Effect of High Pressure, Calcium Chloride and ZnO-Ag Nanoparticles on Beef Color and Shear Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Packaging: Production Method, Chemical Composition and Migration Assays
2.2. Meat Sampling and pH Measurement
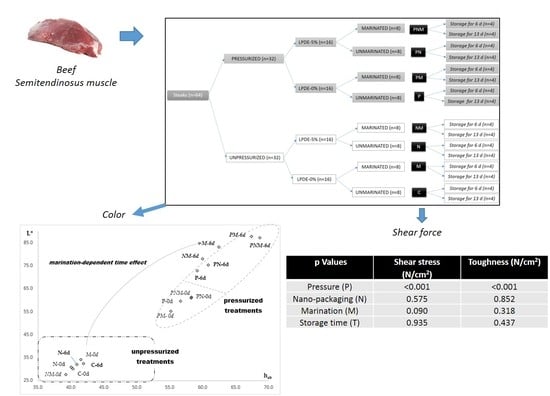
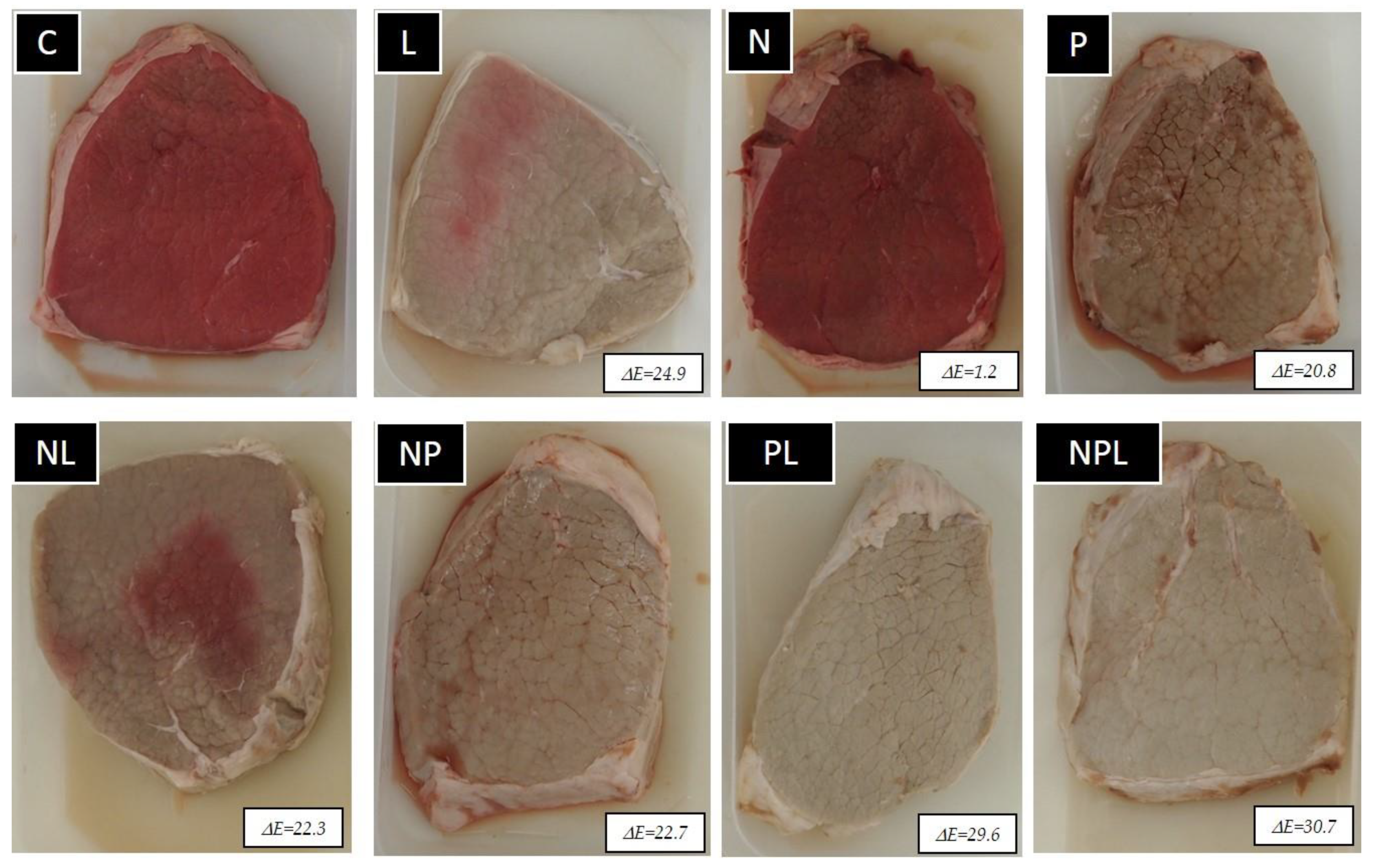
- C: Control (unpressurized-LPDE 0%-unmarinated)
- N: unpressurized-LPDE 5%-unmarinated
- M: unpressurized-LPDE 0%-marinated
- NM: unpressurized-LPDE 5%-marinated
- P: pressurized-LPDE 0%-unmarinated
- PN: pressurized-LPDE 5%-unmarinated
- PM: pressurized-LPDE 0%-marinated
- PNM: pressurized-LPDE 5%-marinated.
2.3. Instrumental Procedures
2.3.1. Color
2.3.2. Texture
2.3.3. Statistics
3. Results
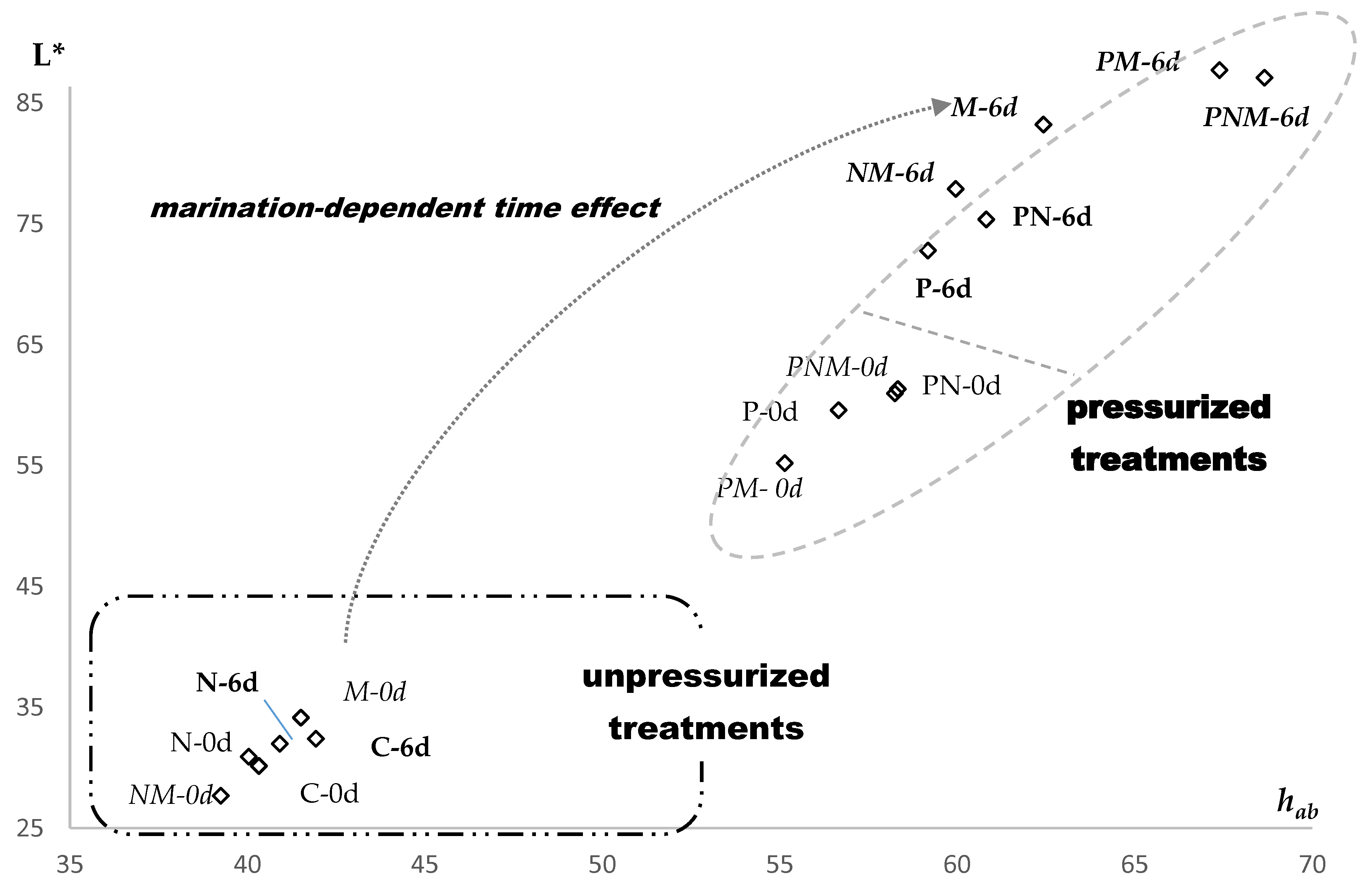
3.1. pH and Color
3.2. Texture
4. Discussion
4.1. Color
4.2. Texture
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bak, K.H.; Bolumar, T.; Karlsson, A.H.; Lindahl, G.; Orlien, V. Effect of high pressure treatment on the color of fresh and processed meats: A review. Crit Rev. Food Sci. Nutr. 2019, 59, 228–252. [Google Scholar] [CrossRef] [PubMed]
- Bhat, Z.F.; Morton, J.D.; Mason, S.L.; Bekhit, A.E.-D.A. Applied and Emerging Methods for Meat Tenderization: A Comparative Perspective. Compr. Rev. Food Sci. Food Saf. 2018, 17, 841–859. [Google Scholar] [CrossRef]
- Zybert, A.; Tarczyński, K.; Sieczkowska, H. A meta-analysis of the effect of high pressure processing on four quality traits of fresh pork. J. Food Process. Preserv. 2019, 43, e13895. [Google Scholar] [CrossRef]
- Morton, J.D.; Pearson, R.G.; Lee, H.Y.; Smithson, S.; Mason, S.L.; Bickerstaffe, R. High pressure processing improves the tenderness and quality of hot-boned beef. Meat Sci. 2017, 133, 69–74. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Ma, H.J.; Ledward, D.A. High pressure processing of fresh meat--is it worth it? Meat Sci. 2013, 95, 897–903. [Google Scholar] [CrossRef]
- McKenna, D.R.; Mies, P.D.; Baird, B.E.; Pfeiffer, K.D.; Ellebracht, J.W.; Savell, J.W. Biochemical and physical factors affecting discoloration characteristics of 19 bovine muscles. Meat Sci. 2005, 70, 665–682. [Google Scholar] [CrossRef]
