The Effect of Sprouting in Lentil (Lens culinaris) Nutritional and Microbiological Profile
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant material and Seed Germination
2.2. Nutritional Analysis
2.3. Preparation of Inocula and Seed Inoculation
2.4. Seed Contamination Evaluation
2.5. Seed and Seed Sprout Disinfection Methods
2.6. Statistical Analysis
3. Results and Discussion
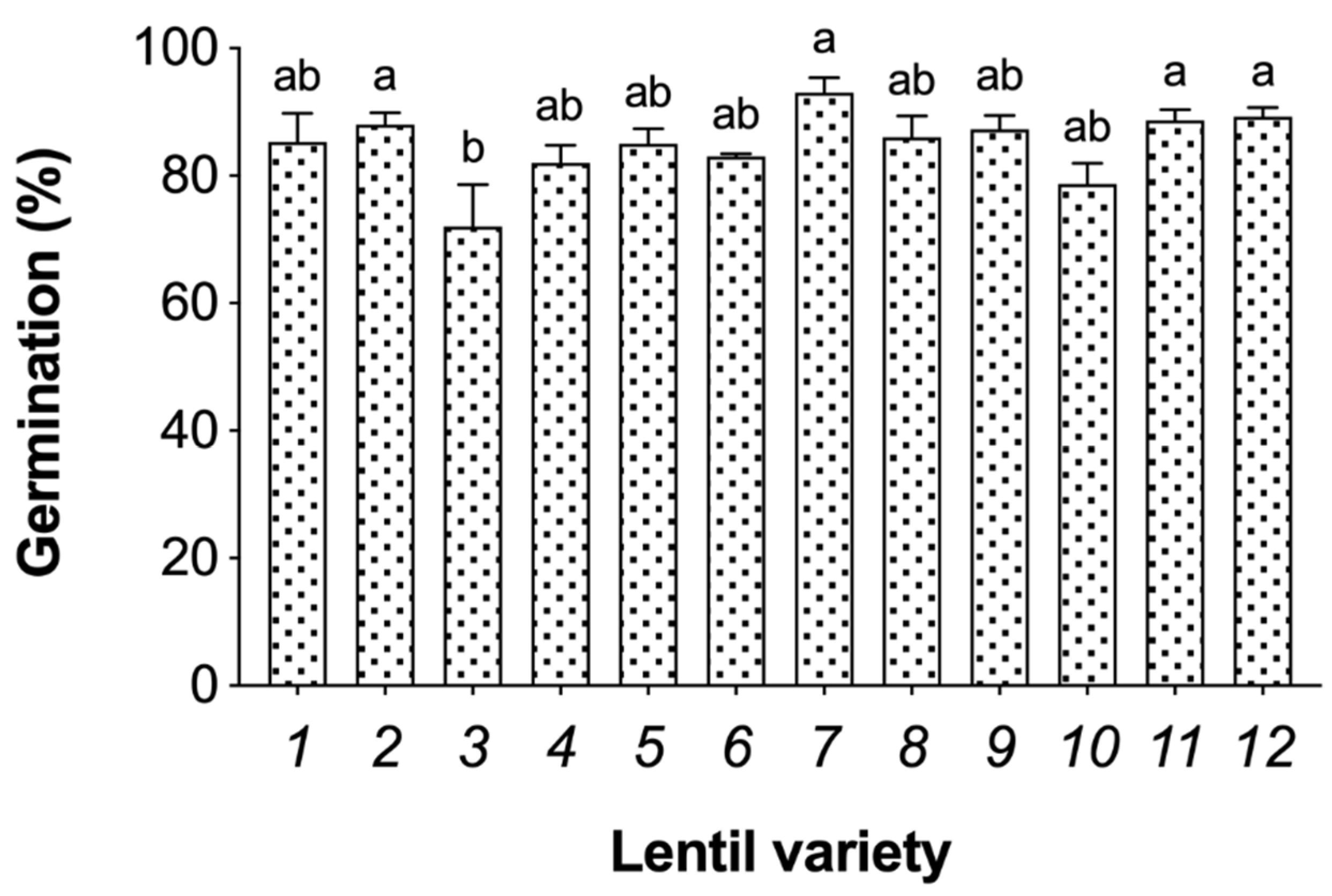
3.1. Germination Efficiency
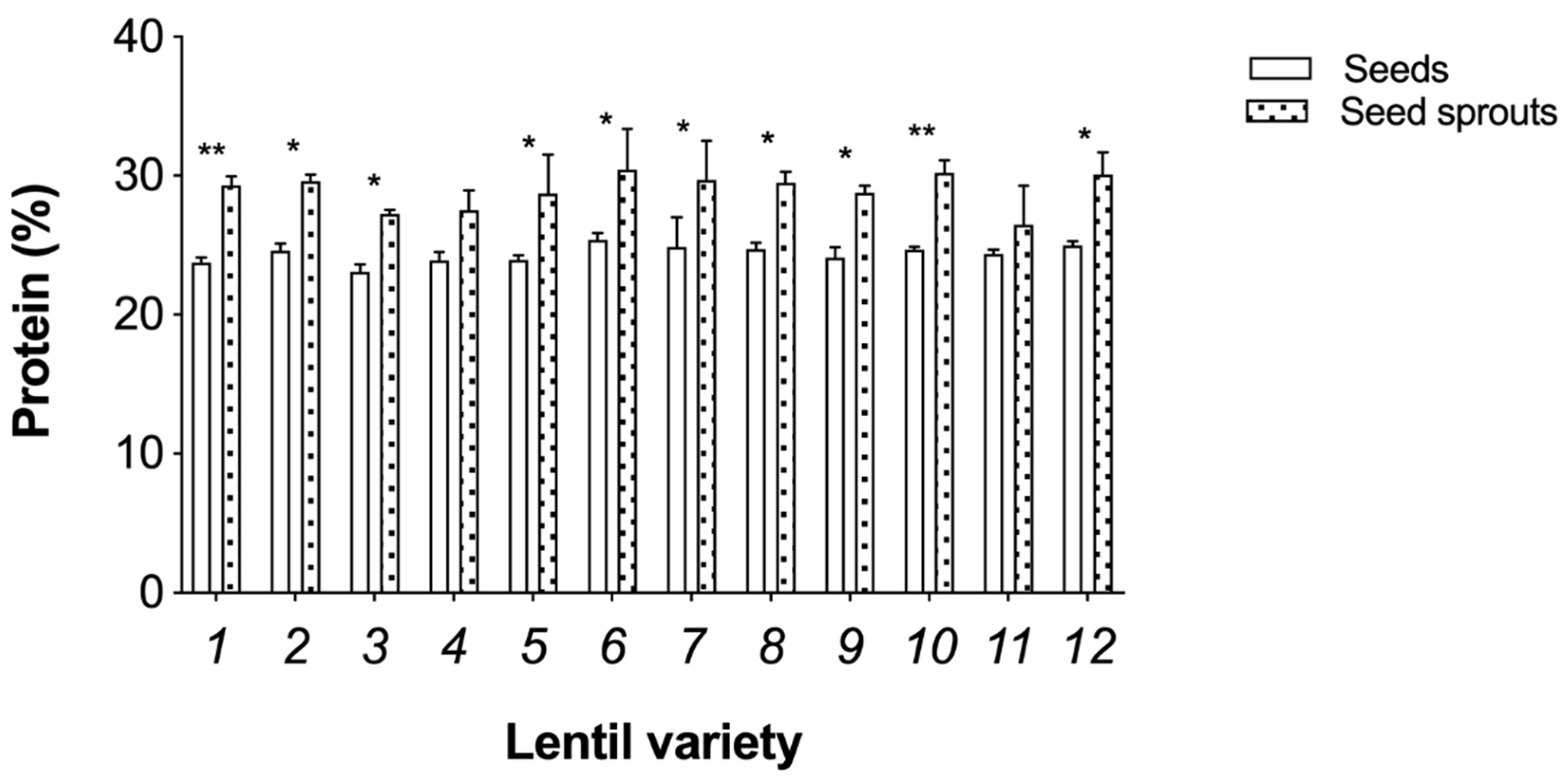
3.2. Nutritional Analysis
3.3. Microbial Counting and Disinfecting Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Magkos, F.; Tetens, I.; Bugel, S.G.; Felby, C.; Schacht, S.R.; Hill, J.O.; Ravussin, E.; Astrup, A. A perspective on the transition to plant-based diets: A diet change may attenuate climate change, but can it also attenuate obesity and chronic disease risk? Adv. Nutr. 2019, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted grains: A comprehensive review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [PubMed]
- Hoover, R.; Zhou, Y. In vitro and in vivo hydrolysis of legume starches by α-amylase and resistant starch formation in legumes—A review. Carbohydr. Polym. 2003, 54, 401–417. [Google Scholar] [CrossRef]
- Bari, M.L.; Nei, D.; Enomoto, K.; Todoriki, S.; Kawamoto, S. Combination Treatments for Killing Escherichia coli O157:H7 on Alfalfa, Radish, Broccoli, and Mung Bean Seeds. J. Food Prot. 2009, 72, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Thavarajah, D.; Thavarajah, P.; Wejesuriya, A.; Rutzke, M.; Glahn, R.P.; Combs, G.F.; Vandenberg, A. The potential of lentil (Lens culinaris L.) as a whole food for increased selenium, iron, and zinc intake: Preliminary results from a 3 year study. Euphytica 2011, 180, 123–128. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K. Phenolic substance characterization and chemical and cell-based antioxidant activities of 11 lentils grown in the Northern United States. J. Agric. Food Chem. 2010, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Khazaei, H.; Subedi, M.; Nickerson, M.; Martínez-Villaluega, C.; Frias, J.; Vandenberg, A. Seed protein of lentils: Current status, progress, and food applications. Foods 2019, 8, 391. [Google Scholar] [CrossRef]
- Siva, N.; Johnson, C.R.; Richard, V.; Jesch, E.D.; Whiteside, W.; Abood, A.A.; Thavarajah, P.; Duckett, S.; Thavarajah, D. Lentil (Lens culinaris Medikus) diet affects the gut microbiome and obesity markers in rat. J. Agric. Food Chem. 2018, 66, 8805–8813. [Google Scholar] [CrossRef]
- García-Mora, P.; Martín-Martínez, M.; Bonache, M.A.; González-Múniz, R.; Peñas, E.; Frias, J.; Martinez-Villaluenga, C. Identification, functional grastrointestinal stability and molecular docking studies of lentil peptides with dual antioxidant and angiotensin I converting enzyme inhibitory activities. Food Chem. 2017, 221, 464–472. [Google Scholar] [CrossRef]
- EFSA. EFSA Assesses the Public Health Risk of Seeds and Sprouted Seeds. Available online: https://www.efsa.europa.eu/en/press/news/111115 (accessed on 10 February 2020).
