Fat Inclusion Level, NaCl Content and LAB Starter Cultures in the Manufacturing of Italian-Type Ostrich Salami: Weight Loss and Nutritional Traits †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal, Diet and Meat
2.2. Experimental Design and Salami Preparation
2.3. Drying and Ripening
2.4. Weight Loss Determination and Chemical Analyses
2.5. Chemical Composition of the Diet
2.6. Statistical Analysis
3. Results
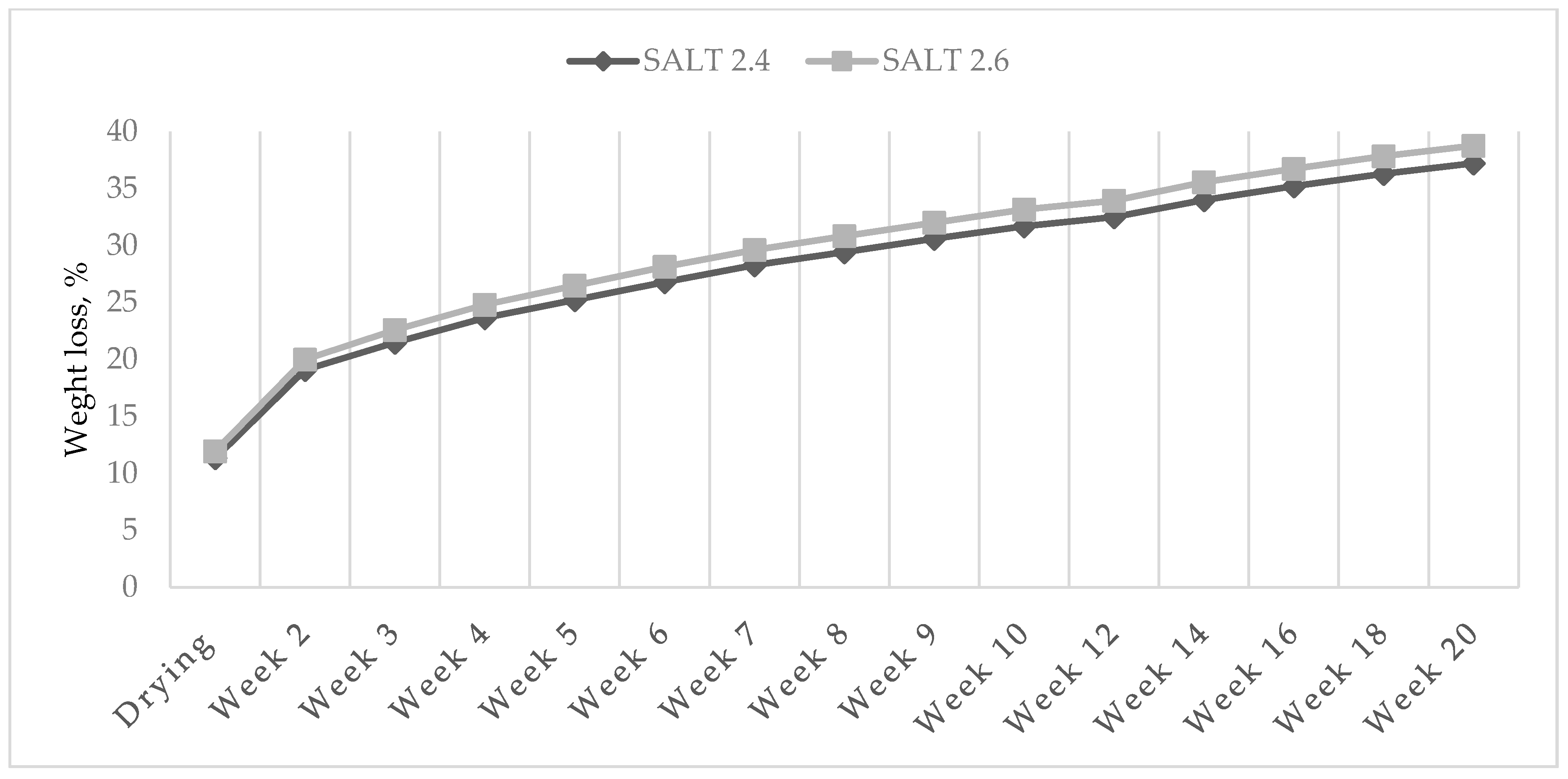
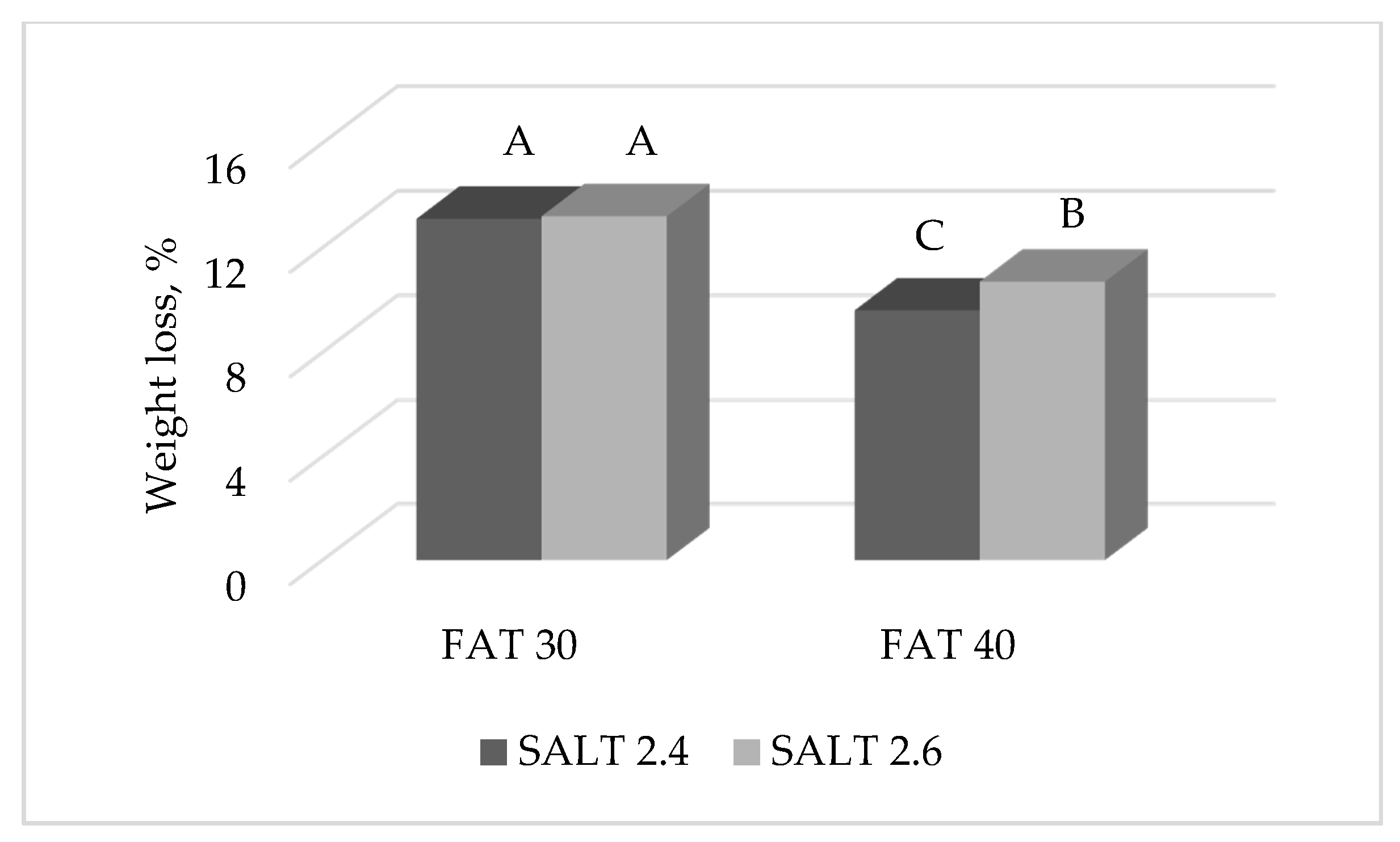
3.1. Weight Loss of Ostrich Salami
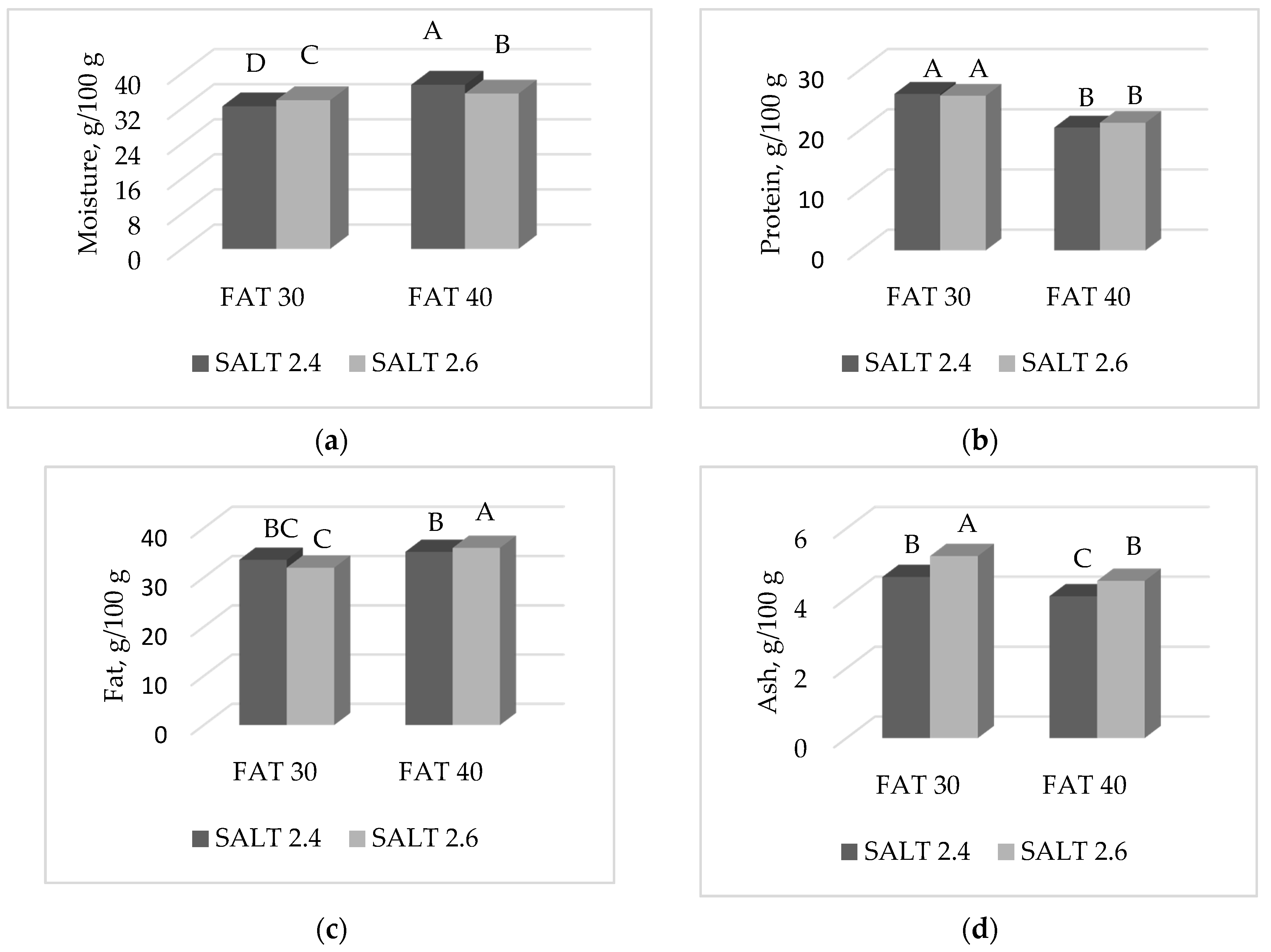
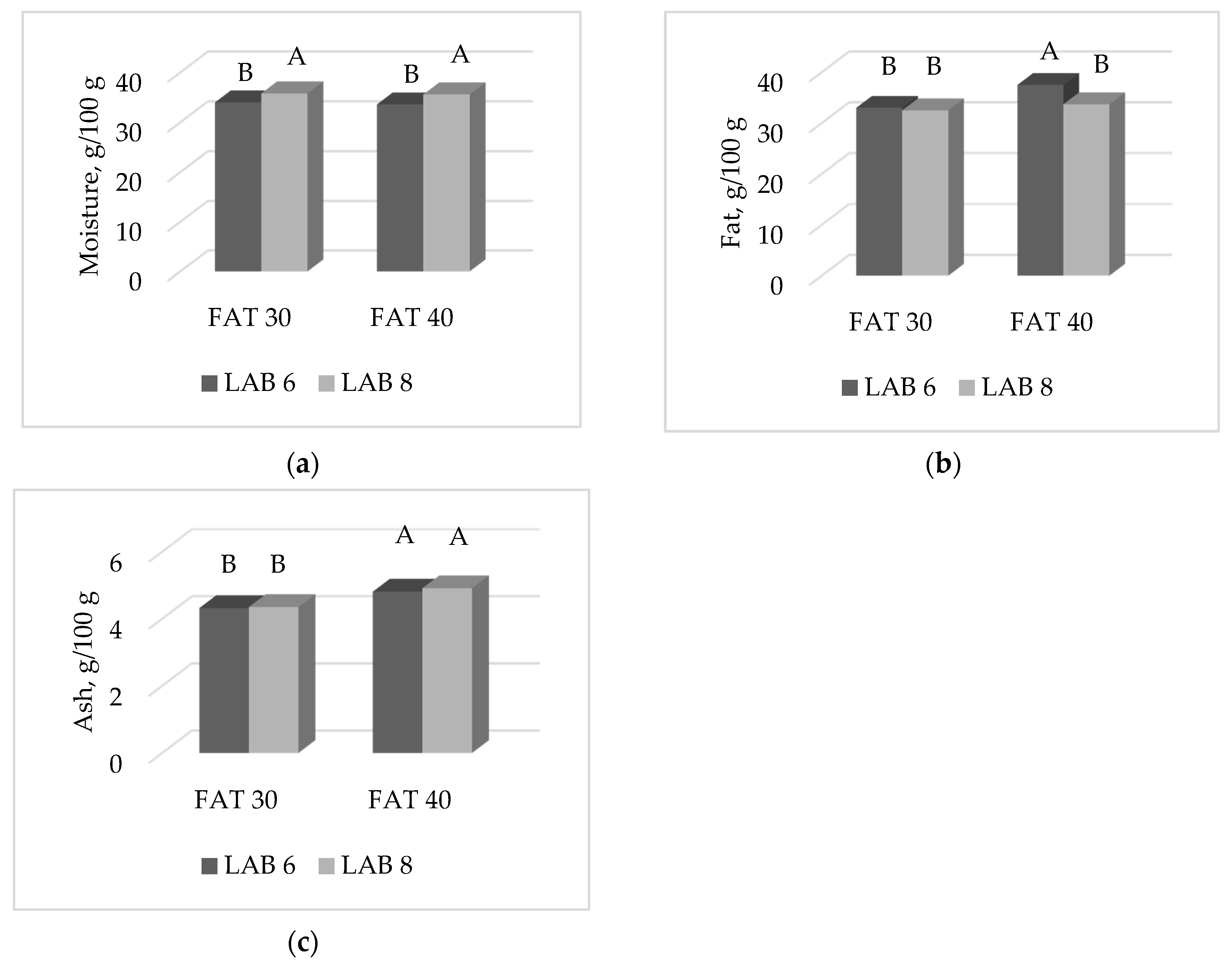
3.2. Proximate Composition and Cholesterol Content of Ostrich Salami
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Comi, G.; Urso, R.; Iacumin, L.; Rantsiou, K.; Cataneo, P.; Cantoni, C.; Cocolin, L. Characterisation of naturally fermented sausages produced