- Kerry, J.; O’grady, M.; Hogan, S. Past, current and potential utilisation of active and intelligent packaging systems for meat and muscle-based products: A review. Meat Sci. 2006, 74, 113–130. [Google Scholar] [CrossRef]
- Stoimenov, P.K.; Klinger, R.L.; Marchin, G.L.; Klabunde, K.J. Metal oxide nanoparticles as bactericidal agents. Langmuir 2002, 18, 6679–6686. [Google Scholar] [CrossRef]
- Emamifar, A.; Kadivar, M.; Shahedi, M.; Soleimanian-Zad, S. Evaluation of nanocomposite packaging containing Ag and ZnO on shelf life of fresh orange juice. Innov. Food Sci. Emerg. Technol. 2010, 11, 742–748. [Google Scholar] [CrossRef]
- Commission, E. COMMISSION REGULATION (EC) No 450/2009 of on active and intelligent materials and articles intended to come into contact with food. Off. J. Eur. Union 2009, 135, 3–11. [Google Scholar]
- Llorens, A.; Lloret, E.; Picouet, P.A.; Trbojevich, R.; Fernandez, A. Metallic-based micro and nanocomposites in food contact materials and active food packaging. Trends Food Sci. Technol. 2012, 24, 19–29. [Google Scholar] [CrossRef]
- Torrescano, G.; Sánchez-Escalante, A.; Giménez, B.; Roncales, P.; Beltrán, J.A. Shear values of raw samples of 14 bovine muscles and their relation to muscle collagen characteristics. Meat Sci. 2003, 64, 85–91. [Google Scholar] [CrossRef]
- Sharedeh, D.; Gatellier, P.; Astruc, T.; Daudin, J.D. Effects of pH and NaCl levels in a beef marinade on physicochemical states of lipids and proteins and on tissue microstructure. Meat Sci. 2015, 110, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Lee, E.-J.; Lee, N.-H.; Kim, Y.-H.; Yamamoto, K. Effects of hydrostatic pressure treatment on the physicochemical, morphological, and textural properties of bovine semitendinosus muscle. Food Sci. Biotechnol. 2007, 16, 49–54. [Google Scholar]
