Lemon Juice, Sesame Paste, and Autoclaving Influence Iron Bioavailability of Hummus: Assessment by an In Vitro Digestion/Caco-2 Cell Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Hummus Preparation and Iron Determination
2.1.2. In Vitro Digestion
2.1.3. Cell Growth and Maintenance
2.1.4. Iron Uptake by Caco-2 Cells Experiment
2.1.5. Ferritin and Protein Determination from Caco-2 Cells
2.2. Methods
2.2.1. Preparation of the Eight Samples of Hummus with Different Processing and Formulation (HDPFI->VIII)
2.2.2. Preparation of the Eight Samples of Hummus with Different Acids (HDA1->8)
2.2.3. In Vitro Digestion and Iron Dialysis through the Dialysis Membrane
2.2.4. Determination of the Total and Dialyzable Iron in the Samples
2.2.5. Cell Culture
2.2.6. In Vitro Digestion of the Four Hummus Samples and Iron Uptake by the Caco-2 Cells
2.2.7. Cell Harvest
2.2.8. Ferritin and Protein Determination
2.2.9. Definition of Total Iron, Dialyzable Iron, Percentages of Bioaccessible Iron and Bioavailable Iron
2.2.10. Statistical Analysis
3. Results
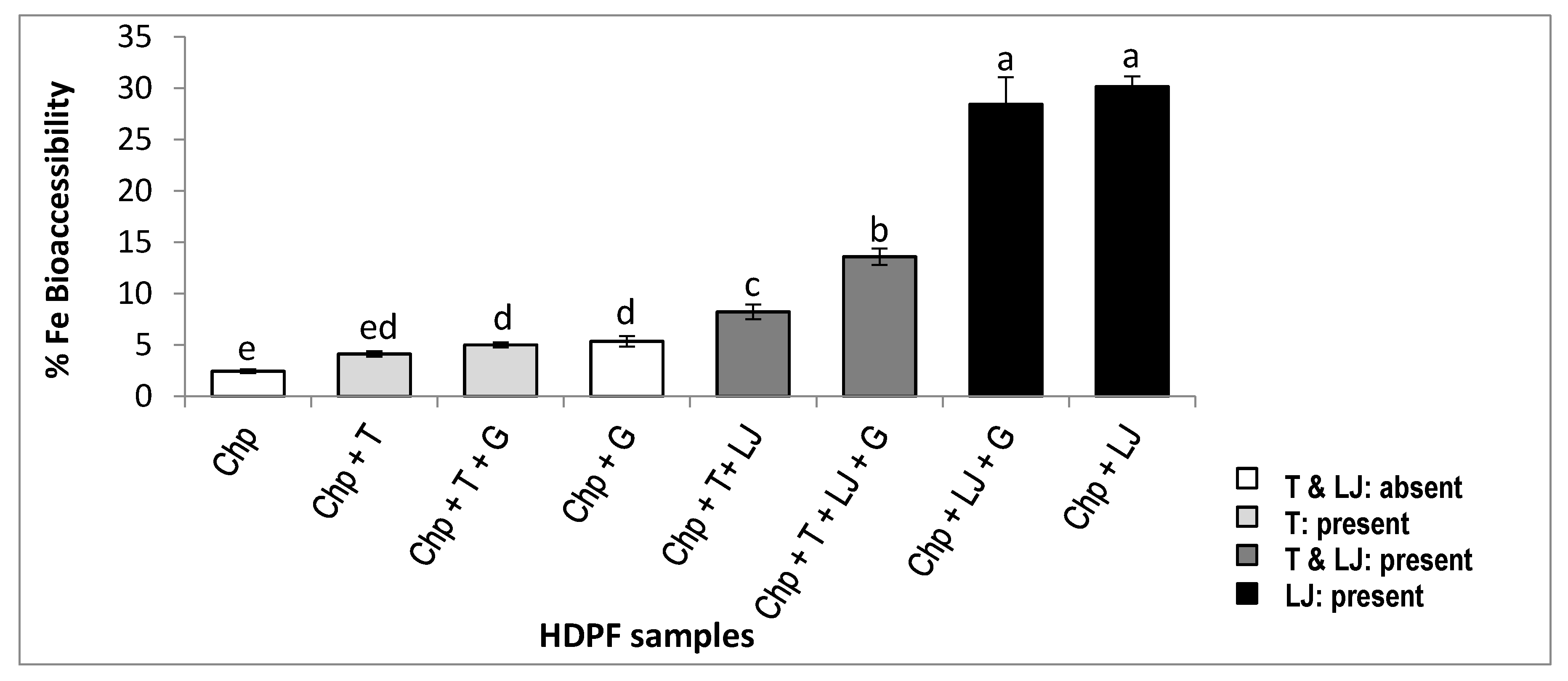
3.1. Amount of the Total Iron, the Dialyzable Iron, and the Percentage of Bioaccessible Iron in the HDPF Samples
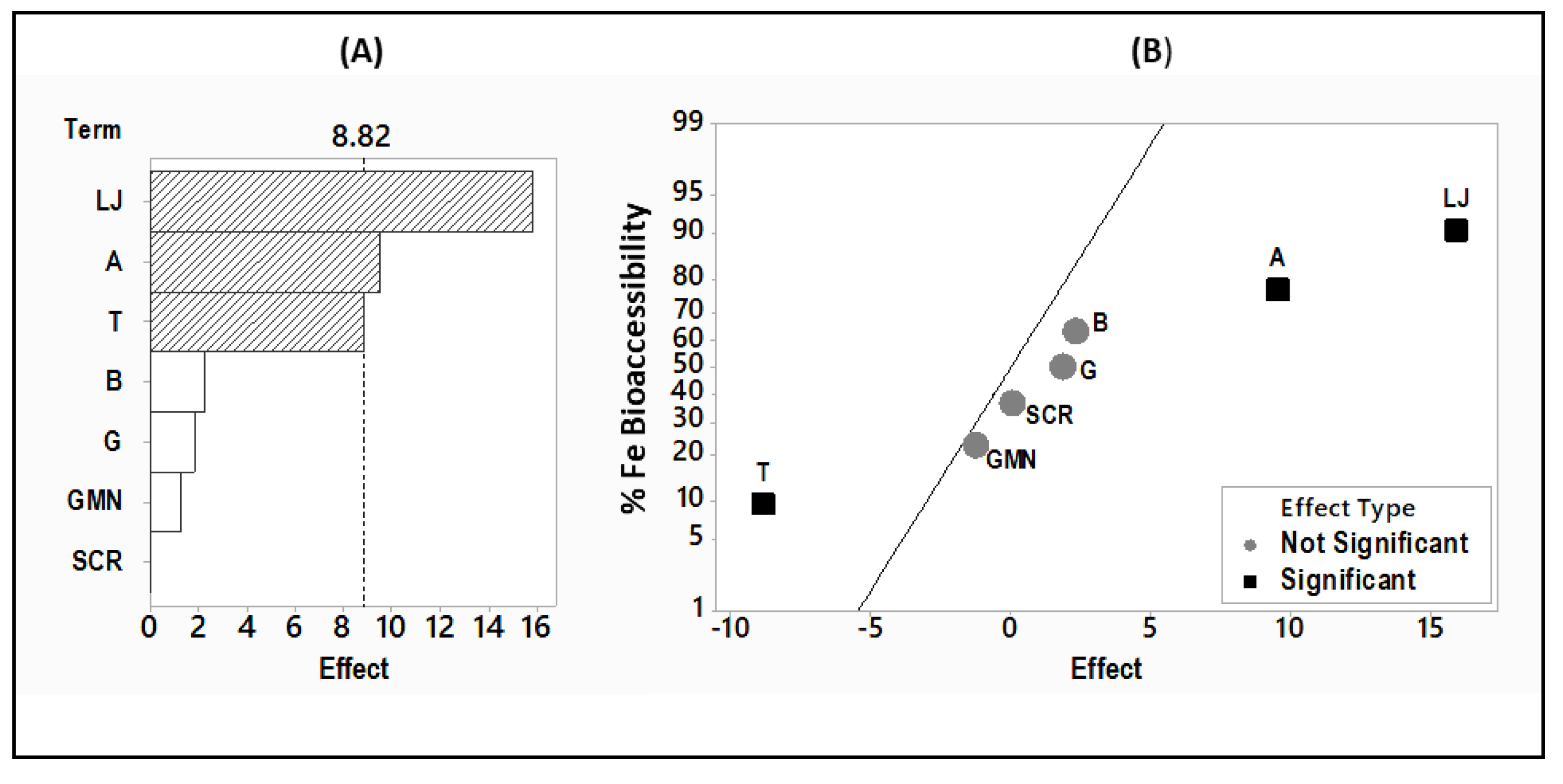
3.2. Effects of the Processing and Formulation Factors on Iron Absorption from Hummus
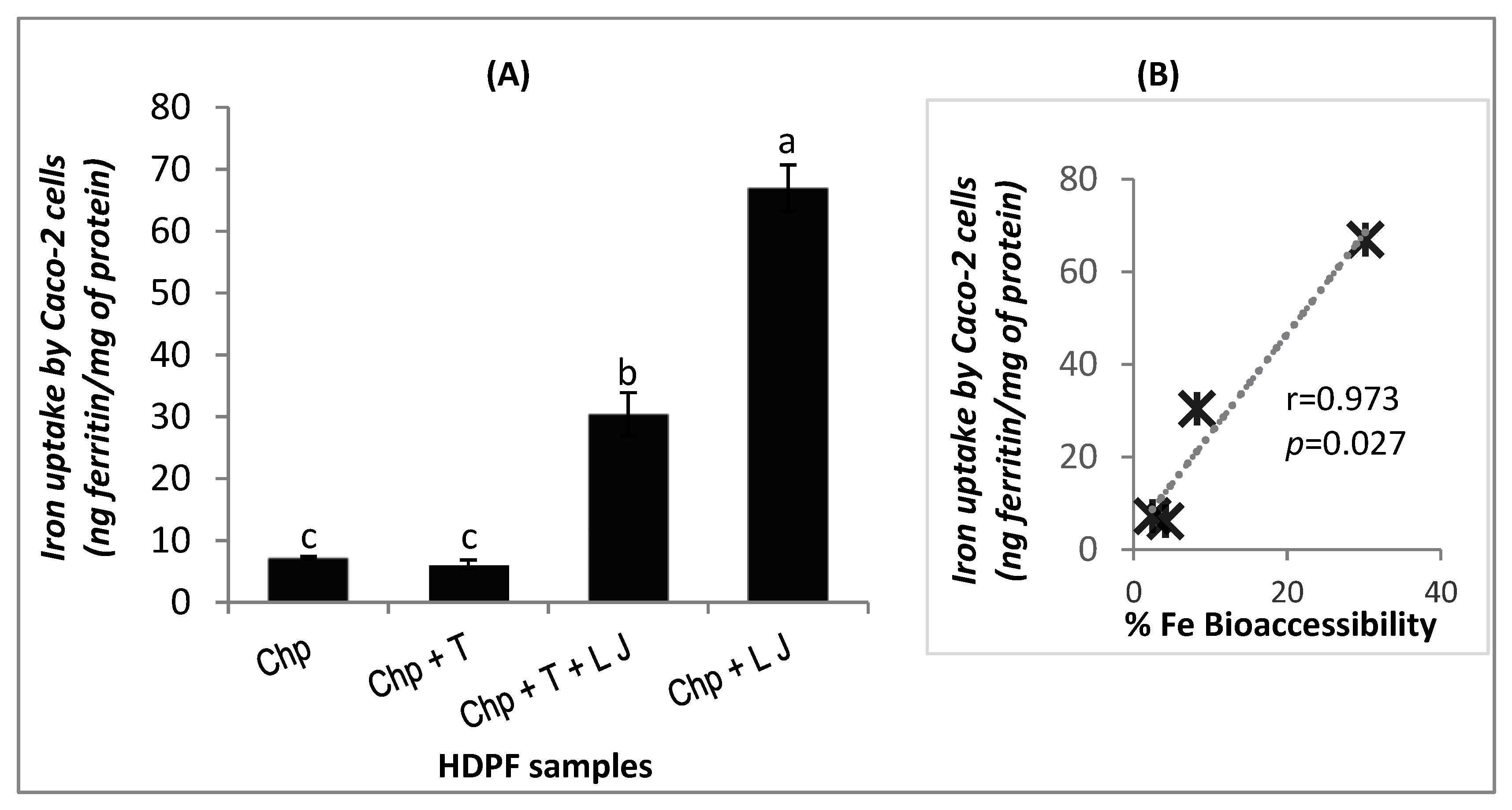
3.3. Validation with Ferritin Uptake by Caco-2 Cells
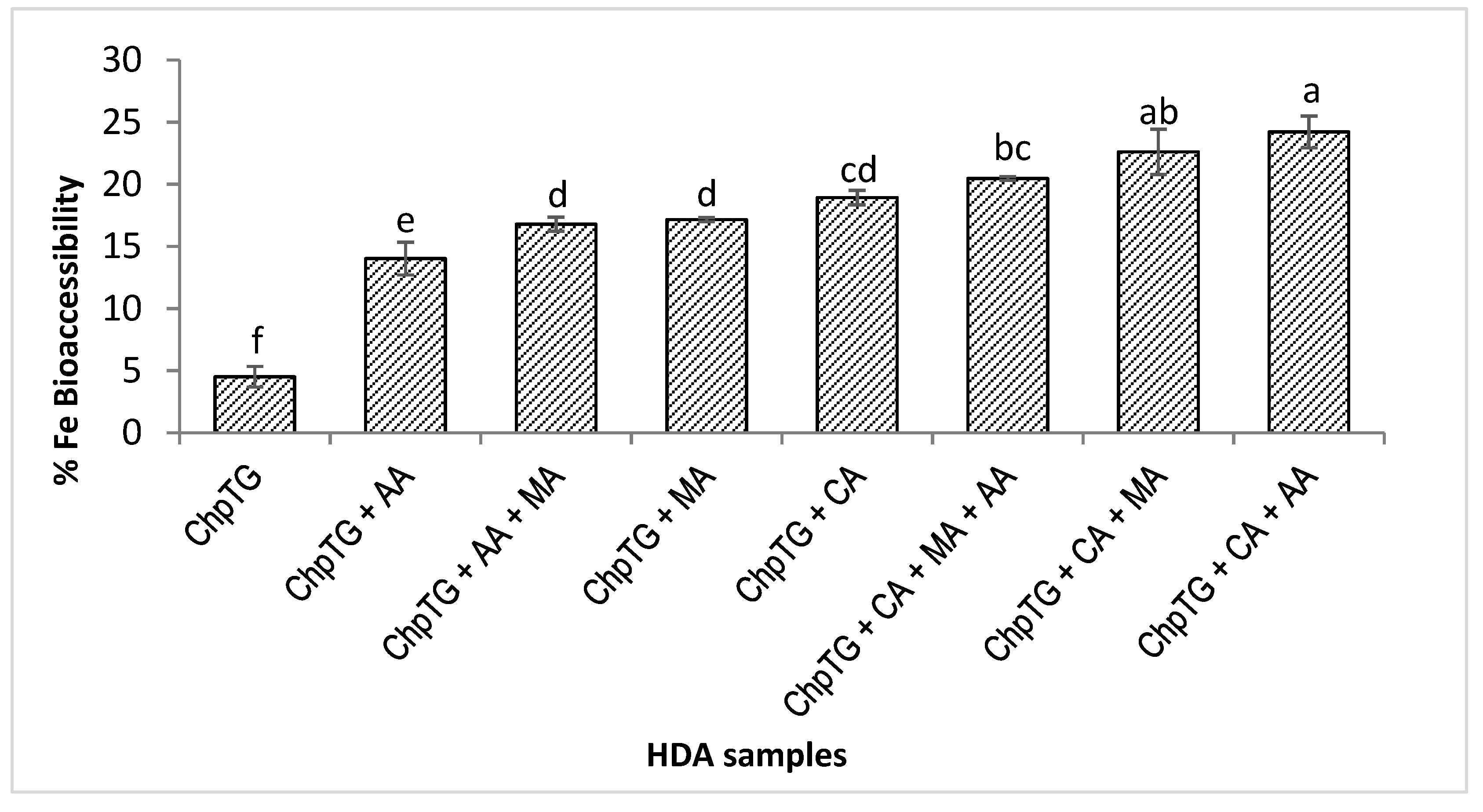
3.4. Amount of Total Iron, Dialyzable Iron, and Percentage Accessible Iron in the HDA Samples
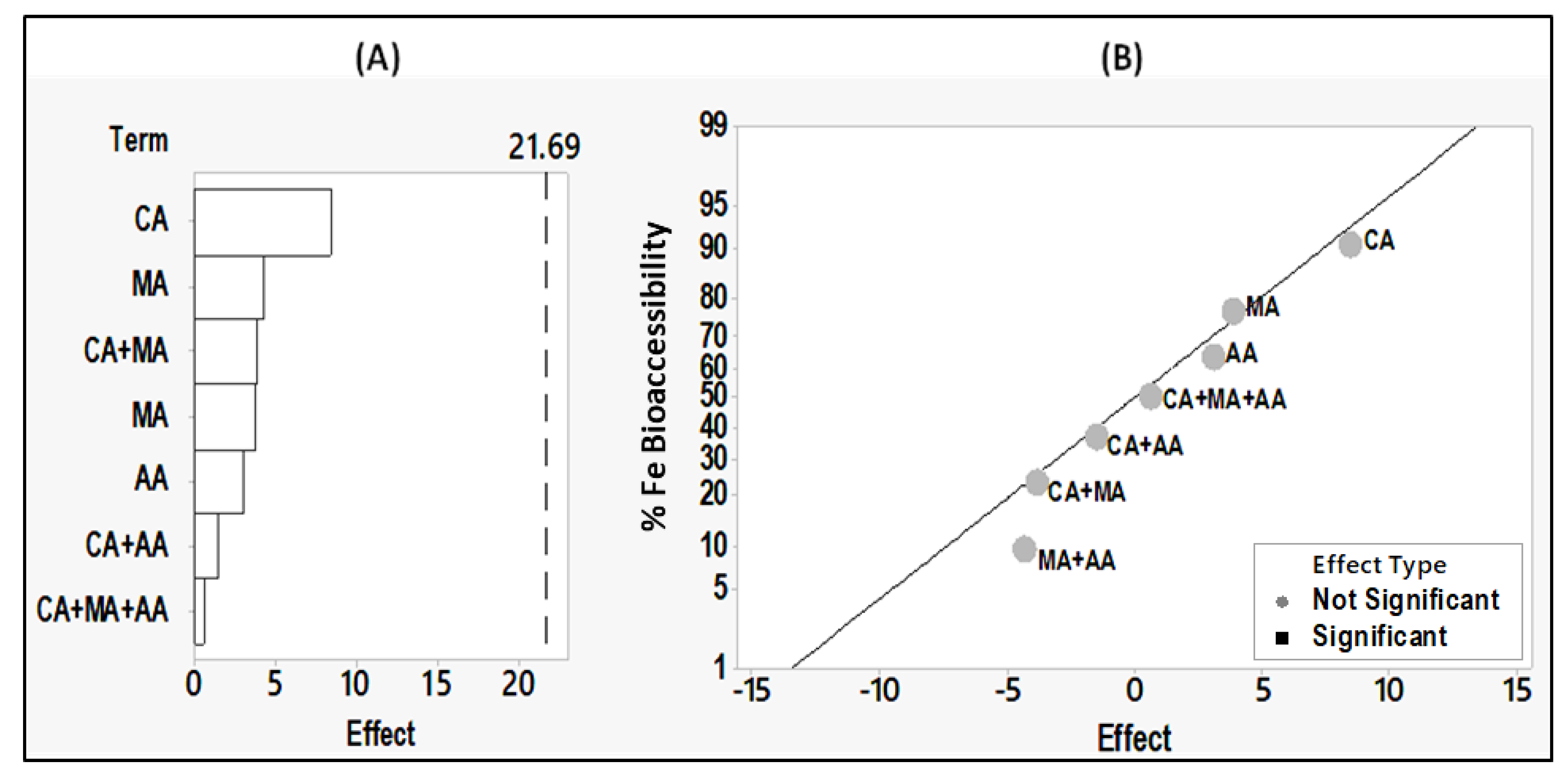
3.5. Effects of Citric, Malic and Ascorbic Acids on Iron Absorption from Hummus
4. Discussion
4.1. Total Iron Content in Hummus
4.2. Lemon Juice, Autoclaving, and Tahini: Principal Modulators
4.3. Acidic Components of Lemon Juice and Iron Bioaccessibility
4.4. Iron Bioaccessibility and Iron Bioavailability
4.5. Iron Bioaccessibility vs. Effect of Factors on Iron Bioaccessibility
4.6. Hummus as a Promising Source of Iron
