Assessment of Water Mobility in Surf Clam and Soy Protein System during Gelation Using LF-NMR Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
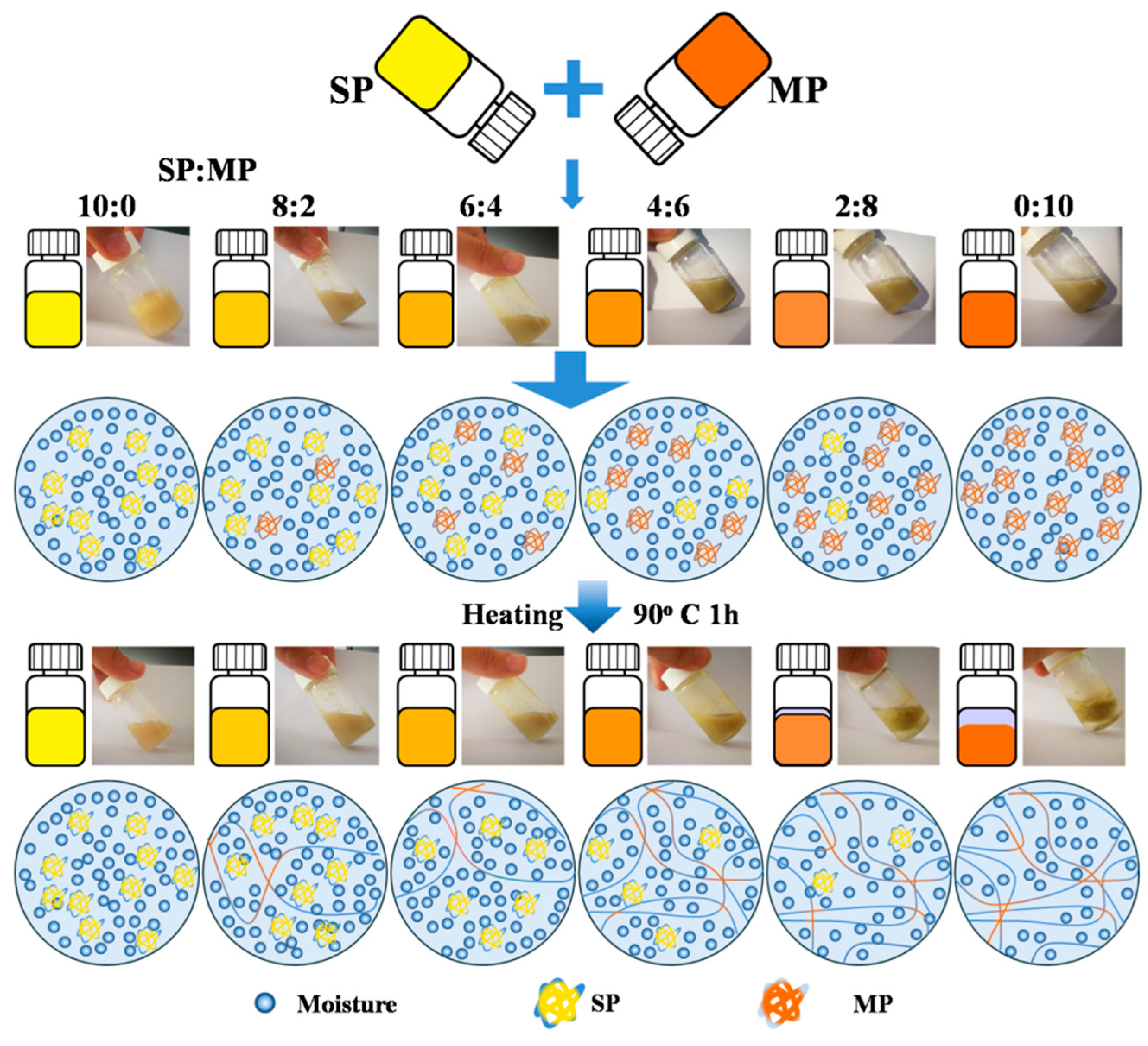
2.2. Preparation of Clam MP and SP Solution
2.3. Preparation of Protein Gels
2.4. Relaxation Properties of Protein Gelation
2.5. Rheological Analysis
2.6. Cooking Loss and Gel Strength Analysis
2.7. Hydrophobicity of the MP
2.8. WHC
2.9. Microstructure of the Gel
2.10. Statistical Analysis
3. Results
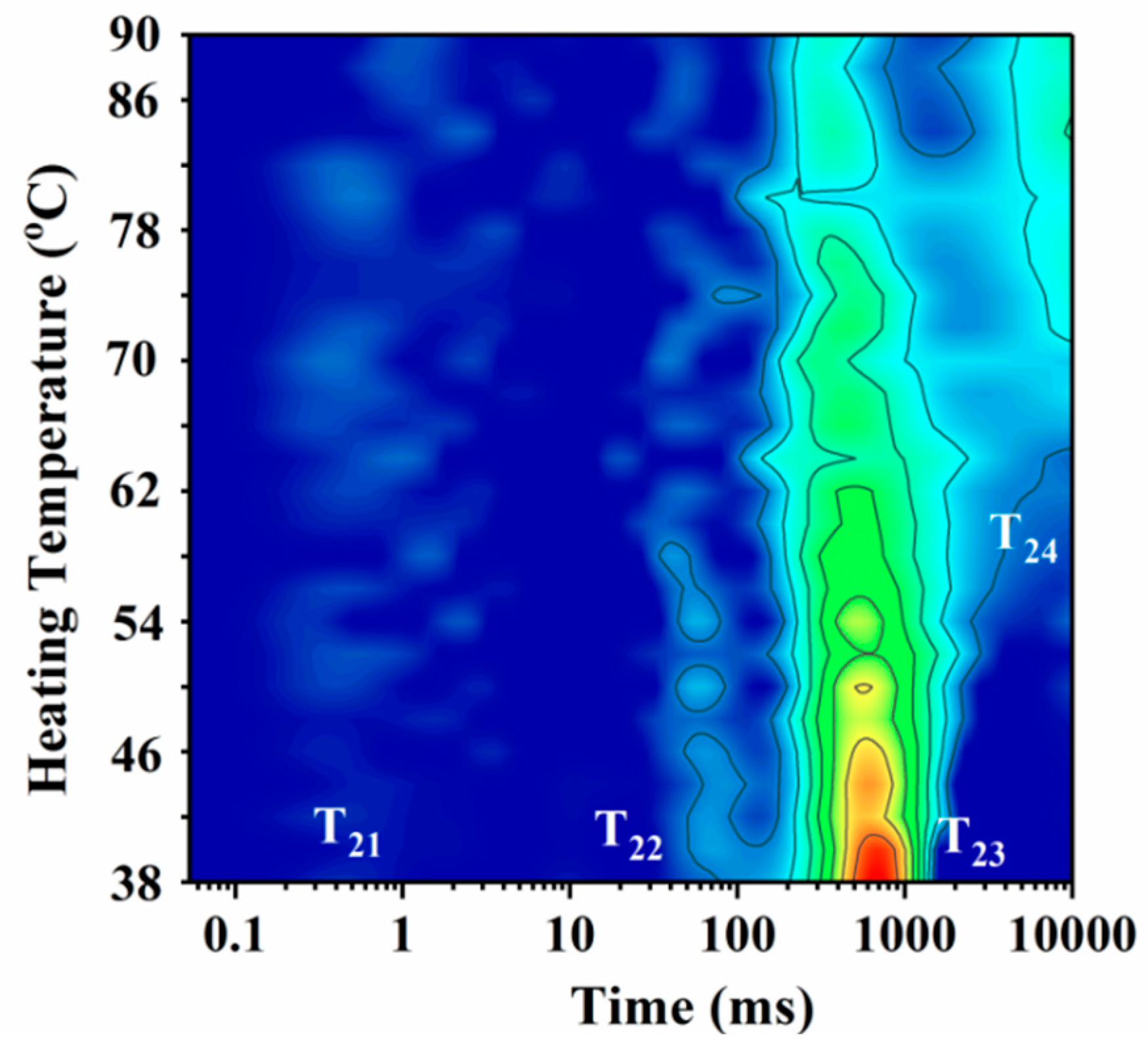
3.1. Moisture Mobility Analysis with LF-NMR
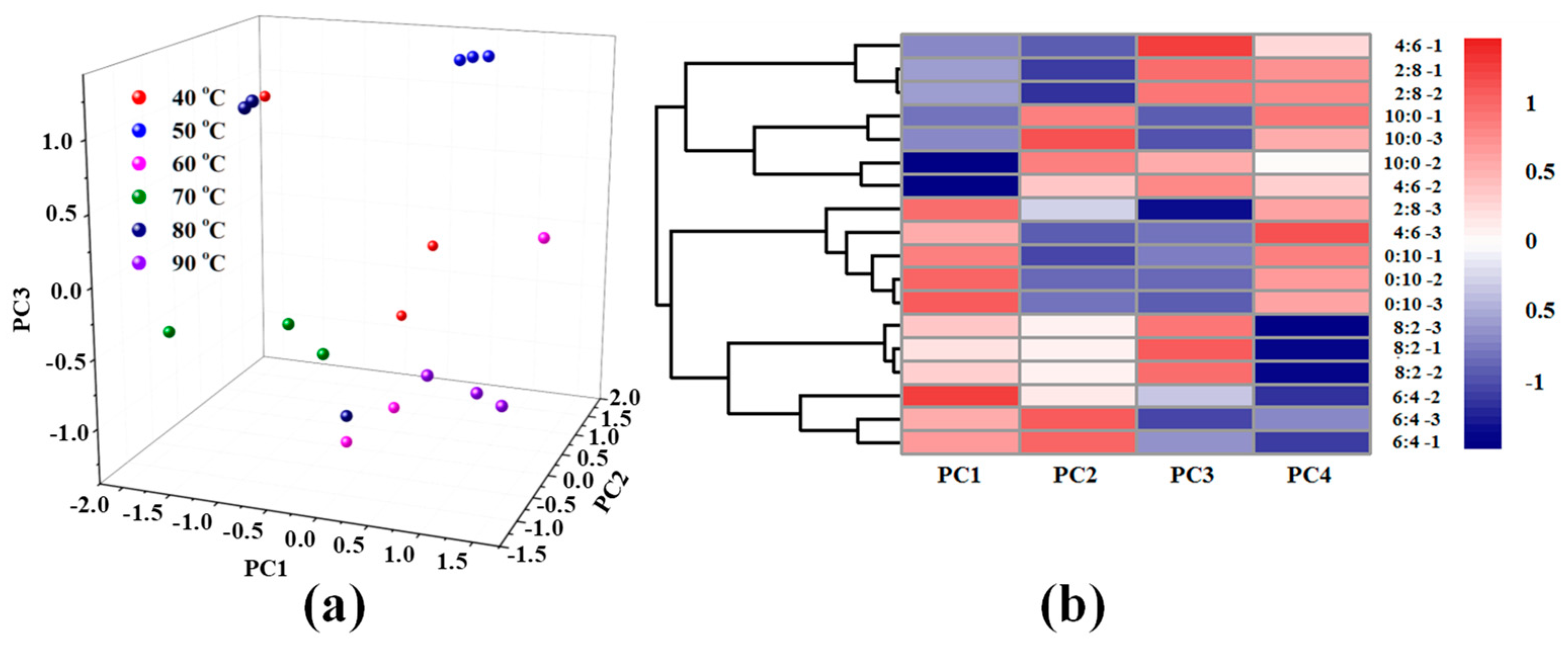
3.2. Principal Component Analysis (PCA)
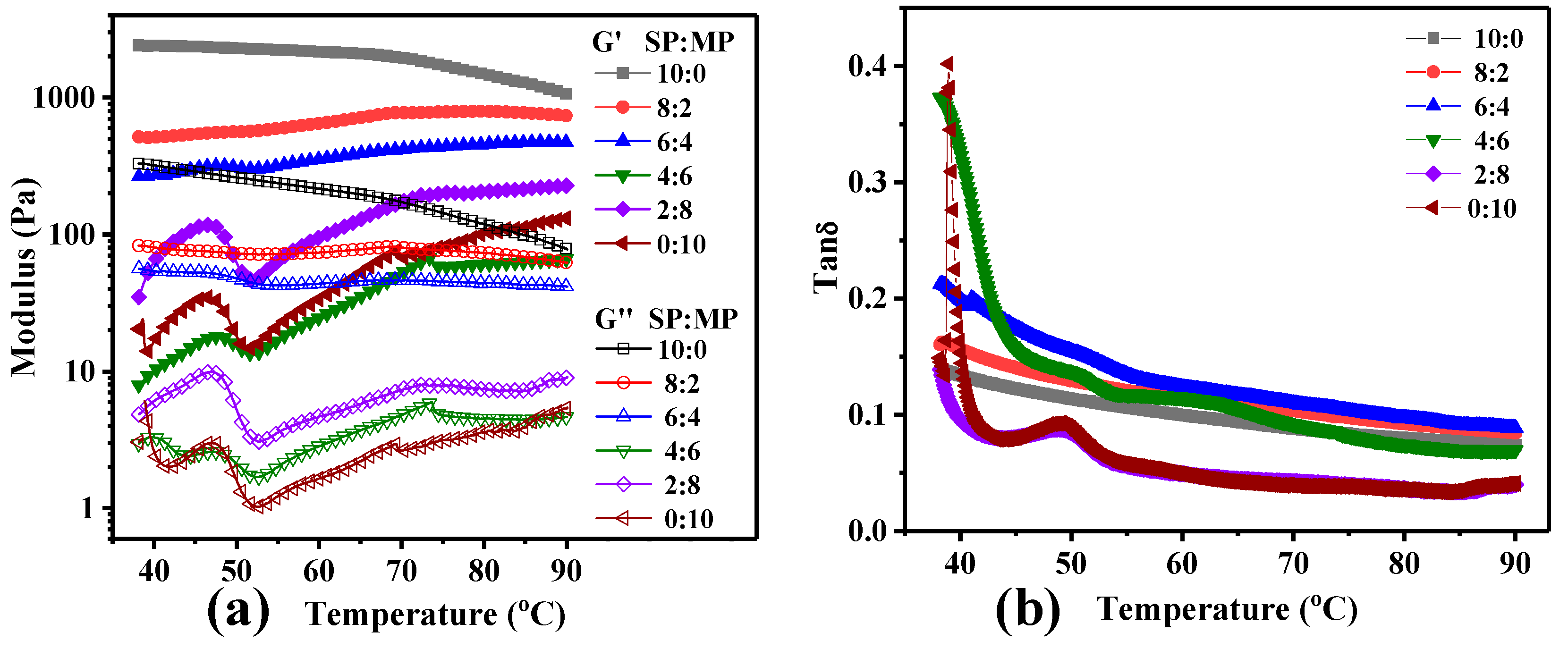
3.3. Characterization of Rheology Property
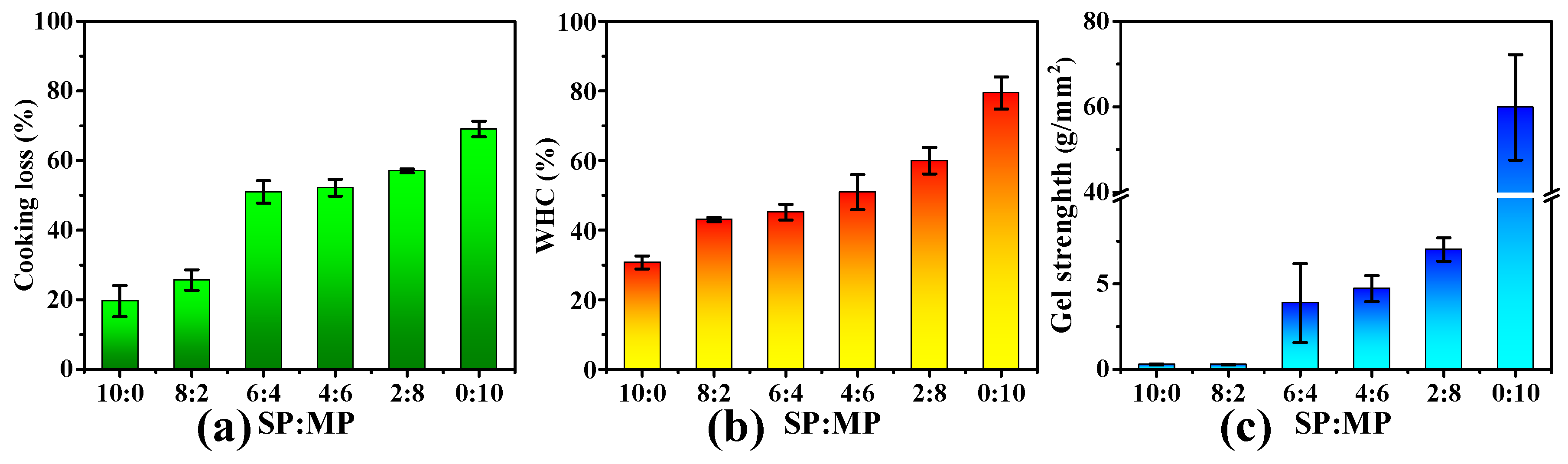
3.4. Cooking Loss, Water Holding Capability (WHC), and Gel Strength Analysis
3.5. Microstructure of Mixed Protein
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, J.; Li, H.; Qin, Y.; Ye, S.; Liu, M. Identification of functional genes involved in Cd(2+) response of Chinese surf clam (Mactra chinensis) through transcriptome sequencing. Environ. Toxicol. Pharmacol. 2016, 41, 113–120. [Google Scholar] [CrossRef]
- Niu, H.; Li, Y.; Han, J.; Liu, Q.; Kong, B. Gelation and rheological properties of myofibrillar proteins influenced by the addition of soybean protein isolates subjected to an acidic pH treatment combined with a mild heating. Food Hydrocoll. 2017, 70, 269–276. [Google Scholar] [CrossRef]