in the North east of Italy. Meat Sci. 2005, 69, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Rantsiou, K.; Drosino, E.H.; Gialitaki, M.; Urso, R.; Krommer, J.; Gasparik-Reichardt, J.; Tóth, S.; Metaxopoulos, I.; Comi, G.; Cocolin, L. Molecular characterization of Lactobacillus species isolated from naturally fermented sausages produced in Greece, Hungary and Italy. Food Microbiol. 2005, 22, 19–28. [Google Scholar] [CrossRef]
- Talon, R.; Leroy, S.; Lebert, I. Microbial ecosystems of traditional fermented meat products: The importance of indigenous starters. Meat Sci. 2007, 77, 55–62. [Google Scholar] [CrossRef]
- Reckem, E.V.; Geeraerts, W.; Charmpi, C.; Van der Veken, D.; De Vuyst, L.; Leroy, F. Exploring the link between the geographical origin of European fermented foods and the diversity of their bacterial communities: The case of fermented meats. Front. Microbiol. 2019, 10, 2302. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.M.T.; Mellet, F.D.; Hoffman, L.C. Use of bacteriocin-producing starter cultures of Lactobacillus plantarum and Lactobacillus curvatus in production of ostrich salami. Meat Sci. 2004, 66, 703–708. [Google Scholar] [CrossRef]
- Olesen, P.T.; Meyer, A.S.; Stahnke, L.H. Generation of flavor compounds in fermented sausages-the influence of curing ingredients, Staphylococcus starter culture and ripening time. Meat Sci. 2004, 66, 675–687. [Google Scholar] [CrossRef]
- Leroy, F.; Verluyten, J.; De Vuyst, L. Functional meat starter cultures for improved sausage fermentation. Int. J. Food Microbiol. 2006, 106, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Todorov, S.D.; Koep, K.S.C.; Van Reenen, C.A.; Hoffman, L.C.; Slinde, E.; Dicks, L.M.T. Production of salami from beef, horse, mutton, Blesbok (Damaliscus dorcas phillipsi) and Springbok (Antidorcas marsupialis) with bacteriocinogenic strains of Lactobacillus plantarum and Lactobacillus curvatus. Meat Sci. 2007, 77, 405–412. [Google Scholar] [CrossRef]
- Hoppu, U.; Hopia, A.; Ponhjanheimo, T.; Rotola-Pukkila, M.; Mäkinen, S.; Pihlanto, A.; Sandell, M. Efefct of salt reduction on consumer acceptance and senssory quality of food. Foods 2017, 6, 103. [Google Scholar] [CrossRef] [Green Version]
- Ruusunen, M.; Puolanne, E. Reducing sodium intake from meat products. Meat Sci. 2005, 70, 531–541. [Google Scholar] [CrossRef] [Green Version]
- Zanardi, E.; Ghidini, S.; Conter, M.; Ianieri, A. Mineral composition of Italian salami and effect of NaCl partial replacement on compositional, physico-chemical and sensory parameters. Meat Sci. 2010, 86, 742–747. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 23 October 2018).