- Cruzen, S.M.; Kim, Y.H.; Lonergan, S.M.; Grubbs, J.K.; Fritchen, A.N.; Huff-Lonergan, E. Effect of early postmortem enhancement of calcium lactate/phosphate on quality attributes of beef round muscles under different packaging systems. Meat Sci. 2015, 101, 63–72. [Google Scholar] [CrossRef]
- Panea, B.; Ripoll, G.; González, J.; Fernández-Cuello, Á.; Albertí, P. Effect of nanocomposite packaging containing different proportions of ZnO and Ag on chicken breast meat quality. J. Food Eng. 2014, 123, 104–112. [Google Scholar] [CrossRef]
- EC. Council Regulation (EC) No 1183/2006 of 24 July 2006 concerning the Community scale for the classification of carcasses of adult bovine animals. Off. J. Eur. Union 2006, 124. [Google Scholar]
- Gerelt, B.; Rusman, H.; Nishiumi, T.; Suzuki, A. Changes in calpain and calpastatin activities of osmotically dehydrated bovine muscle during storage after treatment with calcium. Meat Sci. 2005, 70, 55–61. [Google Scholar] [CrossRef]
- Lawrence, T.; Dikeman, M.; Hunt, M.; Kastner, C.; Johnson, D. Effects of calcium salts on beef longissimus quality. Meat Sci. 2003, 64, 299–308. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C.; Kropf, D.H. Reflectance at 610 nanometers estimates oxymyoglobin content on the surface of ground beef. Meat Sci. 2003, 64, 157–162. [Google Scholar] [CrossRef]
- Stewart, M.R.; Zipser, M.W.; Watts, B.M. The use of reflectance spectrohotometry for the assay of raw meat pigments. J. Food Sci. 1965, 30, 464–469. [Google Scholar] [CrossRef]
- Hunt, M.C.; Acton, J.C.; Benedict, R.C.; Calkins, C.R.; Cornforth, D.P.; Jeremiah, L.E.; Olson, D.G.; Salm, C.P.; Savell, J.W.; Shivas, S.D. Guidelines for Meat Color Evaluation; National Livestock and Meat Board: Chicago, IL, USA, 1991; pp. 1–17. [Google Scholar]
- Strange, E.D.; Benedict, R.C.; Gugger, R.E.; Metzger, V.G.; Swift, C.E. Simplified methodology for measuring meat color. J. Food Sci. 1974, 39, 988–992. [Google Scholar] [CrossRef]
- AMSA. Meat Color. Measurement Guidelines; American Meat Science Association: Savoy, IL, USA, 2012. [Google Scholar]
- Lepetit, J.; Culioli, J. Mechanical properties of meat. Meat Sci. 1994, 36, 203–237. [Google Scholar] [CrossRef]