- Ma, Y.; Xiong, S.; You, J.; Hu, Y.; Huang, Q.; Yin, T. Effects of vacuum chopping on physicochemical and gelation properties of myofibrillar proteins from silver carp (Hypophthalmichthys molitrix). Food Chem. 2018, 245, 557–563. [Google Scholar] [CrossRef]
- Chan, J.T.; Omana, D.A.; Betti, M. Functional and rheological properties of proteins in frozen turkey breast meat with different ultimate pH. Poult. Sci. 2011, 90, 1112–1123. [Google Scholar] [CrossRef] [PubMed]
- Egelandsdal, B. Heat-induced gelling in solutions of ovalbumin. J. Food Sci. 2010, 45, 570–574. [Google Scholar] [CrossRef]
- Zhou, F.; Zhao, M.; Su, G.; Cui, C.; Sun, W. Gelation of salted myofibrillar protein under malondialdehyde-induced oxidative stress. Food Hydrocoll. 2014, 40, 153–162. [Google Scholar] [CrossRef]
- Renkema, J.M.S.; Vliet, T.V. Heat-induced gel formation by soy proteins at neutral pH. J. Agric. Food Chem. 2002, 50, 1569–1573. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.L.; Jang, H.S.; Kang, I.; Chin, K.B. Effect of red bean protein isolate and salt levels on pork myofibrillar protein gels mediated by microbial transglutaminase. LWT-Food Sci. Technol. 2017, 76, 95–100. [Google Scholar]