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moll, R.; Davis, B. Iron, vitamin B 12 and folate. Medicine 2017, 45, 198–203. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 164–174. [Google Scholar]
- Gupta, P.; Perrine, C.; Mei, Z.; Scanlon, K. Iron, Anemia, and Iron Deficiency Anemia among Young Children in the United States. Nutrients 2016, 8, 330. [Google Scholar] [CrossRef] [PubMed]
- Hulthén, L. Iron deficiency and cognition. Scand. J. Nutr. 2003, 47, 152–156. [Google Scholar] [CrossRef]
- Hwalla, N.; Al Dhaheri, A.; Radwan, H.; Alfawaz, H.; Fouda, M.; Al-Daghri, N.; Zaghloul, S.; Blumberg, J. The Prevalence of Micronutrient Deficiencies and Inadequacies in the Middle East and Approaches to Interventions. Nutrients 2017, 9, 229. [Google Scholar] [CrossRef]
- Hunt, J.R. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am. J. Clin. Nutr. 2003, 78, 633S–639S. [Google Scholar] [CrossRef]
- West, A.R.; Oates, P.S. Mechanisms of heme iron absorption: Current questions and controversies. World J. Gastroenterol. 2008, 14, 4101–4110. [Google Scholar] [CrossRef]
- Hurrell, R.F.; Reddy, M.; Cook, J.D. Inhibition of non-haem iron absorption in man by polyphenolic-containing beverages. Br. J. Nutr. 1999, 81, 289–295. [Google Scholar] [CrossRef]
- Leip, A.; Billen, G.; Garnier, J.; Grizzetti, B.; Lassaletta, L.; Reis, S.; Simpson, D.; Sutton, M.A.; de Vries, W.; Weiss, F.; et al. Impacts of European livestock production: Nitrogen, sulphur, phosphorus and greenhouse gas emissions, land-use, water eutrophication and biodiversity. Environ. Res. Lett. 2015, 10, 115004–115016. [Google Scholar] [CrossRef]
- Martinez, J.; Dabert, P.; Barrington, S.; Burton, C. Livestock waste treatment systems for environmental quality, food safety, and sustainability. Bioresour. Technol. 2009, 100, 5527–5536. [Google Scholar] [CrossRef]
- UNEP United Nations Environment Programme. Available online: https://www.theguardian.com/environment/2010/jun/02/un-report-meat-free-diet (accessed on 12 February 2020).
