The Effect of a New Coating on the Drying Performance of Fruit and Vegetables Products: Experimental Investigation and Artificial Neural Network Modeling
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Procedures
2.1.1. Sample Preparation
2.1.2. Osmotic Solutions
2.1.3. Osmotic Dehydration
2.2. Air Drying
2.3. C. Data Analysis
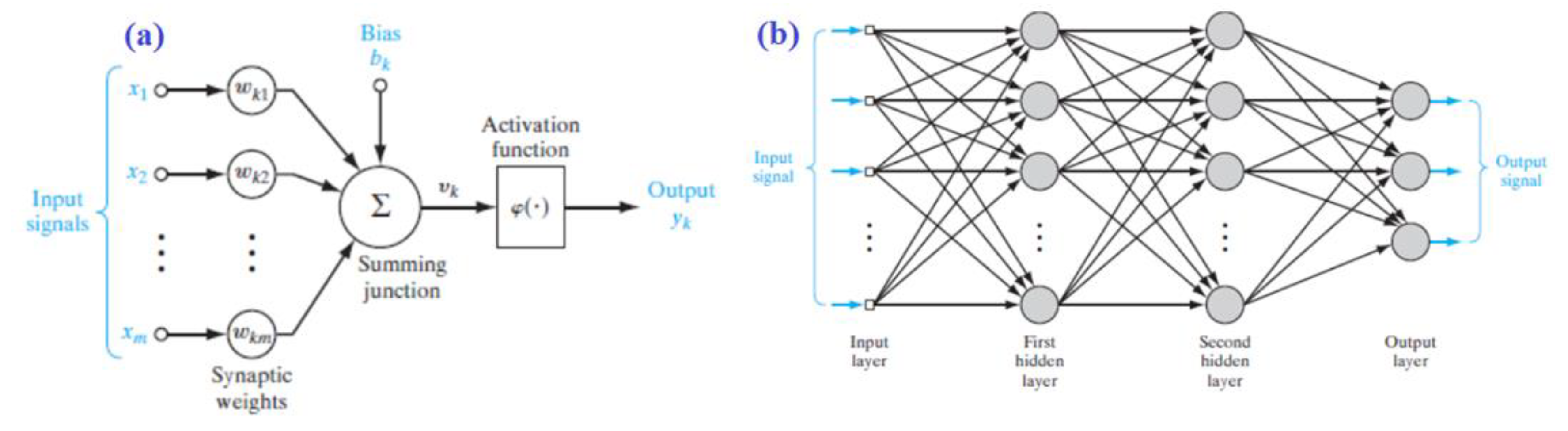
3. Artificial Neural Network (ANN)
4. Results and Discussions
4.1. Choice of Better Osmotic Solutions
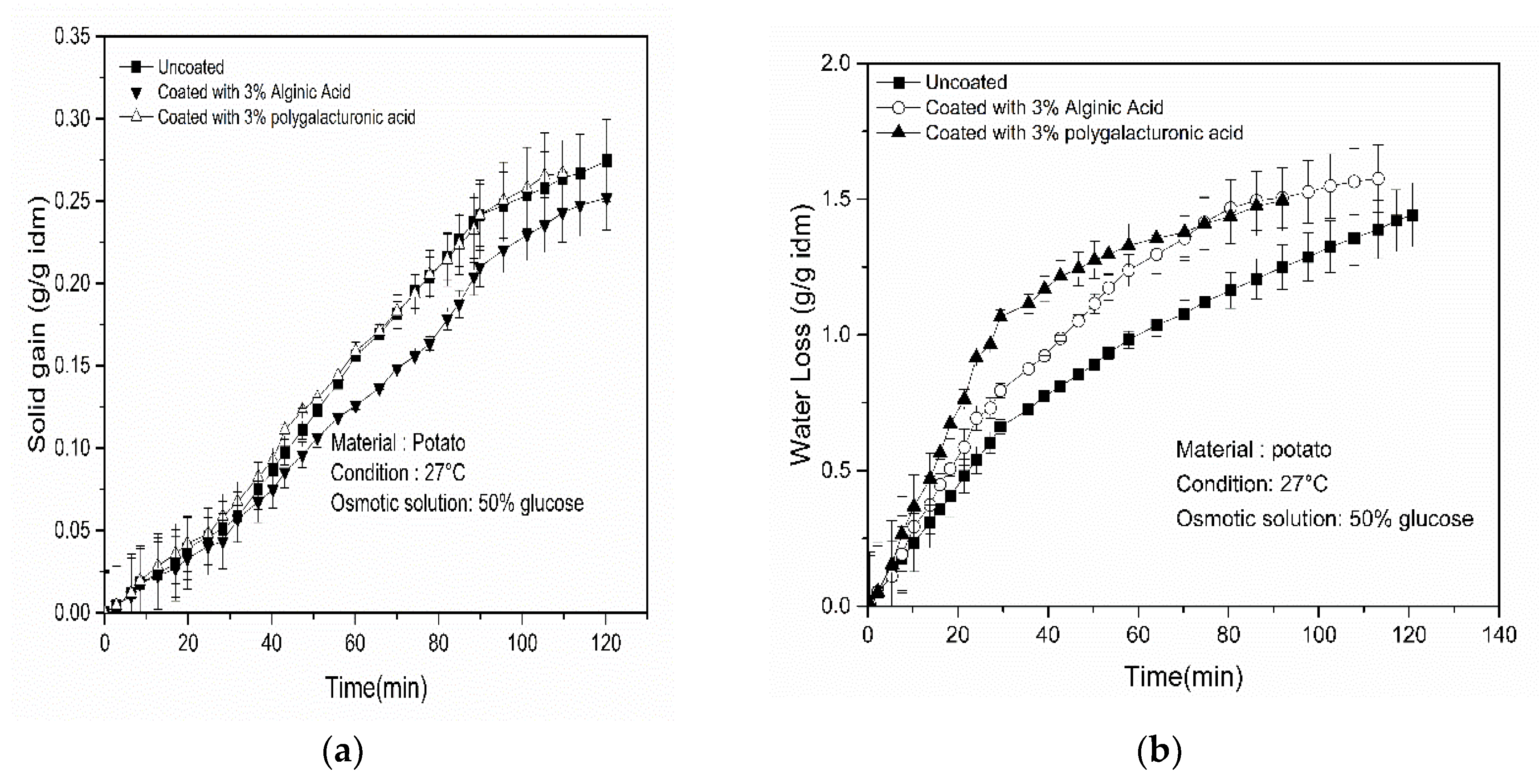
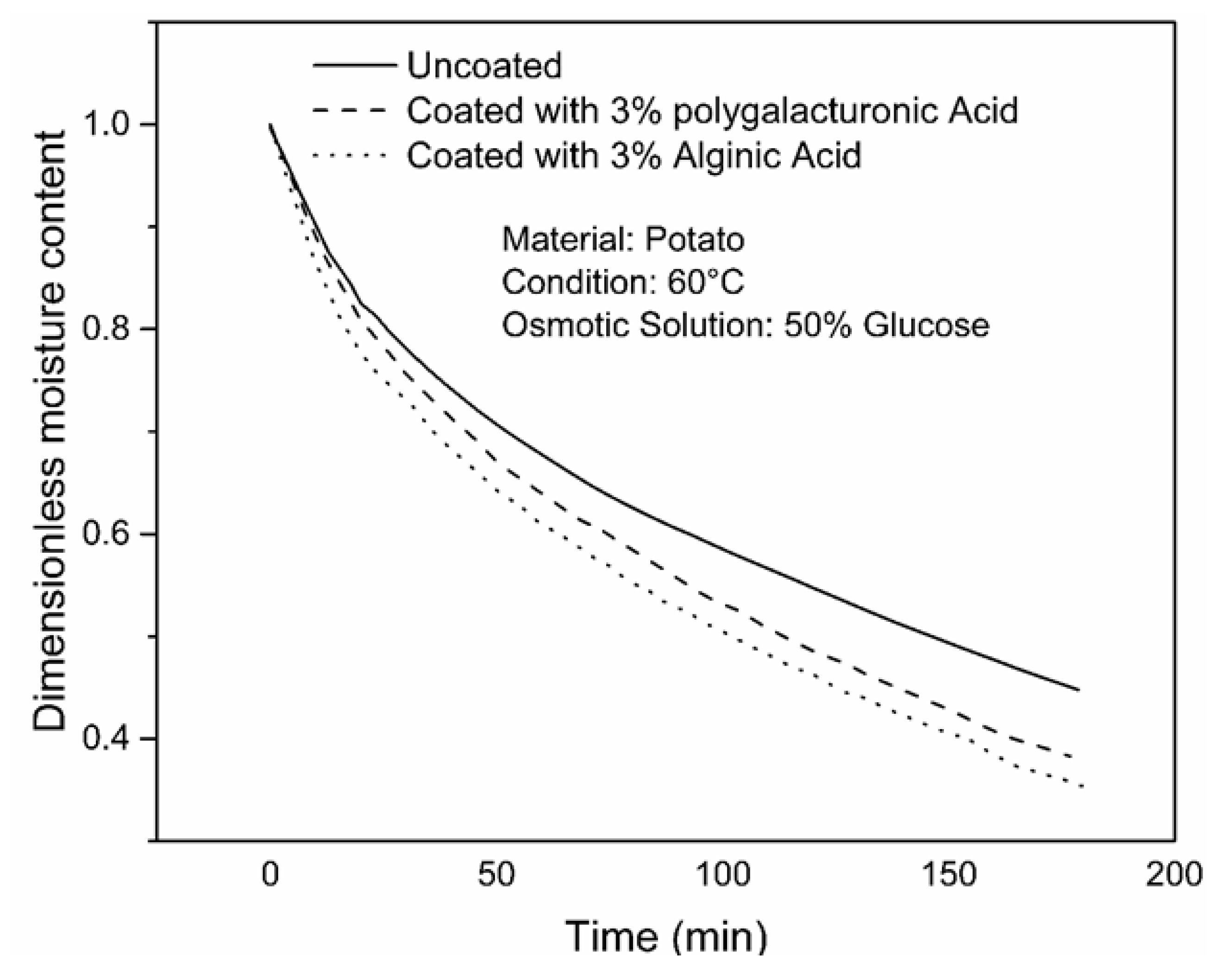
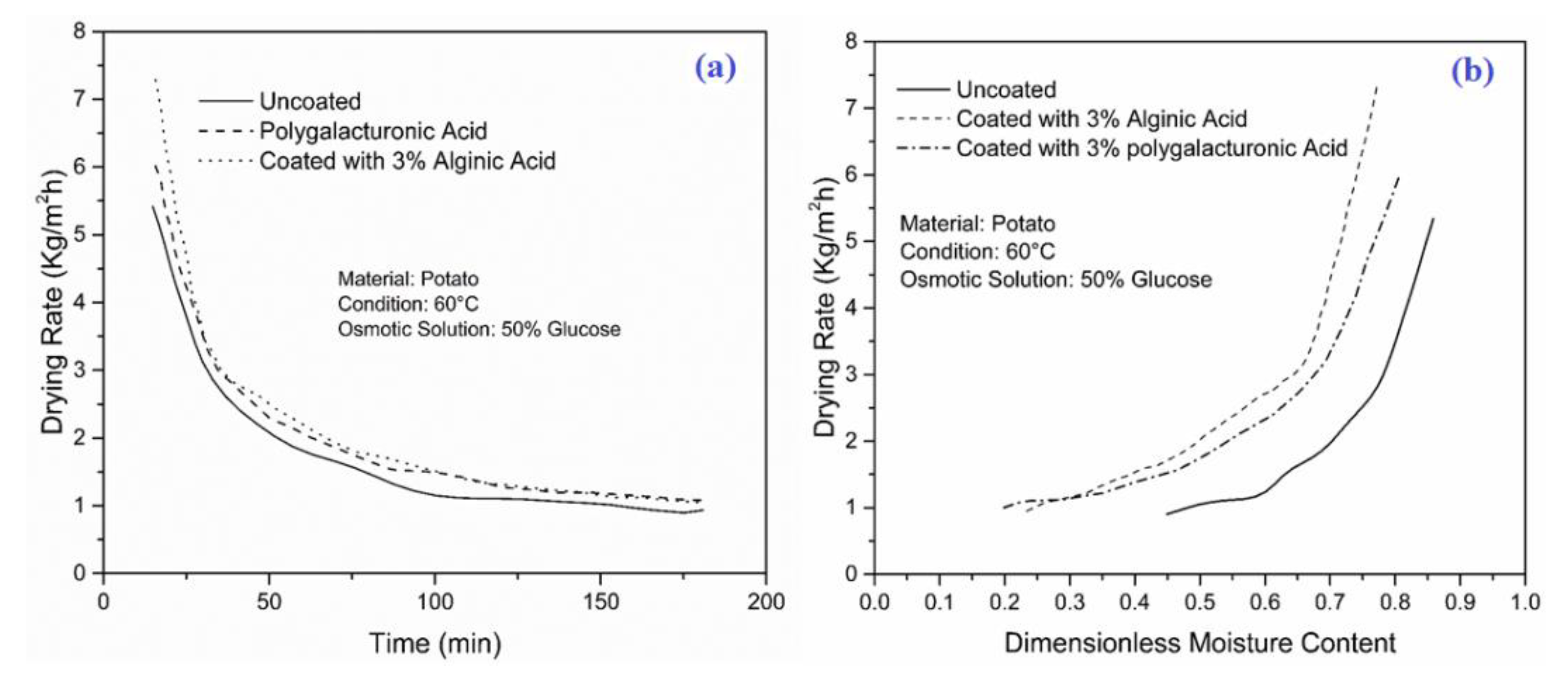
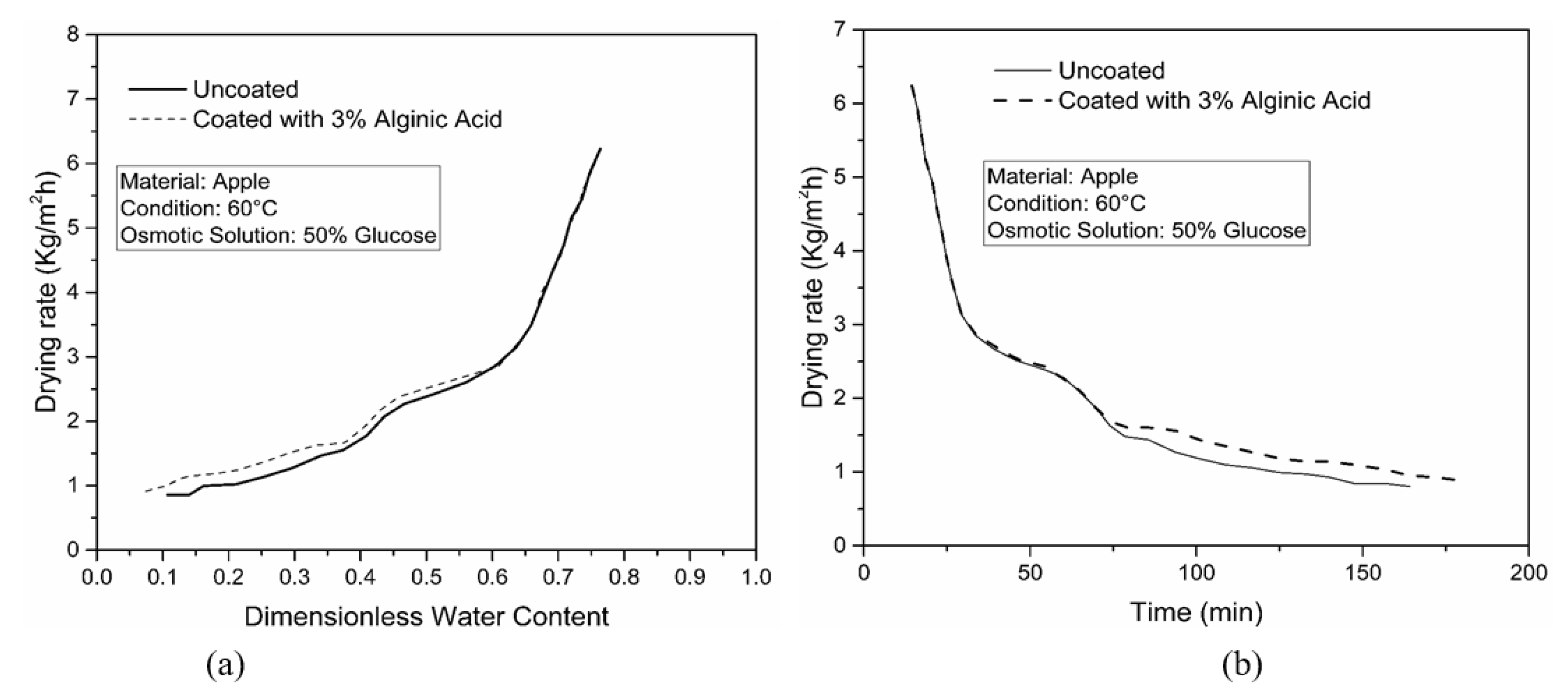
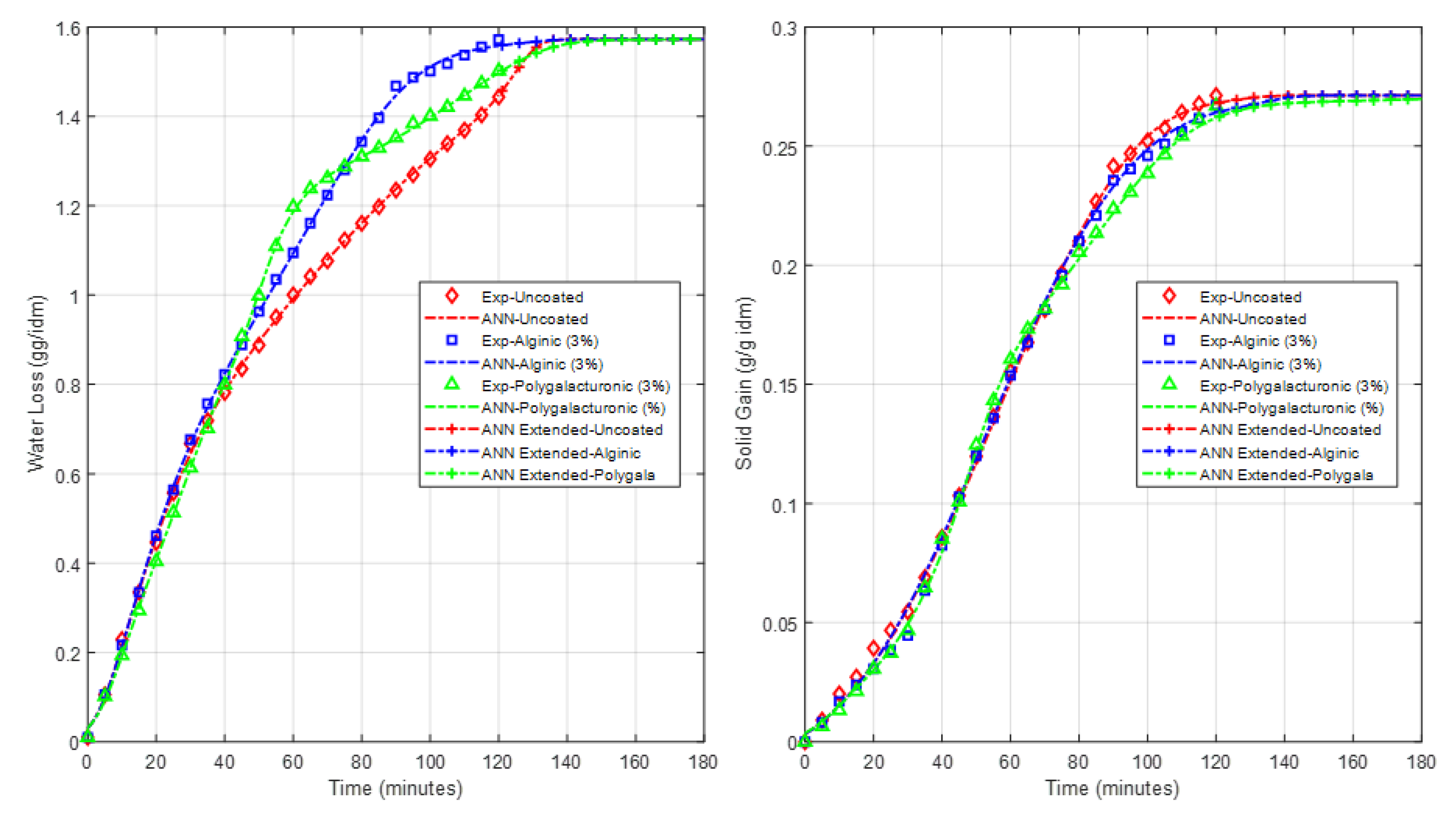
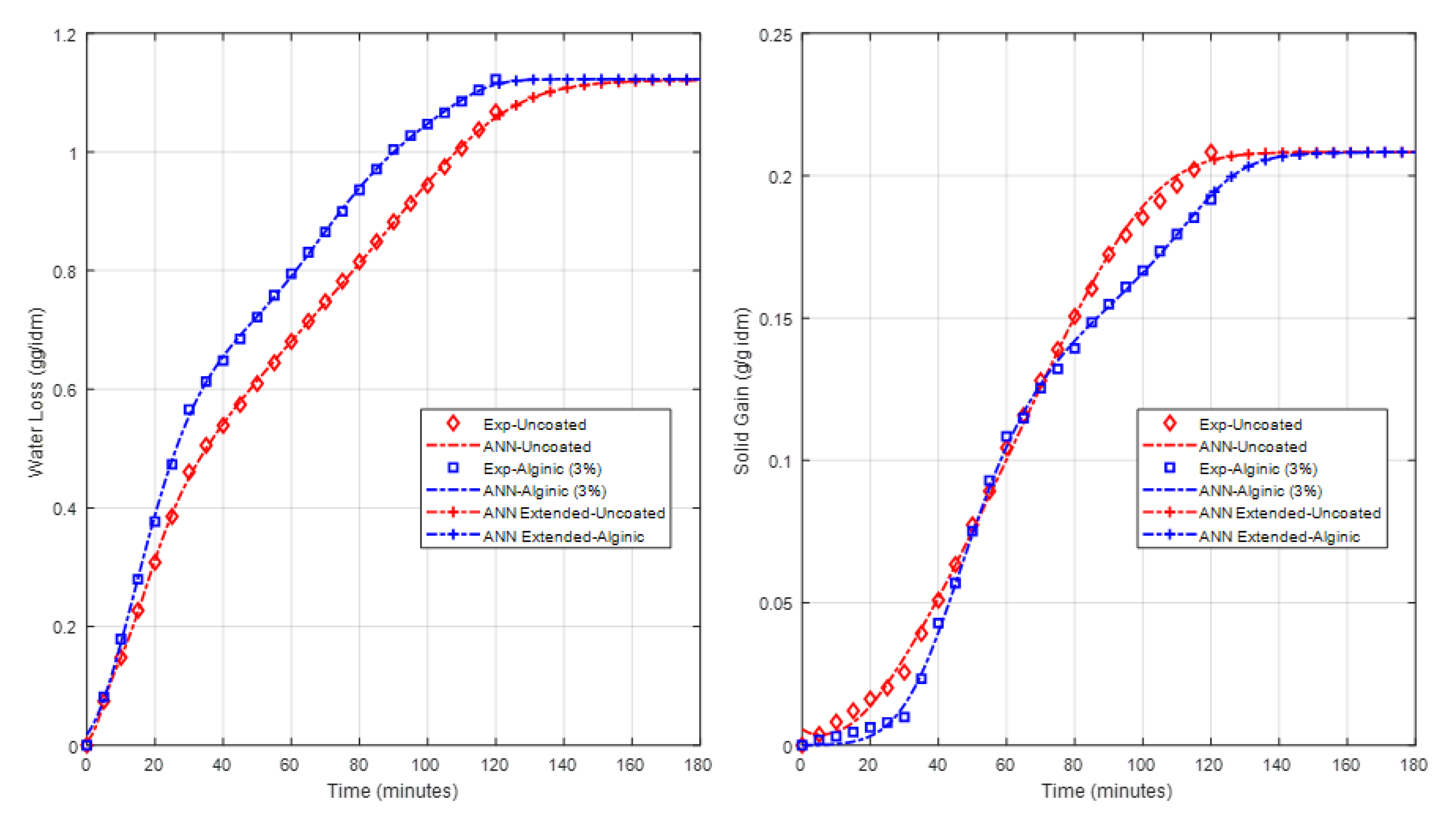
4.2. Effect of Coating on WL, SG and Drying Rate
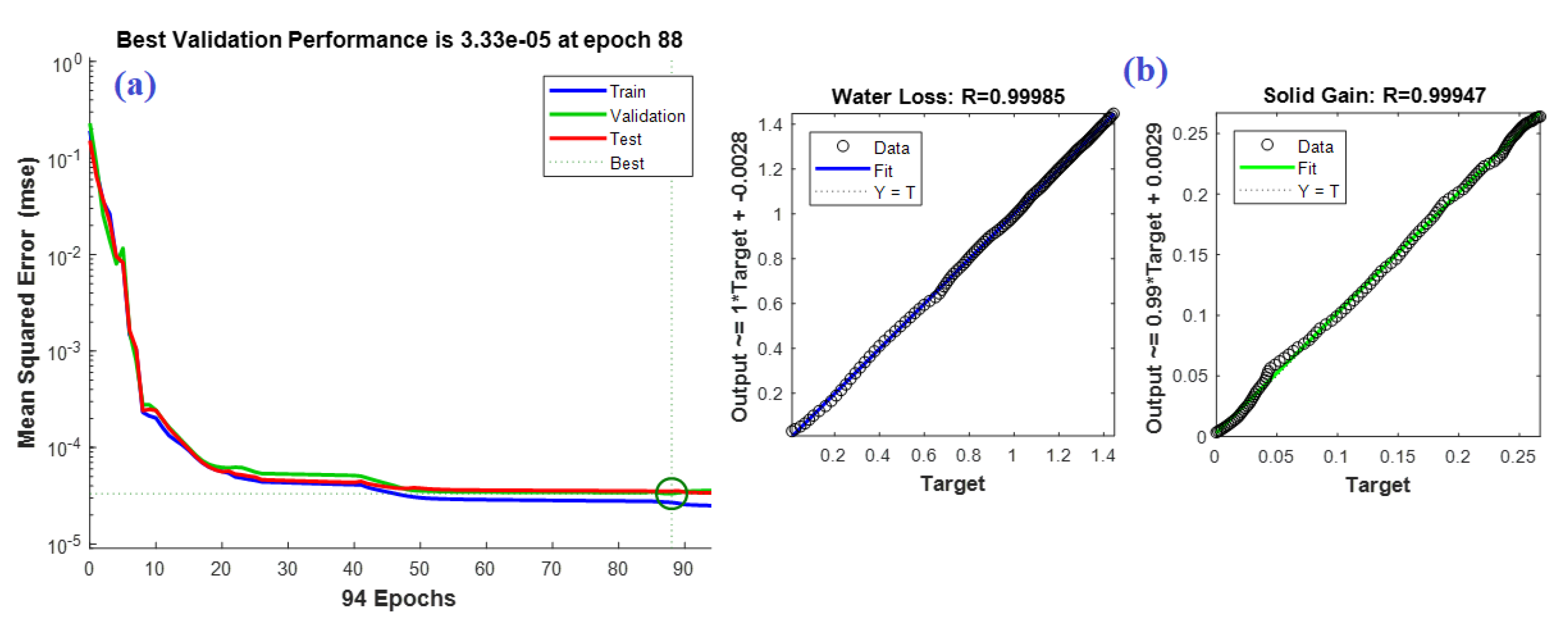
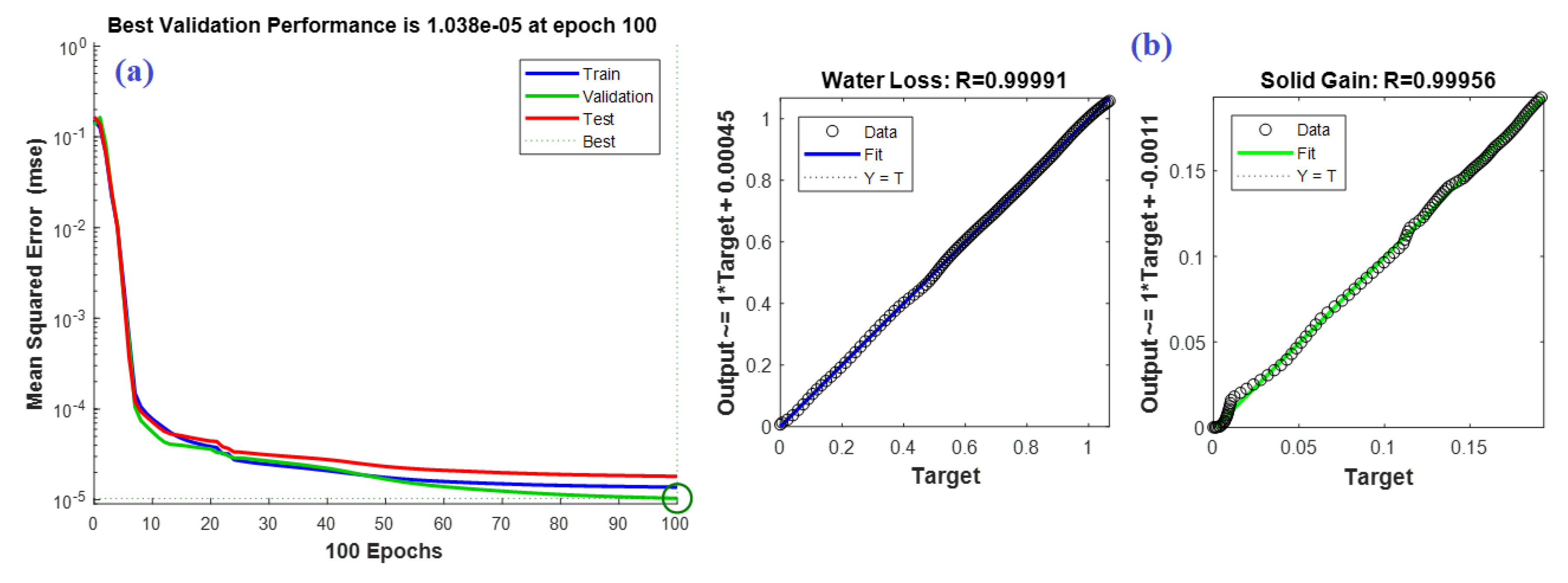
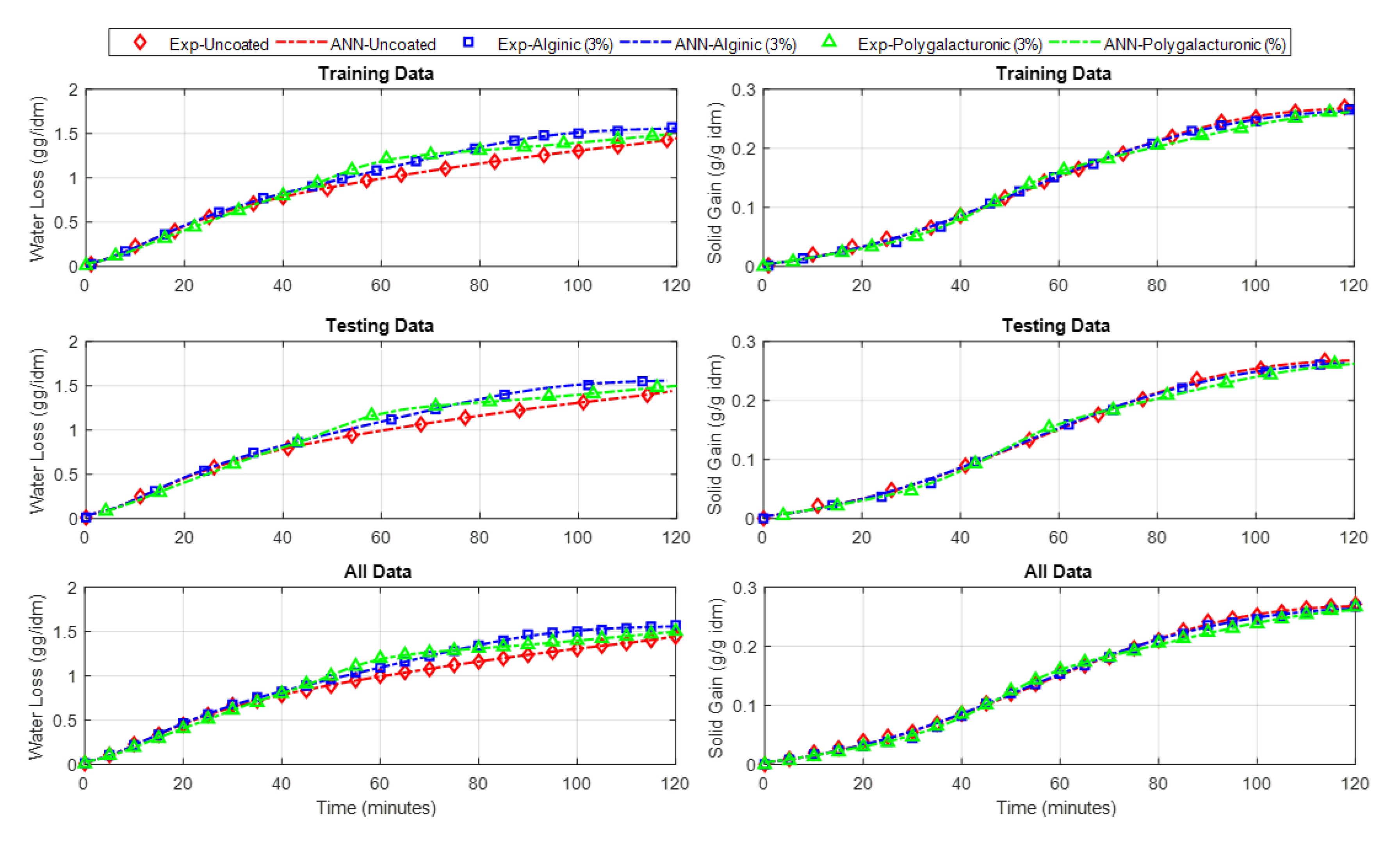
4.3. C. ANN Based Model
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kanagaratnam, S.; Hoque, M.E.; Sahri, M.M.; Spowage, A. Investigating the effect of deforming temperature on the oil-binding capacity of palm oil based shortening. J. Food Eng. 2013, 118, 90–99. [Google Scholar] [CrossRef]