- Rawdkuen, S.; Saiut, S.; Khamsorn, S.; Chaijan, M.; Benjakul, S. Biochemical and gelling properties of tilapia surimi and protein recovered using an acid-alkaline process. Food Chem. 2009, 112, 112–119. [Google Scholar] [CrossRef]
- Puolanne, E.; Halonen, M. Theoretical aspects of water-holding in meat. Meat Sci. 2010, 86, 151–165. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, W.; Li, T.; Zheng, H.; Khan, M.A.; Xu, X.; Sun, J.; Zhou, G. Effect of protein structure on water and fat distribution during meat gelling. Food Chem. 2016, 204, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Idag, A.; Ulf, E.; Emil, V. Water properties and salt uptake in Atlantic salmon fillets as affected by ante-mortem stress, rigor mortis, and brine salting: A low-field 1H NMR and 1H/23Na MRI study. Food Chem. 2010, 120, 482–489. [Google Scholar]
- Carneiro, C.D.S.; Mársico, E.T.; Júnior, C.A.C.; Álvares, T.S.; Jesus, E.F.O.D. Studies of the effect of sodium tripolyphosphate on frozen shrimp by physicochemical analytical methods and low field nuclear magnetic resonance (LF 1 H NMR). LWT-Food Sci. Technol. 2013, 50, 401–407. [Google Scholar] [CrossRef]
- Cao, M.J.; Jiang, X.J.; Zhong, H.C.; Zhang, Z.J.; Su, W.J. Degradation of myofibrillar proteins by a myofibril-bound serine proteinase in the skeletal muscle of crucian carp (Carasius auratus). Food Chem. 2006, 94, 7–13. [Google Scholar] [CrossRef]