- Hall, C.; Hillen, C.; Garden Robinson, J. Composition, Nutritional Value, and Health Benefits of Pulses. Cereal Chem. J. 2017, 94, 11–31. [Google Scholar] [CrossRef]
- Kenawi, M.A. Chemical composition, nutritional value, and in-vitro protein digestibility of three traditional breakfast foods in Jordan. Plant Foods Hum. Nutr. 2003, 58, 1–6. [Google Scholar] [CrossRef]
- Wallace, T.; Murray, R.; Zelman, K. The Nutritional Value and Health Benefits of Chickpeas and Hummus. Nutrients 2016, 8, 766. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, J.; Thomas, L.; Mulla, M. Dielectric and microstructural properties of high-pressure treated hummus in the selected packaging materials. LWT 2020, 118, 108885–108892. [Google Scholar] [CrossRef]
- Sprake, E.F.; Russell, J.M.; Cecil, J.E.; Cooper, R.J.; Grabowski, P.; Pourshahidi, L.K.; Barker, M.E. Dietary patterns of university students in the UK: A cross-sectional study. Nutr. J. 2018, 17, 90–106. [Google Scholar] [CrossRef]
- Yahya, H.M.; Day, A.; Lawton, C.; Myrissa, K.; Croden, F.; Dye, L.; Williamson, G. Dietary intake of 20 polyphenol subclasses in a cohort of UK women. Eur. J. Nutr. 2016, 55, 1839–1847. [Google Scholar] [CrossRef]
- MRFR Market Research Future. Hummus Market. Available online: https://www.marketresearchfuture.com/reports/hummus-market-1585 (accessed on 12 February 2020).
- O’Neil, C.; Nicklas, T.; Fulgoni, V.L. Chickpeas and Hummus are associated with Better Nutrient Intake, Diet Quality, and Levels of Some Cardiovascular Risk Factors: National Health and Nutrition Examination Survey 2003–2010. J. Nutr. Food Sci. 2014, 4, 1–7. [Google Scholar]
- Dasa, F.; Abera, T. Factors Affecting Iron Absorption and Mitigation Mechanisms: A review. Int. J. Agric. Sci. Food Technol. 2018, 4, 24–30. [Google Scholar]
- Gibson, R.S.; Perlas, L.; Hotz, C. Improving the bioavailability of nutrients in plant foods at the household level. Proc. Nutr. Soc. 2006, 65, 160–168. [Google Scholar] [CrossRef]
- Hotz, C.; Gibson, R.S. Traditional Food-Processing and Preparation Practices to Enhance the Bioavailability of Micronutrients in Plant-Based Diets. J. Nutr. 2007, 137, 1097–1100. [Google Scholar] [CrossRef]
- Haileslassie, H.A.; Henry, C.J.; Tyler, R.T. Impact of household food processing strategies on antinutrient (phytate, tannin and polyphenol) contents of chickpeas (Cicer arietinum L.) and beans (Phaseolus vulgaris L.): A review. Int. J. Food Sci. Technol. 2016, 51, 1947–1957. [Google Scholar] [CrossRef]
- Ballot, D.; Baynes, R.D.; Bothwell, T.H.; Gillooly, M.; Macfarlane, J.; Macphail, A.P.; Lyons, G.; Derman, D.P.; Bezwoda, W.R.; Torrance, J.D.; et al. The effects of fruit juices and fruits on the absorption of iron from a rice meal. Br. J. Nutr. 1987, 57, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Doumani, N.; Maalouly, J.; Bou-Maroun, E.; Sok, N.; Cayot, P.; Tueni, M. Iron intake among Lebanese women and assessment of iron absorption modulators in general and in particular for hummus, a Mediterranean food source of iron. 2020. manuscript in preparation. [Google Scholar]
- Fernandes, A.C.; Nishida, W.; Da Costa Proença, R.P. Influence of soaking on the nutritional quality of common beans (Phaseolus vulgaris L.) cooked with or without the soaking water: A review: Soaking and nutritional quality of beans. Int. J. Food Sci. Technol. 2010, 45, 2209–2218. [Google Scholar] [CrossRef]
- Vadivel, V.; Pugalenthi, M. Effect of soaking in sodium bicarbonate solution followed by autoclaving on the nutritional and antinutritional properties of velvet bean seeds. J. Food Process. Preserv. 2009, 33, 60–73. [Google Scholar] [CrossRef]
- Alajaji, S.A.; El-Adawy, T.A. Nutritional composition of chickpea (Cicer arietinum L.) as affected by microwave cooking and other traditional cooking methods. J. Food Compos. Anal. 2006, 19, 806–812. [Google Scholar] [CrossRef]
- Sebastiá, V.; Barberá, R.; Farré, R.; Lagarda, M.J. Effects of legume processing on calcium, iron and zinc contents and dialysabilities: Ca, Fe and Zn contents and dialysabilities in legumes. J. Sci. Food Agric. 2001, 81, 1180–1185. [Google Scholar] [CrossRef]
- Singh, P.K.; Shrivastava, N.; Sharma, B.; Bhagyawant, S.S. Effect of Domestic Processes on Chickpea Seeds for Antinutritional Contents and Their Divergence. Am. J. Food Sci. Technol. 2015, 111–117. [Google Scholar]
- Glahn, R.P.; Tako, E.; Cichy, K.; Wiesinger, J. The cotyledon cell wall and intracellular matrix are factors that limit iron bioavailability of the common bean (Phaseolus vulgaris). Food Funct. 2016, 7, 3193–3200. [Google Scholar] [CrossRef]
- Ghavidel, R.A.; Prakash, J. The impact of germination and dehulling on nutrients, antinutrients, in vitro iron and calcium bioavailability and in vitro starch and protein digestibility of some legume seeds. LWT Food Sci. Technol. 2007, 40, 1292–1299. [Google Scholar] [CrossRef]
- Gowri, B.S.; Platel, K.; Prakash, J.; Srinivasan, K. Influence of amla fruits (Emblica officinalis) on the bio-availability of iron from staple cereals and pulses. Nutr. Res. 2001, 21, 1483–1492. [Google Scholar] [CrossRef]
- Sørensen, A.D.; Bukhave, K. Iron uptake by Caco-2 cells following in vitro digestion: Effects of heat treatments of pork meat and pH of the digests. J. Trace Elem. Med. Biol. 2010, 24, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Platel, K.; Srinivasan, K. Higher Bioaccessibility of Iron and Zinc from Food Grains in the Presence of Garlic and Onion. J. Agric. Food Chem. 2010, 58, 8426–8429. [Google Scholar] [CrossRef]