- Ponting, J. Osmotic dehydration of fruits: Recent modifications and applications. Process Biochem. 1973, 18–20. [Google Scholar]
- Torreggiani, D. Osmotic dehydration in fruit and vegetable processing. Food Res. Int. 1993, 26, 59–68. [Google Scholar] [CrossRef]
- Rahman, M.S. Handbook of Food Preservation; CRC Press: New York, NY, USA, 2007; pp. 8–12. [Google Scholar]
- Pan, Y.; Zhao, L.; Zhang, Y.; Chen, G.; Mujumdar, A.S. Osmotic dehydration pretreatment in drying of fruits and vegetables. Dry. Technol. 2003, 21, 1101–1114. [Google Scholar] [CrossRef]
- Arevalo, P.; Ngadi, M.; Bazhal, M.; Raghavan, G. Impact of pulsed electric fields on the dehydration and physical properties of apple and potato slices. Dry. Technol. 2004, 22, 1233–1246. [Google Scholar] [CrossRef]
- Shynkaryk, M.; Lebovka, N.; Vorobiev, E. Pulsed electric fields and temperature effects on drying and rehydration of red beetroots. Dry. Technol. 2008, 26, 695–704. [Google Scholar] [CrossRef]
- Amami, E.; Khezami, L.; Vorobiev, E.; Kechaou, N. Effect of pulsed electric field and osmotic dehydration pretreatment on the convective drying of carrot tissue. Dry. Technol. 2008, 26, 231–238. [Google Scholar] [CrossRef]