- Kintz, E.; Byrne, L.; Jenkins, C.; McCarthy, N.; Vivancos, R.; Hunter, P. Outbreaks of Shiga toxin-producing Escherichia coli linked to sprouted seeds, salad, and leafy greens: A systematic review. J. Food Prot. 2019, 82, 1950–1958. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Reducing Microbial Food Safety Hazards in the Production of Seed for Sprouting: Guidance for Industry; Office of Food Safet: College Park, MD, USA, 2019. [Google Scholar]
- Scouten, A.; Beuchat, L. Combined effects of chemical, heat and ultrasound treatments to kill Salmonella and Escherichia coli O157:H7 on alfalfa seeds. J. Appl. Microbiol. 2001, 92, 668–674. [Google Scholar] [CrossRef] [PubMed]
- CIDRAP. Sprouts Blamed in Big Ontario Salmonella Outbreak. Regents of the University of Minesotta. Available online: http://www.cidrap.umn.edu/news-perspective/2005/12/sprouts-blamed-big-ontario-salmonella-outbreak (accessed on 10 February 2020).
- Bergspica, I.; Ozola, A.; Miltina, E.; Alksne, L.; Meistere, I.; Cibrovska, A.; Grantina-Ievina, L. Occurence of pathogenic and potentially pathogenic bacteria in microgreens, sprouts, and sprouted seeds on retail market in Riga, Latvia. Foodborne Pathog. Dis. 2020, 17, 6. [Google Scholar]
- Ding, H.; Fu, T.; Smith, M.A. Microbial contamination in sprouts: How effective is seed disinfection treatment? J. Food Sci. 2013, 78, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Standard Operating Procedure (SOP) for Laboratory Disinfection New Orleans LSU Health. 2013. Available online: https://www.lsuhsc.edu/admin/pfm/ehs/docs/decon.pdf (accessed on 4 April 2019).