- Kumari, M.; Platel, K. Effect of sulfur-containing spices on the bioaccessibility of trace minerals from selected cereals and pulses: Sulphur containing spices affect trace mineral bioaccessibility. J. Sci. Food Agric. 2017, 97, 2842–2848. [Google Scholar] [CrossRef] [PubMed]
- Cowan, J.W.; Esfahani, M.; Salji, J.P.; Nahapetian, A. Nutritive value of middle eastern foodstuffs. III.—Physiological availability of iron in selected foods common to the middle east. J. Sci. Food Agric. 1967, 18, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Etcheverry, P.; Grusak, M.A.; Fleige, L.E. Application of in vitro bioaccessibility and bioavailability methods for calcium, carotenoids, folate, iron, magnesium, polyphenols, zinc, and vitamins B6, B12, D, and E. Front. Physiol. 2012, 3, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Alandy, V.; Fleige, L.; Glahn, R. Iron Bioavailability of Fortified Maize and Sorghum Porridges. J. Nutr. Health Food Sci. 2019, 7, 1–6. [Google Scholar]
- Glahn, R.P.; Lee, O.A.; Yeung, A.; Goldman, M.I.; Miller, D.D. Caco-2 Cell Ferritin Formation Predicts Nonradiolabeled Food Iron Availability in an In Vitro Digestion/Caco-2 Cell Culture Model. J. Nutr. 1998, 128, 1555–1561. [Google Scholar] [CrossRef]
- Glahn, R.P.; Wortley, G.M.; South, P.K.; Miller, D.D. Inhibition of Iron Uptake by Phytic Acid, Tannic Acid, and ZnCl2: Studies Using an in Vitro Digestion/Caco-2 Cell Model. J. Agric. Food Chem. 2002, 50, 390–395. [Google Scholar] [CrossRef]
- Rodriguez-Ramiro, I.; Brearley, C.A.; Bruggraber, S.F.A.; Perfecto, A.; Shewry, P.; Fairweather-Tait, S. Assessment of iron bioavailability from different bread making processes using an in vitro intestinal cell model. Food Chem. 2017, 228, 91–98. [Google Scholar] [CrossRef]
- Salovaara, S.; Sandberg, A.-S.; Andlid, T. Organic Acids Influence Iron Uptake in the Human Epithelial Cell Line Caco-2. J. Agric. Food Chem. 2002, 50, 6233–6238. [Google Scholar] [CrossRef] [PubMed]
- Amagloh, F.; Atuna, R.; McBride, R.; Carey, E.; Christides, T. Nutrient and Total Polyphenol Contents of Dark Green Leafy Vegetables, and Estimation of Their Iron Bioaccessibility Using the in Vitro Digestion/Caco-2 Cell Model. Foods 2017, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.J.; Tako, E.; Kochian, L.V.; Glahn, R.P. Identification of Black Bean (Phaseolus vulgaris L.) Polyphenols That Inhibit and Promote Iron Uptake by Caco-2 Cells. J. Agric. Food Chem. 2015, 63, 5950–5956. [Google Scholar] [CrossRef] [PubMed]
- Nikooyeh, B.; Neyestani, T.R. Evaluation of Iron Bioavailability in Caco-2 cell Culture Model: Modification of the Original Method. Nutr. Food Sci. Res. 2016, 3, 11–16. [Google Scholar] [CrossRef]
- Perfecto, A.; Elgy, C.; Valsami-Jones, E.; Sharp, P.; Hilty, F.; Fairweather-Tait, S. Mechanisms of Iron Uptake from Ferric Phosphate Nanoparticles in Human Intestinal Caco-2 Cells. Nutrients 2017, 9, 359. [Google Scholar] [CrossRef]
- Glahn, R.; Tako, E.; Hart, J.; Haas, J.; Lung’aho, M.; Beebe, S. Iron Bioavailability Studies of the First Generation of Iron-Biofortified Beans Released in Rwanda. Nutrients 2017, 9, 787. [Google Scholar] [CrossRef]
- Glahn, R. The use of Caco-2 cells in defining nutrient bioavailability: Application to iron bioavailability of foods. In Designing Functional Foods; Cornell University: Ithaca, NY, USA, 2009; pp. 340–361. ISBN 978-1-84569-432-6. [Google Scholar]
- Carbonell-Capella, J.M.; Buniowska, M.; Barba, F.J.; Esteve, M.J.; Frígola, A. Analytical Methods for Determining Bioavailability and Bioaccessibility of Bioactive Compounds from Fruits and Vegetables: A Review: Bioavailability of bioactive compounds…. Compr. Rev. Food Sci. Food Saf. 2014, 13, 155–171. [Google Scholar] [CrossRef]
- Bryszewska, M. Comparison Study of Iron Bioaccessibility from Dietary Supplements and Microencapsulated Preparations. Nutrients 2019, 11, 273. [Google Scholar] [CrossRef]
- Hunt, J.R. Dietary and Physiological Factors That Affect the Absorption and Bioavailability of Iron. Int. J. Vitam. Nutr. Res. 2005, 75, 375–384. [Google Scholar] [CrossRef]
- El Hajj, A. Ma kul el Hana; Hachette Antoine Publishing: Beirut, Lebanon, 2014; ISBN 978-9953-26-440-0. [Google Scholar]
- Kamal, S.; Othman, S. Expanded Alphabet of Cooking; Dar El-Ilm Lilmalayin: Beirut, Lebanon, 2015; ISBN 978-9953-63-001-4. [Google Scholar]
- Tōnikean, A.P. Sfrah A’nahid Ash-Shhiah; Academia: Beirut, Lebanon, 2014; ISBN 978-9953-37-593-9. [Google Scholar]
- Chiocchetti, G.; De Nadai Fernandes, E.; Wawer, A.; Fairweather-Tait, S.; Christides, T. In Vitro Iron Bioavailability of Brazilian Food-Based by-Products. Medicines 2018, 5, 45. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in Vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Feitosa, S.; Greiner, R.; Meinhardt, A.-K.; Müller, A.; Almeida, D.; Posten, C. Effect of Traditional Household Processes on Iron, Zinc and Copper Bioaccessibility in Black Bean (Phaseolus vulgaris L.). Foods 2018, 7, 123. [Google Scholar] [CrossRef] [PubMed]
- Table of Pearson’s Correlation Critical Values. Available online: https://ww138.droots.co.uk/pearson-product-moment-correlation-coefficient-table.html (accessed on 2 February 2020).