- Rahman, S.M.A.; Hoque, M.E.; Rahman, S.; Rahman, M.M. A Novel Vortex Tube-Assisted Atmospheric Freeze-Drying System: Effect of Osmotic Pretreatment on Biological Products. J. Food Process Eng. 2017, 40, e12449. [Google Scholar] [CrossRef]
- Rahman, S.; Mujumdar, A. Effect of osmotic treatment with concentrated sugar and salt solutions on kinetics and color in vacuum contact drying. J. Food Process. Preserv. 2007, 31, 671–687. [Google Scholar] [CrossRef]
- Lenart, A.; Piotrowski, D. Drying characteristics of osmotically dehydrated fruits coated with semipermeable edible films. Dry. Technol. 2001, 19, 849–877. [Google Scholar] [CrossRef]
- Khoyi, M.R.; Hesari, J. Osmotic dehydration kinetics of apricot using sucrose solution. J. Food Eng. 2007, 78, 1355–1360. [Google Scholar] [CrossRef]
- Dehghannya, J.; Emam-Djomeh, Z.; Sotudeh-Gharebagh, R.; Ngadi, M. Osmotic dehydration of apple slices with carboxy-methyl cellulose coating. Dry. Technol. 2006, 24, 45–50. [Google Scholar] [CrossRef]