- Ripoll, G.; Panea, B.; Albertí, P. Visual appraisal of beef: Relationship with CIELab color space. Itea Inf. Tec. Econ. Agrar. 2012, 108, 222–232. [Google Scholar]
- Ripoll, G.; Albertí, P.; Panea, B.; Failla, S.; Hocquette, J.F.; Dunner, S.; Sañudo, C.; Olleta, J.L.; Christensen, M.; Ertbjerg, P.; et al. Color variability of beef in young bulls from fifteen European breeds. Int. J. Food Sci. Technol. 2018. [CrossRef]
- Ha, M.; Dunshea, F.R.; Warner, R.D. A meta-analysis of the effects of shockwave and high pressure processing on color and cook loss of fresh meat. Meat Sci. 2017, 132, 107–111. [Google Scholar] [CrossRef]
- Bajovic, B.; Bolumar, T.; Heinz, V. Quality considerations with high pressure processing of fresh and value added meat products. Meat Sci. 2012, 92, 280–289. [Google Scholar] [CrossRef]
- Warner, R.D.; McDonnell, C.K.; Bekhit, A.E.D.; Claus, J.; Vaskoska, R.; Sikes, A.; Dunshea, F.R.; Ha, M. Systematic review of emerging and innovative technologies for meat tenderisation. Meat Sci. 2017, 132, 72–89. [Google Scholar] [CrossRef]
- Buckow, R.; Sikes, A.; Tume, R. Effect of high pressure on physicochemical properties of meat. Crit. Rev. Food Sci. Nutr. 2013, 53, 770–786. [Google Scholar] [CrossRef]
- Hood, D.E. Pre-slaughter injection of sodium ascorbate as a method of inhibiting metmyoglobin formation in fresh beef. J. Sci. Food Agric. 1975, 26, 85–90. [Google Scholar] [CrossRef]
- Ripoll, G.; Albertí, P.; Casasús, I.; Blanco, M. Instrumental meat quality of veal calves reared under three management systems and color evolution of meat stored in three packaging systems. Meat Sci. 2013, 93, 336–343. [Google Scholar] [CrossRef]
- Hughes, J.; Oiseth, S.; Purslow, P.; Warner, R. A structural approach to understanding the interactions between color, water-holding capacity and tenderness. Meat Sci. 2014, 98, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Ghoul, M.; de Lamballerie-Anton, M. Influence of high pressure on the color and microbial quality of beef meat. LWT-Food Sci. Technol. 2003, 36, 625–631. [Google Scholar] [CrossRef]