- Hooper, L.; Abdelhamid, A.; Bunn, D.; Brown, T.; Summerbell, C.D.; Skeaff, C.M. Effects of total fat intake on body weight. Cochrane Database Syst. Rev. 2015, 8, CD011834. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization (WHO). Regional Office for Europe Using Dietary Intake Modelling to Achieve Population Salt Reduction: A Guide to Developing a Country-Specific Salt Reduction Model; World Health Organization (WHO): Geneva, Switzerland, 2018. [Google Scholar]
- Flores, M.; Olivares, A.; Corral, S. Healthy trends affect the quality of traditional meat products in Mediterranean area. Acta Agr. Slov. 2013, 4, 183–188. [Google Scholar]
- Leroy, F.; Geyzen, A.; Janssens, M.; De Vuyst, L.; Scholliers, P. Meat fermentation at the crossroads of innovation and tradition: A historical outlook. Trends Food Sci. Tech. 2013, 31, 130–137. [Google Scholar] [CrossRef]
- Olmedilla-Alonso, B.; Jiménez-Colmenero, F.; Sánchez-Muniz, F. Development and assessment of healthy properties of meat and meat products designed as functional foods. Meat Sci. 2013, 95, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Cullere, M.; Hoffman, L.C.; Dalle Zotte, A. First evaluation of unfermented and fermented rooibos (Aspalathus linearis) in preventing lipid oxidation in meat products. Meat Sci. 2013, 95, 72–77. [Google Scholar] [CrossRef]
- COUNCIL REGULATION (EC). No 1099/2009 of 24 September 2009 on the protection of animals at the time of killing. Off. J. Eur. Union 2009, 303, 30. [Google Scholar]
- AOAC. Official methods of analysis of AOAC International. In Association of Official Analytical Chemists, 15th ed.; AOAC: Washington, DC, USA, 1995. [Google Scholar]
- Casiraghi, E.; Lucisano, M.; Pompei, C.; Dellea, C. Cholesterol determination in butter by high performance chromatography. Milchwissenschaft 1994, 49, 194–196. [Google Scholar]
- AOAC. Official methods of analysis of AOAC International. In Association of Official Analytical Chemists, 17th ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- EC 1998. Commission Directive 98/64/EC of 3 September 1998 establishing Community methods of analysis for the determination of amino acids, crude oils and fats, and olaquindox in feeding stuffs and amending Directive 71/393/EEC. Off. J. Eur. Union L257 1998, 257, 0014–0028. [Google Scholar]
- ISO-International Organization for Standardization 1998. Animal Feeding Stuffs, Animal Products and Faeces or Urine. Determination of Gross Calorific Value—Bomb Calorimetric Method; Reference number 9831, prepared by Technical Committee ISO/TC 34, Agricultural food products, Subcommittee SC 10, Animal feeding stuffs; ISO: Geneva, Switzerland, 1998. [Google Scholar]