- Chandra, S.; Kumari, D. Recent development in osmotic dehydration of fruit and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 552–561. [Google Scholar] [CrossRef] [PubMed]
- McSweeney, M.; Seetharaman, K. State of polyphenols in the drying process of fruits and vegetables. Crit. Rev. Food Sci. Nutr. 2015, 55, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Peressini, D. Effects of osmotic dehydration (with and without sonication) and pectin-based coating pretreatments on functional properties and color of hot-air dried apricot cubes. Food Chem. 2020, 311, 125978. [Google Scholar] [CrossRef]
- Nottagh, S.; Hesari, J.; Peighambardoust, S.H.; Rezaei-Mokarram, R.; Jafarizadeh-Malmiri, H. Effectiveness of edible coating based on chitosan and Natamycin on biological, physico-chemical and organoleptic attributes of Iranian ultra-filtrated cheese. Biologia 2019, in press. [Google Scholar] [CrossRef]
- Silva, K.; Garcia, C.; Amado, L.; Mauro, M. Effects of edible coatings on convective drying and characteristics of the dried pineapple. Food Bioprocess Technol. 2015, 8, 1465–1475. [Google Scholar] [CrossRef]
- Dhall, R. Advances in edible coatings for fresh fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef]
- Khin, M.M.; Zhou, W.; Perera, C.O. A study of the mass transfer in osmotic dehydration of coated potato cubes. J. Food Eng. 2006, 77, 84–95. [Google Scholar] [CrossRef]
- Askari, G.; Emam-Djomeh, Z.; Mousavi, S. Effects of combined coating and microwave assisted hot-air drying on the texture, microstructure and rehydration characteristics of apple slices. Food Sci. Technol. Int. 2006, 12, 39–46. [Google Scholar] [CrossRef]
- García, M.; Díaz, R.; Martínez, Y.; Casariego, A. Effects of chitosan coating on mass transfer during osmotic dehydration of papaya. Food Res. Int. 2010, 43, 1656–1660. [Google Scholar] [CrossRef]
- Sabetghadam, M.; Tavakolipour, H. Osmo-coating and ultrasonic dehydration as pre-treatment for hot air-drying of flavored apple. Eng. Agric. Environ. Food 2015, 8, 318–327. [Google Scholar] [CrossRef]
- Ochoa-Martínez, C.; Ayala-Aponte, A. Prediction of mass transfer kinetics during osmotic dehydration of apples using neural networks. LWT Food Sci. Technol. 2007, 40, 638–645. [Google Scholar] [CrossRef]