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Shanmugam, V.; Wang, Y.W.; Tsednee, M.; Karunakaran, K.; Yeh, K.C. Glutathione plays an essential role in nitric oxide-mediated iron-deficiency signaling and iron-deficiency tolerance in Arabidopsis. Plant J. 2015, 84, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.S.; Roriz, M.; Carvalho, S.M.P.; Vasconcelos, M.W. Iron partitioning at an early growth stage impacts iron deficiency responses in soybean plants (Glycine max L.). Front. Plant Sci. 2015, 6, 325. [Google Scholar] [CrossRef]
- GraphPad. Available online: https://www.graphpad.com/scientific-software/prism/ (accessed on 21 March 2020).
- Xu, M.; Jin, Z.; Simsek, S.; Hall, C.; Rao, J.; Chen, B. Effect of germination on the chemical composition, thermal, pasting, and moisture sorption properties of flours from chickpea, lentil, and yellow pea. Food Chem. 2019, 295, 579–587. [Google Scholar] [CrossRef]
- Santos, C.S.; Carbas, B.; Castanho, A.; Bronze, M.R.; Serrano, C.; Vasconcelos, M.W.; Vaz Patto, M.C.; Brites, C. Relationship between seed traits and pasting and cooking behavior in a pulse germplasm collection. Crop Pasture Sci. 2018, 69, 892–903. [Google Scholar] [CrossRef]
- Santos, C.S.; Carbas, B.; Castanho, A.; Vasconcelos, M.W.; Vaz Patto, M.C.; Bomoney, C.; Brites, C. Variation in pea (Pisum sativum L.) seed quality traits defined by physicochemical functional properties. Foods 2019, 8, 570. [Google Scholar] [CrossRef]
- Fouad, A.A.; Rehab, F.M.A. Effect of germination time on proximate analysis, bioactive compounds and antioxidant activity of lentil (Lens culinaris Medik.) sprouts. Acta Sci. Pol. Technol. Aliment. 2015, 14, 233–246. [Google Scholar] [CrossRef]
- Márton, M.; Mándoki, Z.; Csapó-Kiss, Z.; Csapó, J. The role of sprouts in human nutrition. A review. Acta Univ. Sapientiae, Aliment. 2010, 3, 81–117. [Google Scholar]
- Zielinska-Dawidziak, M.; Staniek, H.; Król, E.; Piasecka-Kwiatkowska, D.; Twardowski, T. Legume seeds and cereals grains’ capacity to accumulate iron while sprouting in order to obtain food fortificant. Acta Sci. Pol. Technol. Aliment. 2016, 15, 333–338. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, Y.; Xie, W.; Jin, X.; Wang, Q.; He, Y. Effects of germination on iron, zinc, calcium, manganese, and copper availability from cereals and legumes. CyTA J. Food 2014, 12, 22–26. [Google Scholar] [CrossRef]
- Plaza, L.; Ancos, B.; Cano, M.P. Nutritional and health-related compounds in sprouts and seeds of soybean (Glycine max), wheat (Triticum aestivum L.) and alfalfa (Medicago sativa) treated by a new drying method. Eur. Food Res. Technol. 2003, 216, 138–144. [Google Scholar] [CrossRef]
- Mwikya, S.M.; Camp, J.V.; Rodriguez, R.; Huyghebaert, A. Effects of sprouting on nutrient and antinutrient composition of kidney beans (Phaseolus vulgaris var. Rose coco). Eur. Food Res. Technol. 2001, 212, 188–191. [Google Scholar]
- Devi, C.B.; Kushwaha, A.; Kumar, A. Sprouting characteristics and associated changes in nutritional composition of cowpea (Vigna unguiculata). J. Food Sci. Technol. 2015, 52, 6821–6827. [Google Scholar] [CrossRef]
- Zerche, S.; Ewald, A. Seed potassium concentration decline during maturation is inversely related to subsequent germination of primrose. J. Plant Nutr. 2005, 28, 573–603. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Nahar, K.; Hossain, M.S.; Mahmud, J.A.; Hossen, M.S.; Masud, A.A.C.; Moumita; Fujita, M. Potassium: A vital regulator of plant responses and tolerance to abiotic stresses. Agronomy 2018, 8, 31. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Guidance for Industry: Reducing Microbial Food Safety Hazards for Sprouted Seeds; Office of Food Safety: College Park, MD, USA, 1999.