- Chen, F.L.; Wei, Y.M.; Bo, Z. Characterization of water state and distribution in textured soybean protein using DSC and NMR. J. Food Eng. 2010, 99, 522–526. [Google Scholar] [CrossRef]
- Shao, X.; Li, Y. Classification and prediction by LF NMR. Food Bioprocess Technol. 2012, 5, 1817–1823. [Google Scholar] [CrossRef]
- Han, M.Y.; Zhang, Y.J.; Fei, Y.; Xu, X.L.; Zhou, G.H. Effect of microbial transglutaminase on NMR relaxometry and microstructure of pork myofibrillar protein gel. Eur. Food Res. Technol. 2009, 228, 665–670. [Google Scholar] [CrossRef]
- Hansen, C.L.; Thybo, A.K.; Bertram, H.C.; Viereck, N.; van den Berg, F.; Engelsen, S.B. Determination of dry matter content in potato tubers by low-field nuclear magnetic resonance (LF-NMR). J. Agric. Food Chem. 2010, 58, 10300–10304. [Google Scholar] [CrossRef]
- Salvador, P.; Toldrà, M.; Saguer, E.; Carretero, C.; Parés, D. Microstructure-function relationships of heat-induced gels of porcine haemoglobin. Food Hydrocoll. 2009, 23, 1654–1659. [Google Scholar] [CrossRef]
- Pearce, K.L.; Rosenvold, K.; Andersen, H.J.; Hopkins, D.L. Water distribution and mobility in meat during the conversion of muscle to meat and ageing and the impacts on fresh meat quality attributes—A review. Meat Sci. 2011, 89, 111–124. [Google Scholar] [CrossRef]