- USDA U.S. Department of Agriculture, Agricultural Research Service, USDA Nutrient Data Laboratory, FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 1 January 2020).
- Ibáñez, M.V.; Rincón, F.; Amaro, M.; Martínez, B. Intrinsic variability of mineral composition of chickpea (Cicer arietinum, L.). Food Chem. 1998, 63, 55–60. [Google Scholar] [CrossRef]
- Takatera, K.; Miyake, Y.; Hiramitsu, M.; Inoue, T.; Katagiri, T. Effects of Citric Acid and Lemon Juice on Iron Absorption and Improvement of Anemia in Iron-Deficient Rats. Food Sci. Technol. Res. 2012, 18, 127–130. [Google Scholar] [CrossRef]
- Evans, W.J.; McCourtney, E.J.; Shrager, R.I. Titration studies of phytic acid. J. Am. Oil Chem. Soc. 1982, 59, 189–191. [Google Scholar] [CrossRef]
- Rajni, M.; Nagi, H.P.S.; Sharma, P.; Sharma, S. Effect of Processing on Chemical Composition and Antinutritional Factors in Chickpea Flour. J. Food Sci. Eng. 2012, 2, 180–186. [Google Scholar]
- Abebe, Y.; Bogale, A.; Hambidge, K.M.; Stoecker, B.J.; Bailey, K.; Gibson, R.S. Phytate, zinc, iron and calcium content of selected raw and prepared foods consumed in rural Sidama, Southern Ethiopia, and implications for bioavailability. J. Food Compos. Anal. 2007, 20, 161–168. [Google Scholar] [CrossRef]
- Dds, M.B.R.; Kamali-Nejad, M.; Karimi, M.; Dds, H.R.; Dds, F.H. Sesame extraction gel as an agent for prevention of dental caries: An in-vitro study. J Oral Health Oral Epidemiol 2017, 6, 226–230. [Google Scholar]
- Elleuch, M.; Besbes, S.; Roiseux, O.; Blecker, C.; Attia, H. Quality characteristics of sesame seeds and by-products. Food Chem. 2007, 103, 641–650. [Google Scholar] [CrossRef]
- Gadade, B.V.; Kachare, D.P.; Satbhai, R.D.; Naik, R.M. Nutritional Composition and Oil Quality Parameters of Sesame (Sesamum indicum L.) Genotypes. Int. Res. 2017, 3, 1–13. [Google Scholar]
- Suzuki, T.; Clydesdale, F.M.; Pandolf, T. Solubility of Iron in Model Systems Containing Organic Acids and Lignin. J. Food Prot. 1992, 55, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Gangloff, M.B.; Glahn, R.P.; Miller, D.D.; Van Campen, D.R. Assessment of iron availability using combined in vitro digestion and Caco-2 cell culture. Nutr. Res. 1996, 16, 479–487. [Google Scholar] [CrossRef]
- Hallberg, L.; Rossander-Hulthén, L. Hallberg1984.pdf; American Society for Clinical Nutrition: Rockville, MD, USA, 1984; pp. 577–583. [Google Scholar]
- European Chemical Agency Search for Chemicals. Citric Acid (CAS 77-92-9) Registered Substances Factsheets. Physical and Chemical Properties Dissociation Constant. Available online: http://echa.europa.eu/ (accessed on 3 September 2019).
- European Chemical Agency Search for Chemicals. Malic Acid (CAS 6915-15-7) Registered Substances Factsheets. Physical and Chemical Properties Dissociation Constant. Available online: http://echa.europa.eu/ (accessed on 3 September 2019).
- O’Neill, M. The Merck Index 2006; Merck Publishing: Kenilworth, NJ, USA, 2006. [Google Scholar]
- Anses-Ciqual Vitamin C (mg/100g) in Lemon Juice, Pure Juice. Available online: https://ciqual.anses.fr/#/aliments/2028/lemon-juice-pure-juice (accessed on 18 February 2020).