- Neurath, H.; Greenstein, J.P.; Putnam, F.W.; Erickson, J.A. The Chemistry of Protein Denaturation. Chem. Rev. 1944, 34, 157–265. [Google Scholar] [CrossRef]
- Theitler, D.; Nasser, A.; Gerchman, Y.; Kribus, A.; Mamane, H. Synergistic effect of heat and solar UV on DNA damage and water disinfection of E. coli and bacteriophage MS2. J. Water Health 2012, 10, 605–618. [Google Scholar] [CrossRef]
- Barampuram, S.; Allen, G.; Krasnyanski, S. Effect of various sterilization procedures on the in vitro germination of cotton seeds. Plant Cell Tissue Org. 2014, 118, 179–185. [Google Scholar] [CrossRef]
| Lentil Varieties | Nutrient Concentration (µg/g) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | p Value | Zn | p Value | Mn | p Value | Mg | p Value | Ca | p Value | K | p Value | ||
| Dunkelgrün Marmorierte | Seeds | 38 ± 0.3 | n.s. | 31 ± 0.07 | <0.0001 | 11 ± 0.4 | n.s. | 10005 ± 23 | n.s. | 681 ± 23 | 0.0013 | 9994 ± 308 | n.s. |
| Sprouts | 37 ± 0.1 | 40 ± 0.9 | 13 ± 1.8 | 952 ± 11 | 1079 ± 73 | 12,130 ± 372 | |||||||
| Du Puy | Seeds | 37 ± 0.9 | n.s. | 30 ± 0.04 | <0.0001 | 11 ± 0.07 | <0.0001 | 991 ± 22 | n.s. | 619 ± 36 | 0.0109 | 9730 ± 303 | n.s. |
| Sprouts | 39 ± 0.3 | 36 ± 0.2 | 23 ±4.1 | 891 ± 18 | 964 ± 36 | 10,023 ± 187 | |||||||
| Thessalia | Seeds | 50 ± 0.2 | n.s. | 49 ± 0.1 | n.s. | 17 ± 0.2 | n.s. | 1148 ± 48 | n.s. | 933 ± 40 | n.s. | 10,769 ± 889 | n.s. |
| Sprouts | 53 ± 0.5 | 48 ± 0.4 | 18 ± 0.5 | 1247 ± 104 | 774 ± 66 | 10,830 ± 394 | |||||||
| Dimitra | Seeds | 59 ± 0.3 | n.s. | 42 ± 0.2 | n.s. | 13 ± 0.3 | n.s. | 1155 ± 101 | n.s. | 1040 ± 38 | n.s. | 10,296 ± 641 | n.s. |
| Sprouts | 63 ± 0.8 | 44 ± 0.6 | 14 ± 0.8 | 928 ± 25 | 866 ± 35 | 10,830 ± 705 | |||||||
| Samos | Seeds | 53 ± 0.2 | <0.0001 | 39 ± 0.3 | <0.0001 | 13 ± 0.2 | n.s. | 1067 ± 36 | n.s. | 999 ± 64 | n.s. | 9623 ± 303 | n.s. |
| Sprouts | 34 ± 1.6 | 48 ± 0.8 | 14 ± 0.6 | 1039 ± 45 | 1138 ± 52 | 12,299 ± 383 | |||||||
| Kleine Schwarze | Seeds | 47 ± 0.3 | n.s. | 44 ± 0.6 | <0.0001 | 14 ± 0.3 | n.s. | 1201 ± 83 | n.s. | 754 ± 70 | 0.0350 | 11,050 ± 375 | n.s. |
| Sprouts | 50 ± 1.7 | 51 ± 0.3 | 10 ± 0.4 | 1043 ± 25 | 1067 ± 68 | 10,292 ± 169 | |||||||
| Rosana | Seeds | 68 ± 1.5 | n.s. | 35 ± 0.5 | n.s. | 12 ± 0.5 | 0.0082 | 947 ± 70 | n.s. | 775 ± 62 | 0.0026 | 10,149 ± 68 | 0.0419 |
| Sprouts | 64 ± 3.1 | 38 ± 0.6 | 20 ± 0.8 | 1017 ± 52 | 1156 ± 113 | 13,154 ± 1043 | |||||||
| Flora | Seeds | 51 ± 0.3 | n.s. | 37 ± 0.4 | n.s. | 12 ± 0.7 | n.s. | 1107 ± 73 | n.s. | 851 ± 62 | n.s. | 9414 ± 807 | n.s. |
| Sprouts | 52 ± 0.4 | 39 ± 0.09 | 12 ± 0.5 | 1010 ± 55 | 759 ± 55 | 11,350 ± 491 | |||||||
| Santa | Seeds | 43 ± 0.4 | n.s. | 36 ± 1.5 | n.s. | 13 ± 0.9 | n.s. | 1168 ± 67 | n.s. | 766 ± 4 | n.s. | 10,905 ± 668 | n.s. |