- Marcos, B.; Kerry, J.P.; Mullen, A.M. High pressure induced changes on sarcoplasmic protein fraction and quality indicators. Meat Sci. 2010, 85, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Cheah, P.; Ledward, D. Inhibition of metmyoglobin formation in fresh beef by pressure treatment. Meat Sci. 1997, 45, 411–418. [Google Scholar] [CrossRef]
- King, D.A.; Shackelford, S.D.; Wheeler, T.L. Relative contributions of animal and muscle effects to variation in beef lean color stability. J. Anim. Sci. 2011, 89, 1434–1451. [Google Scholar] [CrossRef]
- Klinhom, P.; Klinhom, J.; Senapa, J.; Methawiwat, S. Improving the quality of citric acid and calcium chloride marinated culled cow meat. Int. Food Res. J. 2015, 22, 1410–1416. [Google Scholar]
- Jung, S.; de Lamballerie-Anton, M.; Ghoul, M. Modifications of Ultrastructure and Myofibrillar Proteins of Post-rigor Beef Treated by High Pressure. LWT-Food Sci. Technol. 2000, 33, 313–319. [Google Scholar] [CrossRef]
- Ma, H.J.; Ledward, D.A. High pressure/thermal treatment effects on the texture of beef muscle. Meat Sci. 2004, 68, 347–355. [Google Scholar] [CrossRef]
- Marcos, B.; Aymerich, T.; Dolors Guardia, M.; Garriga, M. Assessment of high hydrostatic pressure and starter culture on the quality properties of low-acid fermented sausages. Meat Sci. 2007, 76, 46–53. [Google Scholar] [CrossRef][Green Version]
- McArdle, R.A.; Marcos, B.; Kerry, J.P.; Mullen, A.M. Influence of HPP conditions on selected beef quality attributes and their stability during chilled storage. Meat Sci. 2011, 87, 274–281. [Google Scholar] [CrossRef]
- Ueno, Y.; Ikeuchi, Y.; Suzuki, A. Effects of high pressure treatments on intramuscular connective tissue. Meat Sci. 1999, 52, 143–150. [Google Scholar] [CrossRef]
- Kwiatkowska, A.; Jankowska, B.; Korzeniowski, W. Changes in solubility of the bovine semitendinosus muscle collagen under the influence of high pressure. Pol. J. Food Nutr. Sci. 2001, 4. [Google Scholar]