- Mertens, D.R. Gravimetric determination of amylase-treated neutral detergent fibre in feeds with refluxing beakers or crucibles: Collaborative study. J. AOAC Int. 2002, 85, 1217–1240. [Google Scholar] [PubMed]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- SAS. Statistical Analysis Software for Windows; Version 9.2; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Claus, J.R.; Hunt, M.C.; Kastner, C.L.; Kropf, D.H. Low-fat, high-added water Bologna: Effects of massaging, preblending, and time of addition of water and fat on physical and sensory characteristics. J. Food Sci. 1990, 55, 338–341. [Google Scholar] [CrossRef]
- Crehan, C.M.; Hughes, E.; Troy, D.J.; Buckley, D.J. Effects of fat level and maltodextrin on the functional properties of frankfurters formulated with 5, 12 and 30% fat. Meat Sci. 2000, 55, 463–469. [Google Scholar] [CrossRef]
- Muguerza, E.; Fista, G.; Ansorena, D.; Astiasaran, I.; Bloukas, J.G. Effect of fat level and partial replacement of pork backfat with olive oil on processing and quality characteristics of fermented sausages. Meat Sci. 2002, 61, 397–404. [Google Scholar] [CrossRef]
- Baldini, P.; Cantoni, E.M.; Colla, F.; Diaferia, C.; Gabba, L.; Spotti, E.; Marchelli, R.; Dossena, A.; Virgili, E.; Sforza, S.; et al. Dry sausages ripening: Influence of thermohygrometric conditions on microbiological, chemical and physico-chemical characteristics. Food Res. Int. 2000, 33, 161–170. [Google Scholar] [CrossRef]
- Olivares, A.; Navarro, J.L.; Salvador, A.; Flores, M. Sensory acceptability of slow fermented sausages based on fat content and ripening time. Meat Sci. 2010, 86, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef]
- Guàrdia, M.D.; Guerrero, L.; Gelabert, J.; Gou, P.; Arnau, J. Consumer attitude towards sodium reduction in meat products and acceptability of fermented sausage with reduced sodium content. Meat Sci. 2006, 73, 484–490. [Google Scholar] [CrossRef]
- Warner, R.D. The Eating Quality of Meat—IV Water-Holding Capacity and Juiciness. In Lawrie’s Meat Science, 8th ed.; Toldrá, F., Ed.; Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, Cambridge, UK, 2017; pp. 419–453. [Google Scholar]
- Kenneally, P.M.; Schwarz, G.; Fransen, N.G.; Arendt, E.K. Lipolytic starter culture effects on production of free fatty acids in fermented sausages. J. Food Sci. 1998, 63, 538–543. [Google Scholar] [CrossRef]
- Chizzolini, R.; Zanardi, E.; Dorigoni, V.; Ghidini, S. Calorific value and cholesterol content of normal and low-fat meat and meat products. Trends Food Sci. Tech. 1999, 10, 119–128. [Google Scholar] [CrossRef]
- Charmpi, C.; Van der Veken, D.; Van Reckem, E.; De Vuyst, L.; Leroy, F. Raw meat quality and salt levels affect the bacterial species diversity and community dynamics during the fermentation of pork mince. Food Microbiol. 2020, 89, 103434. [Google Scholar] [CrossRef] [PubMed]
- Bedia, M.; Méndez, L.; Bañón, S. Evaluation of different starter cultures (Staphylococci plus Lactic Acid Bacteria) in semi-ripened Salami stuffed in swine gut. Meat Sci. 2011, 87, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Casaburi, A.; Di Monaco, R.; Cavella, S.; Toldrá, F.; Ercolini, D.; Villani, F. Proteolytic and lipolytic starter cultures and their effect on traditional fermented sausages ripening and sensory traits. Food Microbiol. 2008, 25, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Baka, A.M.; Papavergou, E.J.; Pragalaki, T.; Bloukas, J.G.; Kotzekidou, P. Effect of selected autochthonous starter cultures on processing and quality characteristics of Greek fermented sausages. LWT-Food Sci. Technol. 2011, 44, 54–61. [Google Scholar] [CrossRef]