- Lifshitz, A.; Stepak, Y. Detection of Adulteration of Fruit Juice. I. Characterization of Israel Lemon Juice. J. AOAC Int. 1971, 54, 1262–1265. [Google Scholar] [CrossRef]
- Penniston, K.L.; Nakada, S.Y.; Holmes, R.P.; Assimos, D.G. Quantitative Assessment of Citric Acid in Lemon Juice, Lime Juice, and Commercially-Available Fruit Juice Products. J. Endourol. 2008, 22, 567–570. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Penella, J.M.; Laparra, J.M.; Sanz, Y.; Haros, M. Assessment of Iron Bioavailability in Whole Wheat Bread by Addition of Phytase-Producing Bifidobacteria. J. Agric. Food Chem. 2012, 60, 3190–3195. [Google Scholar] [CrossRef]
- Abdullah Al-Faris, N. Nutritional Evaluation of Selected Traditional Foods Commonly Consumed in Saudi Arabia. J. Food Nutr. Res. 2017, 5, 168–175. [Google Scholar] [CrossRef]
- Pachón, H.; Stoltzfus, R.J.; Glahn, R.P. Chicken thigh, chicken liver, and iron-fortified wheat flour increase iron uptake in an in vitro digestion/Caco-2 cell model. Nutr. Res. 2008, 28, 851–858. [Google Scholar] [CrossRef]
| Processing of Chickpeas | Formulation of Hummus | ||||||
|---|---|---|---|---|---|---|---|
| HDPF Samples | Pre-Cooking | with Bicarbonate | Cooking | Seed Coat | Tahini | Lemon Juice | Garlic |
| I | +1 | +1 | +1 | −1 | +1 | −1 | −1 |
| II | −1 | +1 | +1 | +1 | −1 | +1 | −1 |
| III | −1 | −1 | +1 | +1 | +1 | −1 | +1 |
| IV | +1 | −1 | −1 | +1 | +1 | +1 | −1 |
| V | −1 | +1 | −1 | −1 | +1 | +1 | +1 |
| VI | +1 | −1 | +1 | −1 | −1 | +1 | +1 |
| VII | +1 | +1 | −1 | +1 | −1 | −1 | +1 |
| VIII | −1 | −1 | −1 | −1 | −1 | −1 | −1 |
| High level +1 | 48 h Germination | 1.4 g | Autoclave 120 °C, 20 min | Removed | 21.5 g | 14 g | 1 g |
| Low level −1 | 12 h Soaking | 0 g | Boiling for 90 min | Conserved | 0 g | 0 g | 0 g |
| Acids Added per 100 g Hummus | |||
|---|---|---|---|
| HDA Samples | Citric Acid (4 mmol) | Malic Acid (4 mmol) | Ascorbic Acid (4 mmol) |
| 1 | −1 | −1 | −1 |
| 2 | +1 | −1 | −1 |
| 3 | −1 | +1 | −1 |
| 4 | +1 | +1 | −1 |
| 5 | −1 | −1 | +1 |
| 6 | +1 | −1 | +1 |
| 7 | −1 | +1 | +1 |
| 8 | +1 | +1 | +1 |
| High Level +1 | Added | Added | Added |
| Low Level −1 | Not added | Not added | Not added |
| HDPF Samples | Ingredients Added to Chickpeas | Total Fe (mg/100 g) | Dialyzable Fe (mg/100 g) | Bioaccessibility of Fe (%) |
|---|---|---|---|---|
| I | Chp + T | 4.67 ± 0.43 a | 0.19 ± 0.01 de | 4.12 ± 0.13 de |
| II | Chp + LJ | 4.39 ± 0.31 a | 1.32 ± 0.02 a | 30.17 ± 0.51 a |
| III | Chp + T + G | 5.02 ± 0.42 a | 0.25 ± 0.01 cd | 5.00 ± 0.13 d |
| IV | Chp + T+ LJ | 4.25 ± 0.32 a | 0.35 ± 0.01 c | 8.23 ± 0.35 c |
| V | Chp + T + LJ + G | 4.60 ± 0.32 a | 0.63 ± 0.02 b | 13.60 ± 0.39 b |
| VI | Chp + LJ + G | 4.70 ± 0.35 a | 1.33 ± 0.06 a | 28.44 ± 1.3 a |
| VII | Chp + G | 4.47 ± 0.26 a | 0.24 ± 0.01 cde | 5.36 ± 0.26 d |
| VIII | Chp | 5.52 ± 0.44 a | 0.13 ± 0.01 e | 2.44 ± 0.09 e |
| HDA Samples | Acids Added to Hummus without Lemon Juice | Total Fe (mg/100 g ChpTG) | Dialyzable Fe (mg/100 g) | Fe Bioaccessibility (%) |
|---|---|---|---|---|
| 1 | ChpTG | 2.24 ± 0.02 | 0.10 ± 0.1 f | 4.50 ± 0.43 f |
| 2 | ChpTG + CA | 2.24 ± 0.02 | 0.42 ± 0.01 cd | 18.92 ± 0.30 cd |
| 3 | ChpTG + MA | 2.24 ± 0.02 | 0.38 ± 0.0 d | 17.15 ± 0.08 d |
| 4 | ChpTG + CA + MA | 2.24 ± 0.02 | 0.51 ± 0.02 ab | 22.61 ± 0.93 ab |
| 5 | ChpTG + AA | 2.24 ± 0.02 | 0.31 ± 0.02 e | 14.02 ± 0.67 e |
| 6 | ChpTG + CA + AA | 2.24 ± 0.02 | 0.54 ± 0.01 a | 24.21 ± 0.66 a |
| 7 | ChpTG + AA + MA | 2.24 ± 0.02 | 0.38 ± 0.01 d | 16.79 ± 0.29 d |
| 8 | ChpTG + CA + MA + AA | 2.24 ± 0.02 | 0.46 ± 0.0 bc | 20.47 ± 0.08 bc |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doumani, N.; Severin, I.; Dahbi, L.; Bou-Maroun, E.; Tueni, M.; Sok, N.; Chagnon, M.-C.; Maalouly, J.; Cayot, P. Lemon Juice, Sesame Paste, and Autoclaving Influence Iron Bioavailability of Hummus: Assessment by an In Vitro Digestion/Caco-2 Cell Model. Foods 2020, 9, 474. https://doi.org/10.3390/foods9040474
Doumani N, Severin I, Dahbi L, Bou-Maroun E, Tueni M, Sok N, Chagnon M-C, Maalouly J, Cayot P. Lemon Juice, Sesame Paste, and Autoclaving Influence Iron Bioavailability of Hummus: Assessment by an In Vitro Digestion/Caco-2 Cell Model. Foods. 2020; 9(4):474. https://doi.org/10.3390/foods9040474
Chicago/Turabian StyleDoumani, Nour, Isabelle Severin, Laurence Dahbi, Elias Bou-Maroun, Maya Tueni, Nicolas Sok, Marie-Christine Chagnon, Jacqueline Maalouly, and Philippe Cayot. 2020. "Lemon Juice, Sesame Paste, and Autoclaving Influence Iron Bioavailability of Hummus: Assessment by an In Vitro Digestion/Caco-2 Cell Model" Foods 9, no. 4: 474. https://doi.org/10.3390/foods9040474
APA StyleDoumani, N., Severin, I., Dahbi, L., Bou-Maroun, E., Tueni, M., Sok, N., Chagnon, M.-C., Maalouly, J., & Cayot, P. (2020). Lemon Juice, Sesame Paste, and Autoclaving Influence Iron Bioavailability of Hummus: Assessment by an In Vitro Digestion/Caco-2 Cell Model. Foods, 9(4), 474. https://doi.org/10.3390/foods9040474