- Bertram, H.C.; Aaslyng, M.D.; Andersen, H.J. Elucidation of the relationship between cooking temperature, water distribution and sensory attributes of pork-a combined NMR and sensory study. Meat Sci. 2005, 70, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Gudjónsdóttir, M.; Arason, S.; Rustad, T. The effects of pre-salting methods on water distribution and protein denaturation of dry salted and rehydrated cod—A low-field NMR study. J. Food Eng. 2011, 104, 23–29. [Google Scholar] [CrossRef]
- Park, J.W.; Lin, T.M.; Yongsawatdigul, J. New developments in manufacturing of surimi and surimi seafood. Food Rev. Int. 1997, 13, 577–610. [Google Scholar] [CrossRef]
- Shao, J.H.; Deng, Y.M.; Song, L.; Batur, A.; Jia, N.; Liu, D.Y. Investigation the effects of protein hydration states on the mobility water and fat in meat batters by LF-NMR technique. LWT-Food Sci. Technol. 2016, 66, 1–6. [Google Scholar] [CrossRef]
- Han, M.; Wang, P.; Xu, X.; Zhou, G. Low-field NMR study of heat-induced gelation of pork myofibrillar proteins and its relationship with microstructural characteristics. Food Res. Int. 2014, 62, 1175–1182. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, X.; Li, R.; Yang, H.; Wang, H.; Wang, H.; Tan, M. Influence of multiple freeze-thaw cycles on quality characteristics of beef semimembranous muscle: With emphasis on water status and distribution by LF-NMR and MRI. Meat Sci. 2019, 147, 44–52. [Google Scholar] [CrossRef]
