | 70;55 | 38.4 | 1759 (ref.: HP-Innowax, 1764 [50]) | Fat b |
| phenylethyl alcohol | 91 | 43.7 | 1905 (ref.: Innowax, 1905 [33]) | Honey, spicy, rose, lilac a |
| Terpenes | ||||
| citronellol | 69;41 | 38.5 | 1762 (ref.: BP-20, 1762 [51]) | Rose b |
| Norisoprenoids | ||||
| β-damascenone | 69 | 40.6 | 1819 (ref.: DB-Wax, 1814 [29]) | Rose, honey, plum a |
| MUSTS | ||||
| Compound Name | Base Peak (*) (m/z) | Retention Time (**) (min) | Linear Retention Index (***) | Odor Descriptor |
| Ethyl Esters | ||||
| ethyl butanoate | 71;43 | 8.9 | 1033 (ref.: DB-Wax, 1035 [27]; PEG -{H2 carrier}, 1044 [28]) | Apple b |
| ethyl octanoate | 88 | 25.4 | 1436 (ref.: DB-Wax, 1433 [29]; PEG {H2 carrier}, 1436 [28]) | Fruit, fat a |
| ethyl decanoate | 88 | 33.8 | 1638 (ref.: SP-1000, 1644 [32]; Innovax, 1630 [33]) | Grape a |
| diethyl succinate | 101 | 35.2 | 1674 (ref.: DB-Wax, 1687 [33]) | Wine, fruit b |
| ethyl dodecanoate | 88 | 41.4 | 1841 (ref.: DB-Wax, 1856 [35]) | Leaf b |
| C6 Compounds | ||||
| hexanal | 44 | 10.5 | 1079 (ref.: DB-Wax, 1077 [27]) | Grass, tallow, fat b |
| 2-(E)-hexenal | 41 | 15.9 | 1216 (ref.: DB-Wax, 1215 [52]) | Green, leaf b |
| n-hexanol | 56 | 21.8 | 1352 (ref.: DB-Wax, 1360 [47]) | Resin, flower, green b |
| 2-hexen-1-ol | 57 | 24.1 | 1405 (ref.: HP-Innowax, 1408 [53]) | Green, leaf, walnut b |
| Other Esters | ||||
| isoamyl octanoate | 70 | 34.5 | 1657 (ref.: ZB-Wax, 1658 [38]) | Fruity c |
| isopropyl dodecanoate | 43 | 41.1 | 1831 (ref.: ZB-Wax, 1832 [41]) | Green c |
| Higher Alcohols | ||||
| phenylethyl alcohol | 91 | 43.7 | 1905 (ref.: Innowax, 1905 [33]) | Honey, spicy, rose, lilac a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dupas de Matos, A.; Longo, E.; Chiotti, D.; Pedri, U.; Eisenstecken, D.; Sanoll, C.; Robatscher, P.; Boselli, E. Pinot Blanc: Impact of the Winemaking Variables on the Evolution of the Phenolic, Volatile and Sensory Profiles. Foods 2020, 9, 499. https://doi.org/10.3390/foods9040499
Dupas de Matos A, Longo E, Chiotti D, Pedri U, Eisenstecken D, Sanoll C, Robatscher P, Boselli E. Pinot Blanc: Impact of the Winemaking Variables on the Evolution of the Phenolic, Volatile and Sensory Profiles. Foods. 2020; 9(4):499. https://doi.org/10.3390/foods9040499
Chicago/Turabian StyleDupas de Matos, Amanda, Edoardo Longo, Danila Chiotti, Ulrich Pedri, Daniela Eisenstecken, Christof Sanoll, Peter Robatscher, and Emanuele Boselli. 2020. "Pinot Blanc: Impact of the Winemaking Variables on the Evolution of the Phenolic, Volatile and Sensory Profiles" Foods 9, no. 4: 499. https://doi.org/10.3390/foods9040499
APA StyleDupas de Matos, A., Longo, E., Chiotti, D., Pedri, U., Eisenstecken, D., Sanoll, C., Robatscher, P., & Boselli, E. (2020). Pinot Blanc: Impact of the Winemaking Variables on the Evolution of the Phenolic, Volatile and Sensory Profiles. Foods, 9(4), 499. https://doi.org/10.3390/foods9040499