| Sprouts | 43 ± 0.3 | 36 ± 0.2 | 16 ± 0.6 | 1044 ± 72 | 668 ± 30 | 10,128 ± 192 | |||||||
| Große Rote | Seeds | 51 ± 0.2 | 0.0374 | 38 ± 1.4 | 0.0186 | 15 ± 2 | 0.0079 | 1006 ± 47 | n.s. | 864 ± 52 | n.s. | 10,905 ± 668 | n.s. |
| Sprouts | 45 ± 0.5 | 42 ± 1.1 | 23 ± 0.6 | 1061 ± 27 | 980 ± 35 | 10,128 ± 192 | |||||||
| Kleine Rote | Seeds | 66 ± 0.2 | n.s. | 43 ± 0.3 | 0.0195 | 17 ± 0.6 | 0.0167 | 1084 ± 17 | n.s. | 1062±70 | n.s. | 10,423 ± 849 | n.s. |
| Sprouts | 63 ± 2.2 | 47 ± 1.1 | 25 ± 1.1 | 1048 ± 28 | 1146 ± 62 | 10,216 ± 308 | |||||||
| Kleine Späths II | Seeds | 44 ± 0.2 | 0.0152 | 38 ± 0.2 | <0.0001 | 11 ± 1.2 | n.s. | 1224 ± 121 | n.s. | 623 ± 52 | n.s. | 10,121 ± 211 | n.s. |
| Sprouts | 38 ± 0.9 | 47 ± 0.1 | 16 ± 1.5 | 1075 ± 54 | 803 ± 21 | 10,473 ± 425 | |||||||
| Treatments | % Germination | ||
|---|---|---|---|
| E. coli | Salmonella spp. | Control | |
| No disinfection/without inoculation | - | - | 98.8 |
| No disinfection/after inoculation | 99.3 | 96.9 | - |
| SDS disinfection | 96.3 | 96.0 | 97.3 |
| Water (80 °C) disinfection | 95.5 | 65.3 | 82.3 |
| Treatments | Seeds | Sprouts | ||
|---|---|---|---|---|
| E. coli | Salmonella | E. coli | Salmonella | |
| No disinfection/without inoculation | ≤1.0 × 108 | ≤1.0 × 108 | ||
| No disinfection/with inoculation | 2.7 × 108 | 1.1 × 108 | 1.8 × 108 | 1.4 × 108 |
| SDS disinfection | 1.1 × 108 | ≤1.0× 108 | 11.4 × 108 | 2.7 × 108 |
| Water (80 °C) disinfection | 2.7 × 107 | >3.0 × 108 | 2.1 × 108 | 1.2 × 108 |
| Water (80 °C) + H2O rinse | 9.0 × 107 | 9.4 × 107 | ||
| Water (80 °C) + Amukine | 9.8 × 107 | 8.0 × 107 | ||
| SDS + H2O rinse | 6.0 × 107 | 9.8 × 108 | ||
| SDS + Amukine | 3.9 × 106 | 5.6 × 107 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
S.Santos, C.; Silva, B.; M.P.Valente, L.; Gruber, S.; W.Vasconcelos, M. The Effect of Sprouting in Lentil (Lens culinaris) Nutritional and Microbiological Profile. Foods 2020, 9, 400. https://doi.org/10.3390/foods9040400
S.Santos C, Silva B, M.P.Valente L, Gruber S, W.Vasconcelos M. The Effect of Sprouting in Lentil (Lens culinaris) Nutritional and Microbiological Profile. Foods. 2020; 9(4):400. https://doi.org/10.3390/foods9040400
Chicago/Turabian StyleS.Santos, Carla, Beatriz Silva, Luísa M.P.Valente, Sabine Gruber, and Marta W.Vasconcelos. 2020. "The Effect of Sprouting in Lentil (Lens culinaris) Nutritional and Microbiological Profile" Foods 9, no. 4: 400. https://doi.org/10.3390/foods9040400
APA StyleS.Santos, C., Silva, B., M.P.Valente, L., Gruber, S., & W.Vasconcelos, M. (2020). The Effect of Sprouting in Lentil (Lens culinaris) Nutritional and Microbiological Profile. Foods, 9(4), 400. https://doi.org/10.3390/foods9040400