- Casquete, R.; Benito, M.J.; Martín, A.; Ruiz-Moyano, S.; Hernández, A.; Córdoba, M.G. Effect of autochthonous starter cultures in the production of “salchichón”, a traditional Iberian dry-fermented sausage, with different ripening processes. LWT-Food Sci. Technol. 2011, 44, 1562–1571. [Google Scholar] [CrossRef]
- Fadda, S.; Sanz, Y.; Vignolo, G.; Aristoy, M.C.; Oliver, G.; Toldrá, F. Hydrolysis of pork muscle sarcoplasmic proteins by Lactobacillus curvatus and Lactobacillus sake. Appl. Environ. Microb. 1999, 65, 578–584. [Google Scholar] [CrossRef] [Green Version]
- Candogan, K.; Wardlaw, F.B.; Acton, J.C. Effect of starter culture on proteolytic changes during processing of fermented beef sausages. Food Chem. 2009, 116, 731–739. [Google Scholar] [CrossRef]
- Ordóñez, J.A.; Hierro, E.M.; Bruna, J.M.; de la Hoz, L. Changes in the components of dry-fermented sausages during ripening. Crit. Rev. Food Sci. 1999, 39, 329–367. [Google Scholar] [CrossRef]
- Leroy, F.; De Vuyst, L. Lactic acid bacteria as functional starter cultures for the food fermentation industry. Trends Food Sci. Tech. 2004, 15, 67–78. [Google Scholar] [CrossRef]


| Analyzed Composition | g/kg |
|---|---|
| Water | 126 |
| Crude protein | 147 |
| Crude fat | 17.8 |
| Ash | 60.7 |
| Crude fiber | 143 |
| Neutral detergent fiber | 231 |
| Acid detergent fiber | 155 |
| Acid detergent lignin | 22.7 |
| Acid Insoluble Ash | 4.74 |
| Starch | 213 |
| Gross Energy, MJ/kg | 8.90 |
| Ca | 10.5 |
| P | 2.50 |
| Fe | 0.12 |
| FAT (F) | SALT (S) | LAB (L) | Significance | RSD 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 40 | 2.4 | 2.6 | 6 | 8 | F | S | L | F × S | F × L | ||
| Drying | 13.2 | 10.1 | 11.3 | 11.9 | 11.5 | 11.7 | <0.0001 | <0.01 | ns | <0.05 | ns | 0.65 |
| Week 2 | 22.2 | 16.5 | 19.1 | 20.0 | 19.4 | 19.7 | <0.0001 | <0.001 | ns | <0.05 | ns | 0.86 |
| Week 3 | 25.6 | 18.5 | 21.5 | 22.6 | 21.9 | 22.2 | <0.0001 | <0.001 | ns | <0.01 | ns | 0.92 |
| Week 4 | 28.1 | 20.3 | 23.6 | 24.8 | 24.1 | 24.3 | <0.0001 | <0.001 | ns | <0.05 | <0.05 | 0.98 |
| Week 5 | 30.1 | 21.6 | 25.2 | 26.5 | 25.7 | 26.0 | <0.0001 | <0.0001 | ns | <0.05 | <0.05 | 0.98 |
| Week 6 | 31.9 | 23.0 | 26.8 | 28.1 | 27.3 | 27.6 | <0.0001 | <0.05 | ns | ns | ns | 1.95 |
| Week 7 | 33.5 | 24.4 | 28.3 | 29.6 | 28.9 | 29.0 | <0.0001 | <0.0001 | ns | <0.05 | <0.05 | 1.04 |
| Week 8 | 34.8 | 25.4 | 29.4 | 30.8 | 30.0 | 30.2 | <0.0001 | <0.0001 | ns | <0.05 | <0.05 | 1.04 |
| Week 9 | 36.1 | 26.5 | 30.6 | 32.0 | 31.2 | 31.4 | <0.0001 | <0.0001 | ns | <0.05 | <0.05 | 1.06 |
| Week 10 | 37.4 | 27.5 | 31.7 | 33.2 | 32.3 | 32.5 | <0.0001 | <0.0001 | ns | <0.05 | ns | 1.06 |