- Ichinoseki, S.; Nishiumi, T.; Suzuki, A. Tenderizing effect of high hydrostatic pressure on bovine intramuscular connective tissue. J. Food Sci. 2006, 71, E276–E281. [Google Scholar] [CrossRef]
- Perez-Chabela, M.L.; Guerrero-Legarreta, L.I.; Gutierrez-Ruiz, M.C.; Betancourt-Rule, J.M.; Perez-Torres, A.; Ustarroz-Cano, M. Effect of Calcium Chloride Marination on Electrophoretical and Structural Characteristics of Beef, Horse, Rabbit and Chicken Meat. Int. J. Food Prop. 2005, 8, 207–219. [Google Scholar] [CrossRef]
- Whipple, G.; Koohmaraie, M. Calcium Chloride Marination Effects on Beef Steak Tenderness and Calpain Proteolytic Activity. Meat Sci. 1993, 33, 265. [Google Scholar] [CrossRef]
| L* | a* | b* | hab | MMb | ||
|---|---|---|---|---|---|---|
| Pressure (P) | <0.001 | <0.001 | <0.001 | 0.011 | <0.001 | <0.001 |
| Nano-packaging (N) | 0.630 | 0.503 | 0.259 | 0.814 | 0.107 | 0.470 |
| Marination (M) | <0.001 | <0.001 | 0.140 | <0.001 | <0.001 | <0.001 |
| Storage time (T) | <0.001 | <0.001 | 0.910 | <0.001 | <0.001 | <0.001 |
| P × N | <0.001 | 0.035 | 0.179 | 0.014 | 0.511 | 0.531 |
| P × M | <0.001 | <0.001 | 0.123 | <0.001 | <0.001 | <0.001 |
| P × T | <0.001 | 0.084 | 0.489 | 0.048 | <0.001 | <0.001 |
| N × M | 0.491 | 0.797 | 0.314 | 0.178 | 0.643 | 0.361 |
| N × T | 0.414 | 0.744 | 0.343 | 0.501 | 0.646 | 0.441 |
| M × T | <0.001 | <0.001 | 0.167 | <0.001 | <0.001 | <0.001 |
| Treatment | Time, d | L* | a* | b* | hab | C* | MMb % |
|---|---|---|---|---|---|---|---|
| C | 0 | 40.3 cdx | 15.4 ax | 8.9 cx | 30.2 cdx | 17.8 abx | 1.43 ax |
| 6 | 41.9 Dx | 15.2 Ax | 9.7 Cx | 32.4 Dx | 18.1 Ax | 1.33 Ay | |
| N | 0 | 40.0 cdx | 16.2 ax | 9.6 bcx | 30.9 cdx | 18.9 ax | 1.41 ax |
| 6 | 40.9 Dx | 15.8 Ax | 9.8 Cxx | 32.0 Dx | 18.6 Ax | 1.29 Ay | |
| M | 0 | 41.5 cy | 15.3 ax | 10.3 bx | 34.1 cy | 18.5 ax | 1.40 ax |
| 6 | 62.4 Bx | 1.1 Cy | 9.2 Cx | 83.2 ABx | 9.3 Dy | 0.91 Cy | |
| NM | 0 | 39.3 dy | 16.8 ax | 8.8 cx | 27.7 dy | 19.1 ax | 1.42 ax |
| 6 | 60.0 BCx | 2.1 Cy | 9.7 Cx | 77.9 BCx | 10.0 Dy | 1.02 By | |
| P | 0 | 56.7 aby | 8.5 bx | 14.3 ax | 59.6 aby | 16.6 bx | 0.96 bx |