- Michaelriis, H.; Andreas, B.; Imad, F.; Lars, N.; Sven, P.; Sørenb, E. Comparative NMR relaxometry of gels of amylomaltase-modified starch and gelatin. Food Hydrocoll. 2009, 23, 2038–2048. [Google Scholar]
- Azilawati, M.I.; Hashim, D.M.; Jamilah, B.; Amin, I. RP-HPLC method using 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate incorporated with normalization technique in principal component analysis to differentiate the bovine, porcine and fish gelatins. Food Chem. 2015, 172, 368–376. [Google Scholar] [CrossRef]
- Karaman, S.; Cengiz, E.; Kayacier, A.; Dogan, M. Exposure to air accelerates the gelation of gelatin: Steady and dynamic shear rheological characterization to see the effect of air on the strength of gelatin gel. Int. J. Food Prop. 2016, 19, 721–730. [Google Scholar] [CrossRef]
- Feng, T.; Ye, R.; Zhuang, H.; Rong, Z.; Fang, Z.; Wang, Y.; Gu, Z.; Jin, Z. Physicochemical properties and sensory evaluation of Mesona Blumes gum/rice starch mixed gels as fat-substitutes in Chinese Cantonese-style sausage. Food Res. Int. 2013, 50, 85–93. [Google Scholar] [CrossRef]
- Jiang, J. Extreme pH treatments enhance the structure-reinforcement role of soy protein isolate and its emulsions in pork myofibrillar protein gels in the presence of microbial transglutaminase. Meat Sci. 2013, 93, 469–476. [Google Scholar] [CrossRef] [PubMed]