| Week 12 | 38.2 | 28.3 | 32.5 | 33.9 | 33.1 | 33.4 | <0.0001 | <0.0001 | ns | <0.01 | <0.05 | 0.95 |
| Week 14 | 39.8 | 29.8 | 34.0 | 35.6 | 34.6 | 35.0 | <0.0001 | <0.0001 | ns | <0.05 | <0.05 | 0.97 |
| Week 16 | 41.0 | 30.9 | 35.2 | 36.7 | 35.8 | 36.2 | <0.0001 | <0.0001 | ns | <0.05 | <0.05 | 0.97 |
| Week 18 | 42.1 | 32.0 | 36.3 | 37.8 | 36.8 | 37.3 | <0.0001 | <0.0001 | ns | <0.01 | ns | 0.99 |
| Week 20 | 43.1 | 32.9 | 37.2 | 38.8 | 37.8 | 38.2 | <0.0001 | <0.0001 | ns | <0.05 | ns | 1.01 |
| FAT (F) | SALT (S) | LAB (L) | Significance | RSD 1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 40 | 2.4 | 2.6 | 6 | 8 | F | S | L | F × S | F × L | ||
| Salami | 4 | 4 | 4 | 4 | 4 | 4 | ||||||
| Moisture | 33.2 | 36.4 | 34.9 | 34.6 | 33.8 | 35.7 | <0.0001 | ns | <0.0001 | <0.0001 | <0.0001 | 0.36 |
| Protein | 25.8 | 20.8 | 23.2 | 23.4 | 23.1 | 23.5 | <0.0001 | ns | <0.05 | <0.05 | ns | 0.55 |
| Fat | 32.8 | 35.6 | 34.4 | 34.0 | 35.3 | 33.1 | <0.0001 | ns | <0.001 | <0.05 | <0.01 | 1.37 |
| Ash | 4.91 | 4.28 | 4.34 | 4.86 | 4.57 | 4.63 | <0.0001 | <0.0001 | ns | <0.05 | <0.01 | 0.07 |
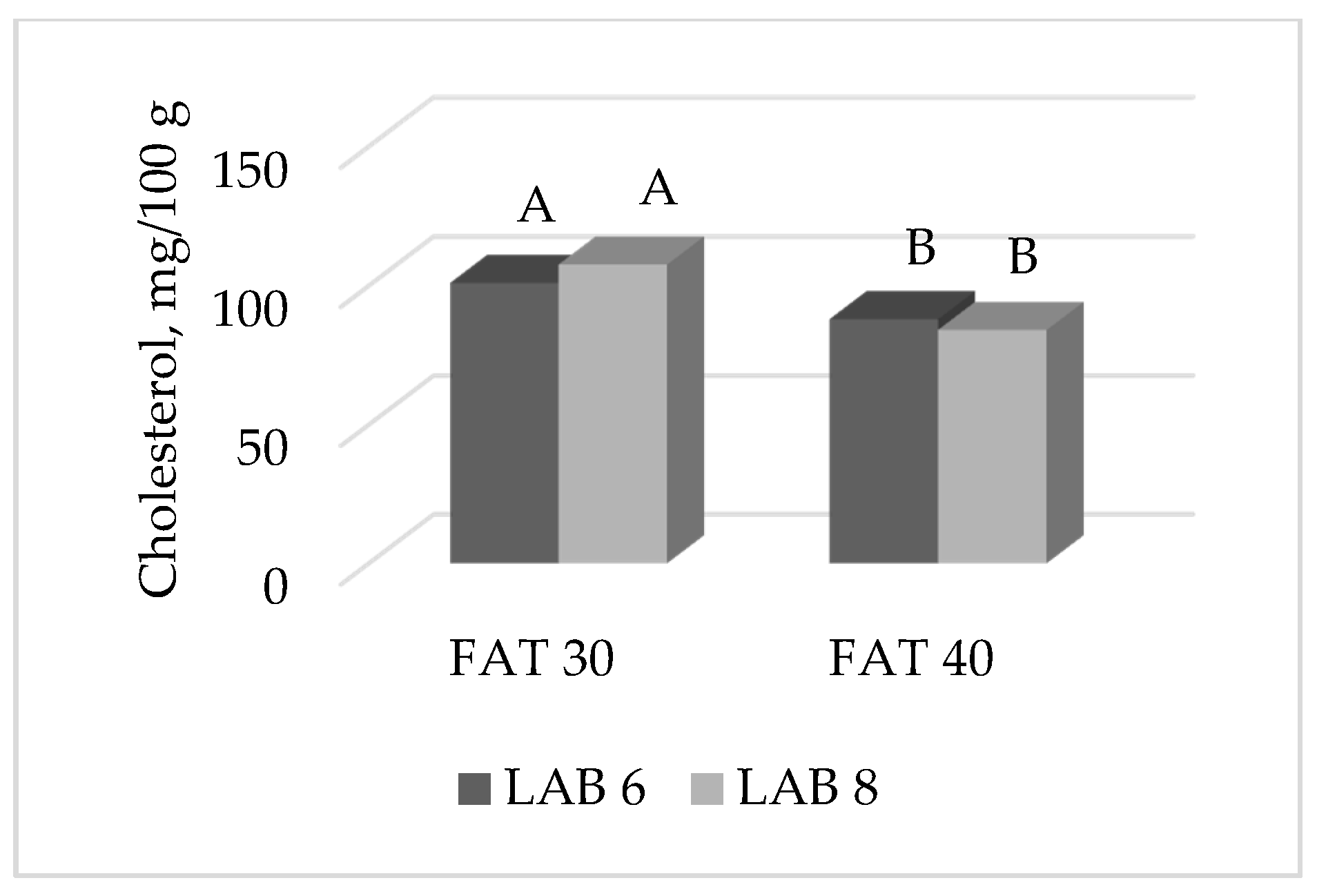
| Cholesterol 2 | 93.4 | 83.1 | 88.6 | 87.9 | 90.8 | 85.7 | <0.0001 | ns | <0.001 | ns | ns | 3.83 |
| FAT (F) | SALT (S) | LAB (L) | Significance | RSD 1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 40 | 2.4 | 2.6 | 6 | 8 | F | S | L | F × L | ||
| Salami | 5 | 5 | 5 | 5 | 5 | 5 | |||||
| Moisture | 26.4 | 31.1 | 29.0 | 28.5 | 28.0 | 29.5 | <0.0001 | ns | ns | ns | 2.61 |
| Protein | 27.5 | 21.5 | 24.3 | 24.7 | 24.2 | 24.8 | <0.0001 | ns | ns | ns | 1.18 |
| Fat | 36.5 | 36.5 | 36.4 | 36.6 | 37.1 | 35.9 | ns | ns | ns | ns | 3.58 |
| Ash | 5.55 | 4.79 | 5.07 | 5.28 | 5.22 | 5.13 | <0.0001 | ns | ns | ns | 0.42 |
| Cholesterol | 104.3 | 86.0 | 95.7 | 94.7 | 94.5 | 95.9 | <0.0001 | ns | ns | <0.05 | 6.54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cullere, M.; Novelli, E.; Dalle Zotte, A. Fat Inclusion Level, NaCl Content and LAB Starter Cultures in the Manufacturing of Italian-Type Ostrich Salami: Weight Loss and Nutritional Traits. Foods 2020, 9, 476. https://doi.org/10.3390/foods9040476
Cullere M, Novelli E, Dalle Zotte A. Fat Inclusion Level, NaCl Content and LAB Starter Cultures in the Manufacturing of Italian-Type Ostrich Salami: Weight Loss and Nutritional Traits. Foods. 2020; 9(4):476. https://doi.org/10.3390/foods9040476
Chicago/Turabian StyleCullere, Marco, Enrico Novelli, and Antonella Dalle Zotte. 2020. "Fat Inclusion Level, NaCl Content and LAB Starter Cultures in the Manufacturing of Italian-Type Ostrich Salami: Weight Loss and Nutritional Traits" Foods 9, no. 4: 476. https://doi.org/10.3390/foods9040476
APA StyleCullere, M., Novelli, E., & Dalle Zotte, A. (2020). Fat Inclusion Level, NaCl Content and LAB Starter Cultures in the Manufacturing of Italian-Type Ostrich Salami: Weight Loss and Nutritional Traits. Foods, 9(4), 476. https://doi.org/10.3390/foods9040476