| 6 | 59.2 Cx | 4.4 By | 14.1 ABx | 72.8 Cx | 14.7 BCy | 0.82 Dy | |
| PN | 0 | 58.3 ay | 8.0 bx | 14.5 ax | 61.3 ay | 16.6 bx | 1.03 bx |
| 6 | 60.8 BCx | 4.0 By | 15.2 Ax | 75.4 Cx | 15.7 Bx | 0.83 Dy | |
| PM | 0 | 55.1 by | 9.5 bx | 13.6 ax | 55.2 by | 16.7 bx | 1.07 bx |
| 6 | 67.4 Ax | 0.5 Cy | 13.3 Bx | 87.7 Ax | 13.3 Cy | 0.92 Cy | |
| PNM | 0 | 58.2 ay | 8.0 bx | 14.4 ax | 61.0 ay | 16.5 bx | 1.02 bx |
| 6 | 68.7 Ax | 0.7 Cy | 13.7 Bx | 87.1 Ax | 13.7 Cy | 0.91 Cy | |
| Standard error | 1.03 | 0.594 | 0.267 | 2.145 | 0.295 | 0.023 |
| Shear Stress (N/cm2) | Toughness (N/cm2) | |
|---|---|---|
| Pressure (P) | <0.001 | <0.001 |
| Nano-packaging (N) | 0.575 | 0.852 |
| Marination (M) | 0.090 | 0.318 |
| Storage time (T) | 0.935 | 0.437 |
| P × N | 0.027 | 0.185 |
| P × M | 0.750 | 0.185 |
| P × T | 0.124 | 0.424 |
| N × M | 0.635 | 0.995 |
| N × T | 0.986 | 0.850 |
| M × T | 0.455 | 0.423 |
| Shear Stress (N/cm2) | Toughness (N/cm2) | |
|---|---|---|
| C | 40.5 bc | 13.0 c |
| N | 48.0 b | 16.1 bc |
| M | 38.7 c | 17.1 bc |
| NM | 43.6 bc | 17.8 abc |
| P | 78.9 a | 22.7 a |
| PN | 71.9 a | 20.1 ab |
| PM | 71.1 a | 21.1 ab |
| PNM | 70.7 a | 20.9 ab |
| s.e. | 2.21 | 0.67 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panea, B.; Albertí, P.; Ripoll, G. Effect of High Pressure, Calcium Chloride and ZnO-Ag Nanoparticles on Beef Color and Shear Stress. Foods 2020, 9, 179. https://doi.org/10.3390/foods9020179
Panea B, Albertí P, Ripoll G. Effect of High Pressure, Calcium Chloride and ZnO-Ag Nanoparticles on Beef Color and Shear Stress. Foods. 2020; 9(2):179. https://doi.org/10.3390/foods9020179
Chicago/Turabian StylePanea, Begoña, Pere Albertí, and Guillermo Ripoll. 2020. "Effect of High Pressure, Calcium Chloride and ZnO-Ag Nanoparticles on Beef Color and Shear Stress" Foods 9, no. 2: 179. https://doi.org/10.3390/foods9020179
APA StylePanea, B., Albertí, P., & Ripoll, G. (2020). Effect of High Pressure, Calcium Chloride and ZnO-Ag Nanoparticles on Beef Color and Shear Stress. Foods, 9(2), 179. https://doi.org/10.3390/foods9020179