- Chauhan, V.; Jindal, V.K. Neural networks approach to modeling food processing operations. In Food Processing Operations Modeling; CRC Press: New York, NY, USA, 2001; pp. 306–338. [Google Scholar]
- Özdemir, M.B.; Aktaş, M.; Şevik, S.; Khanlari, A. Modeling of a convective-infrared kiwifruit drying process. Int. J. Hydrog. Energy 2017, 42, 18005–18013. [Google Scholar] [CrossRef]
- Azadbakht, M.; Torshizi, M.V.; Noshad, F.; Rokhbin, A. Application of artificial neural network method for prediction of osmotic pretreatment based on the energy and exergy analyses in microwave drying of orange slices. Energy 2018, 165, 836–845. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Artificial neural network and response surface methodology modeling in mass transfer parameters predictions during osmotic dehydration of Carica papaya L. Alex. Eng. J. 2013, 52, 507–516. [Google Scholar] [CrossRef]
- Lertworasirikul, S.; Saetan, S. Artificial neural network modeling of mass transfer during osmotic dehydration of kaffir lime peel. J. Food Eng. 2010, 98, 214–223. [Google Scholar] [CrossRef]
- Zenoozian, M.S.; Devahastin, S. Application of wavelet transform coupled with artificial neural network for predicting physicochemical properties of osmotically dehydrated pumpkin. J. Food Eng. 2009, 90, 219–227. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. The human brain in numbers: A linearly scaled-up primate brain. Front. Hum. Neurosci. 2009, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Haykin, S.S. Neural Networks and Learning Machines; McMaster University: Hamilton, ON, Canada, 2009; pp. 1–46. [Google Scholar]
- Nassef, A.M.; Fathy, A.; Sayed, E.T.; Abdelkareem, M.A.; Rezk, H.; Tanveer, W.H.; Olabi, A.G. Maximizing SOFC performance through optimal parameters identification by modern optimization algorithms. Renew. Energy 2019, 138, 458–464. [Google Scholar] [CrossRef]


| Solution | Water Loss (g/g idm) | Standard Deviation | Solid Gain (g/g idm) | Standard Deviation |
|---|---|---|---|---|
| 30% Glucose | 1.056 | 0.07 | 0.219 | 0.02 |
| 50% Glucose | 1.442 | 0.275 | ||
| 30% Sucrose | 1.011 | 0.05 | 0.209 | 0.03 |