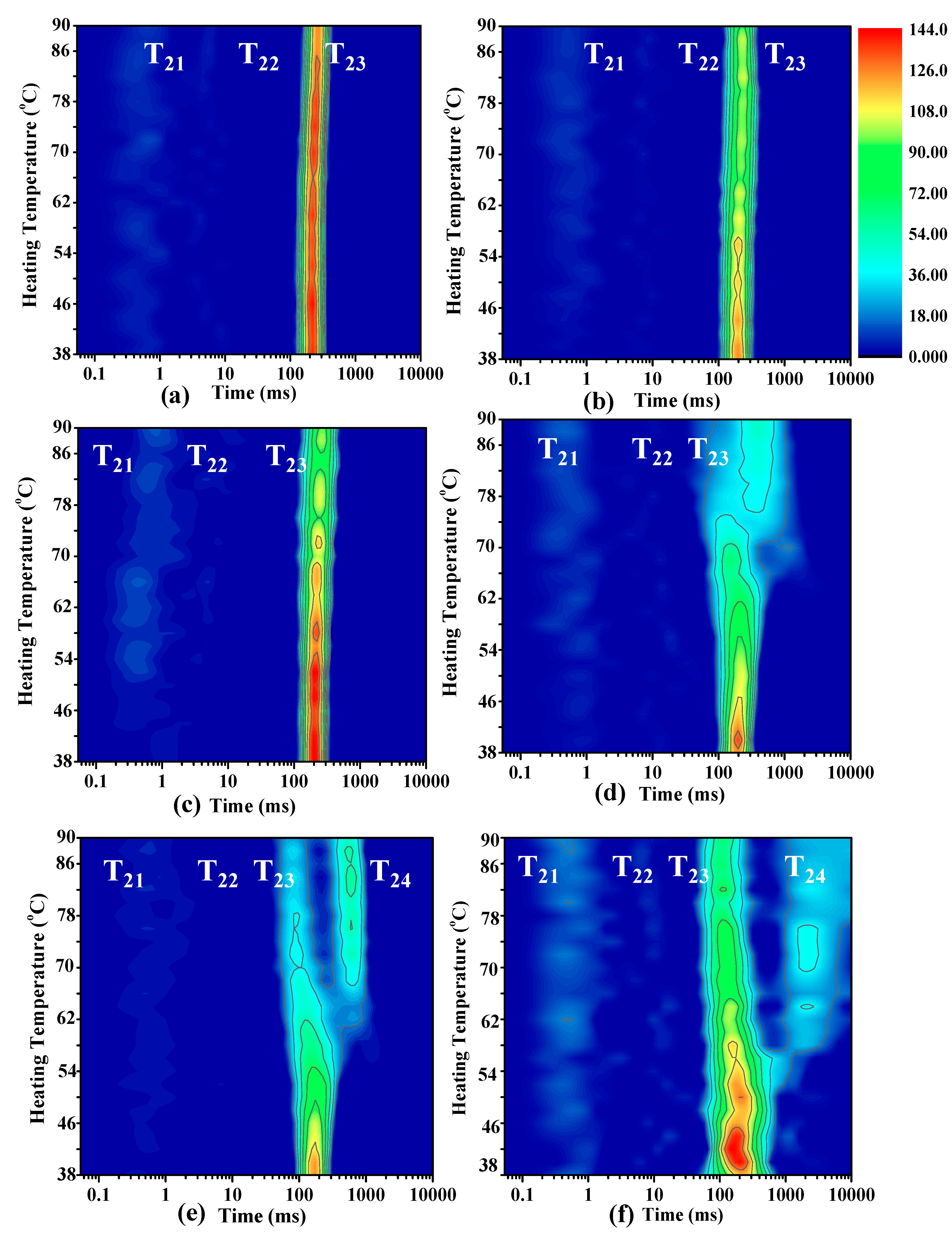

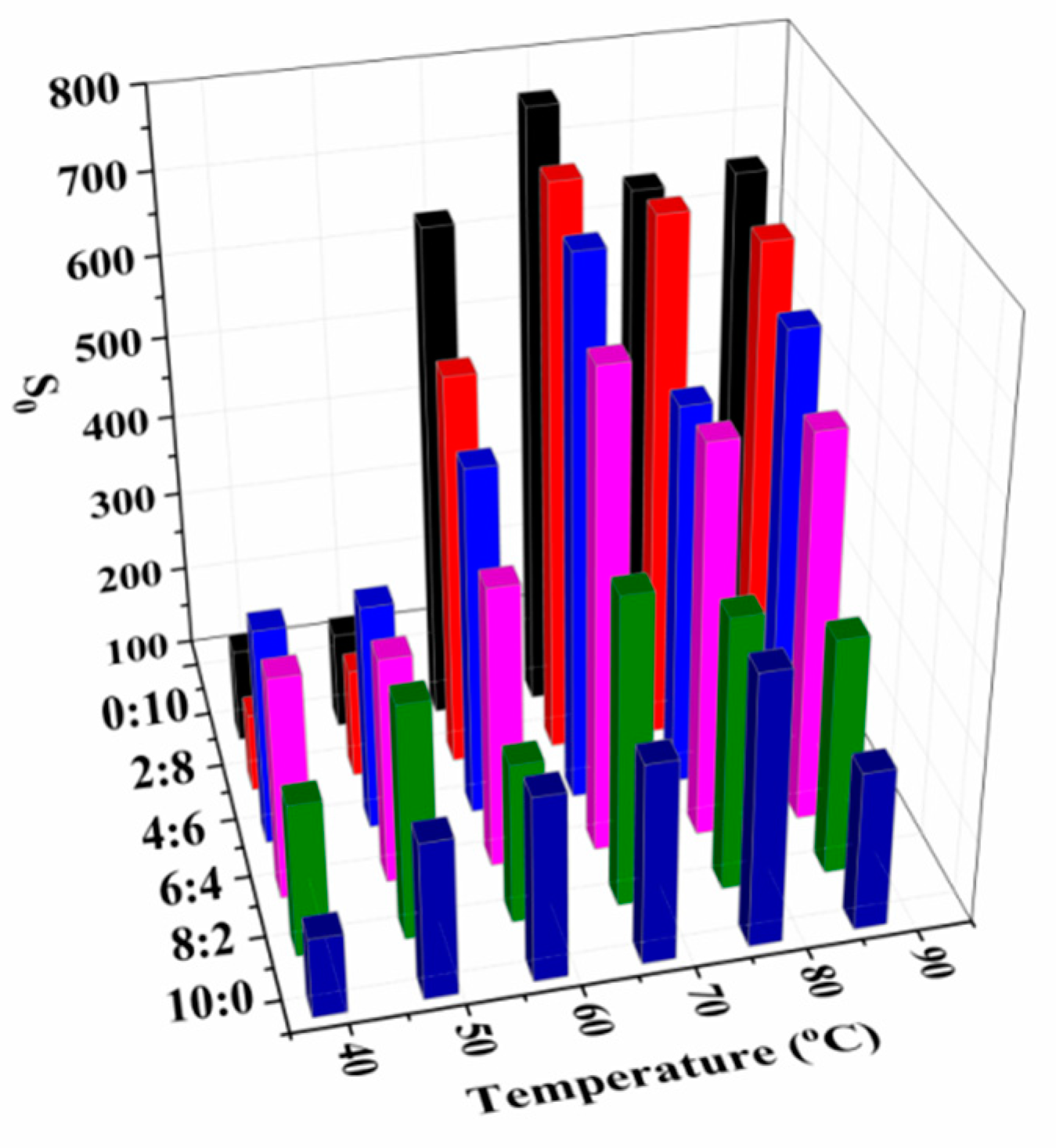

| SP:MP | T21 (ms) | T22 (ms) | T23 (ms) | T24 (ms) | P21 (%) | P22 (%) | P23 (%) | P24 (%) |
|---|---|---|---|---|---|---|---|---|
| Before heating | ||||||||
| 10:0 | 0.54 ± 0.31 a | 2.96 ± 0.54 b | 223.28 ± 0.00 a | - | 3.35 ± 0.82 a | 2.06 ± 1.17 a | 94.58 ± 0.50 a | - |
| 8:2 | 0.89 ± 0.26 a | 6.66 ± 2.41 b | 214.69 ± 14.88 a | - | 4.71 ± 1.35 a | 0.59 ± 0.29 a | 94.70 ± 1.59 a | - |
| 6:4 | 0.97 ± 0.55 a | 3.17 ± 1.09 ab | 206.10 ± 14.88 a | - | 4.53 ± 1.87 a | 0.97 ± 0.33 a | 94.49 ± 2.18 a | - |
| 4:6 | 0.61 ± 0.34 a | 4.30 ± 3.50 ab | 206.10 ± 14.88 a | - | 3.11 ± 2.69 a | 1.73 ± 1.50 a | 94.84 ± 1.34 a | - |
| 2:8 | 0.57 ± 0.28 a | 3.57 ± 1.05 a | 174.72 ± 0.00 a | - | 4.34 ± 0.62 a | 1.20 ± 0.30 a | 94.47 ± 0.92 a | - |
| 0:10 | 0.40 ± 0.05 a | 7.80 ± 2.44 a | 175.59 ± 21.49 a | - | 5.36 ± 0.45 a | 1.35 ± 0.21 a | 91.79 ± 0.36 a | - |
| 90 °C heating | ||||||||
| 10:0 | 0.54 ± 0.14 ab | 5.66 ± 0.69 a | 252.42 ± 0.00 cd | - | 12.86 ± 2.23 ab | 2.29 ± 0.20 a | 84.85 ± 2.43 c | - |
| 8:2 | 0.49 ± 0.11 ab | 9.25 ± 1.13 a | 243.97 ± 35.84 c | - | 15.09 ± 1.91 ab | 2.61 ± 0.4a a | 82.30 ± 2.31 c | - |
| 6:4 | 0.89 ± 0.26 b | 15.85 ± 3.04 b | 285.35 ± 0.00 d | - | 15.49 ± 3.44 ab | 4.00 ± 0.41 a | 80.52 ± 3.34 c | - |