| 50% Sucrose | 1.438 | 0.283 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, S.M.A.; Nassef, A.M.; Al-Dhaifallah, M.; Abdelkareem, M.A.; Rezk, H. The Effect of a New Coating on the Drying Performance of Fruit and Vegetables Products: Experimental Investigation and Artificial Neural Network Modeling. Foods 2020, 9, 308. https://doi.org/10.3390/foods9030308
Rahman SMA, Nassef AM, Al-Dhaifallah M, Abdelkareem MA, Rezk H. The Effect of a New Coating on the Drying Performance of Fruit and Vegetables Products: Experimental Investigation and Artificial Neural Network Modeling. Foods. 2020; 9(3):308. https://doi.org/10.3390/foods9030308
Chicago/Turabian StyleRahman, S. M. Atiqure, Ahmed M. Nassef, Mujahed Al-Dhaifallah, Mohammad Ali Abdelkareem, and Hegazy Rezk. 2020. "The Effect of a New Coating on the Drying Performance of Fruit and Vegetables Products: Experimental Investigation and Artificial Neural Network Modeling" Foods 9, no. 3: 308. https://doi.org/10.3390/foods9030308
APA StyleRahman, S. M. A., Nassef, A. M., Al-Dhaifallah, M., Abdelkareem, M. A., & Rezk, H. (2020). The Effect of a New Coating on the Drying Performance of Fruit and Vegetables Products: Experimental Investigation and Artificial Neural Network Modeling. Foods, 9(3), 308. https://doi.org/10.3390/foods9030308