| 4:6 | 0.78 ± 0.31 ab | 9.05 ± 3.59 a | 396.40 ± 27.47 e | - | 14.22 ± 1.83 ab | 1.68 ± 0.21 a | 82.81 ± 1.46 c | - |
| 2:8 | 0.60 ± 0.04 ab | 5.26 ± 1.01 a | 77.27 ± 5.58 a | 526.86 ± 0.00 | 11.02 ± 0.83 a | 1.94 ± 0.44 a | 31.88 ± 0.60 a | 55.16 ± 1.36 |
| 0:10 | 0.38 ± 0.05 a | 8.01 ± 3.17 a | 126.19 ± 9.11 b | 2948.14 ± 360.89 | 18.71 ± 2.75 b | 4.32 ± 3.37 a | 42.74 ± 2.77 b | 34.22 ± 3.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Lin, R.; Cheng, S.; Wang, Z.; Tan, M. Assessment of Water Mobility in Surf Clam and Soy Protein System during Gelation Using LF-NMR Technique. Foods 2020, 9, 213. https://doi.org/10.3390/foods9020213
Wang S, Lin R, Cheng S, Wang Z, Tan M. Assessment of Water Mobility in Surf Clam and Soy Protein System during Gelation Using LF-NMR Technique. Foods. 2020; 9(2):213. https://doi.org/10.3390/foods9020213
Chicago/Turabian StyleWang, Siqi, Rong Lin, Shasha Cheng, Zhixiang Wang, and Mingqian Tan. 2020. "Assessment of Water Mobility in Surf Clam and Soy Protein System during Gelation Using LF-NMR Technique" Foods 9, no. 2: 213. https://doi.org/10.3390/foods9020213
APA StyleWang, S., Lin, R., Cheng, S., Wang, Z., & Tan, M. (2020). Assessment of Water Mobility in Surf Clam and Soy Protein System during Gelation Using LF-NMR Technique. Foods, 9(2), 213. https://doi.org/10.3390/foods9020213







