Influence of Acid Adaptation on the Probability of Germination of Clostridium sporogenes Spores Against pH, NaCl and Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Conditions
2.2. Preparation of Inoculum
2.3. Acid Adaptation of C. sporogenes Strains
2.4. Experimental Design
2.5. Inoculation Procedure and Germination Assessment
2.6. Development of Logistic Regression Models
2.7. Assessment of Model’s Performance
2.8. Validation of the Logistic Regression Models in Table Olive Brines
3. Results
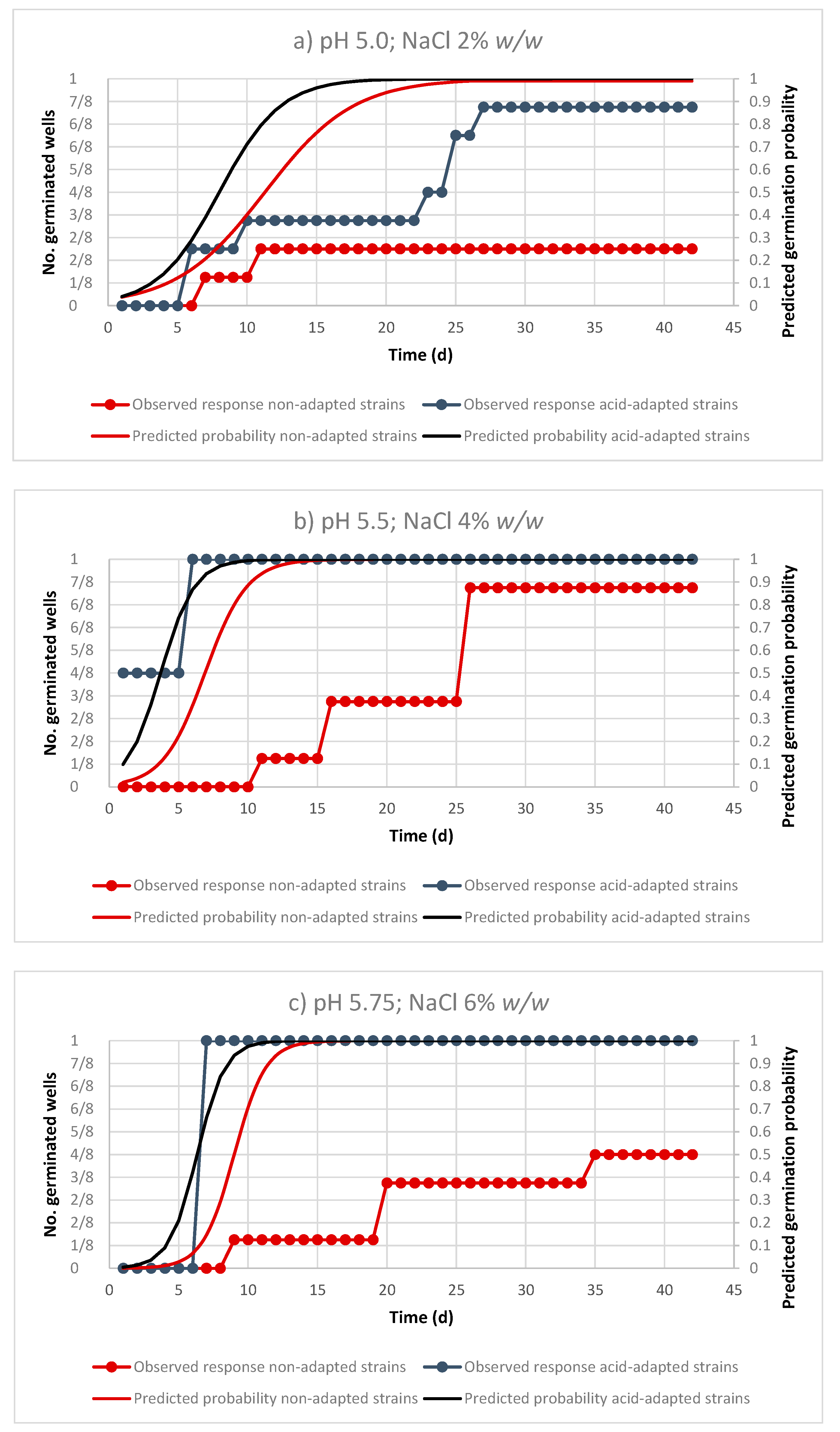
3.1. Performance of the Logistic Regression Models
3.2. Effect of Environmental Factor on the Probability of Germination of Non-Adapted and Acid-Adapted C. sporogenes Strains
3.3. Germination of C. sporogenes Strains in Table Olive Brines
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Songer, J.G. Clostridia as Agents of Zoonotic Disease. Vet. Microbiol. 2010. [Google Scholar] [CrossRef]
- Hong, Y.; Huang, L.; Byong, W. Mathematical Modeling and Growth Kinetics of Clostridium sporogenes in Cooked Beef. Food Control 2016, 60, 471–477. [Google Scholar] [CrossRef]
- Barari, M.; Kalantar, E. An Outbreak of Type A and B Botulism Associated with Traditional Vegetable Pickle in Sanandaj. Iran. J. Clin. Infect. Dis. 2010, 5, 111–112. [Google Scholar]
- Loutfy, M.R.; Austin, J.W.; Blanchfield, B.; Fong, I.W. An Outbreak of Foodborne Botulism in Ontario. Can. J. Infect. Dis. 2003. [Google Scholar] [CrossRef] [PubMed]
- Giraudon, I.; Cathcart, S.; Blomqvist, S.; Littleton, A.; Surman-Lee, S.; Mifsud, A.; Anaraki, S.; Fraser, G. Large Outbreak of Salmonella Phage Type 1 Infection with High Infection Rate and Severe Illness Associated with Fast Food Premises. Public Health 2009. [Google Scholar] [CrossRef] [PubMed]
- Browning, L.M.; Prempeh, H.; Little, C.; Houston, C.; Grant, K.; Cowden, J.M. An Outbreak of Food-Borne Botulism in Scotland, United Kingdom, November 2011. Eurosurveillance 2011. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Juliao, P.C.; Maslanka, S.; Dykes, J.; Gaul, L.; Bagdure, S.; Granzow-Kibiger, L.; Salehi, E.; Zink, D.; Neligan, R.P.; Barton-Behravesh, C.; et al. National Outbreak of Type a Foodborne Botulism Associated with a Widely Distributed Commercially Canned Hot Dog Chili Sauce. Clin. Infect. Dis. 2013. [Google Scholar] [CrossRef]
- Outbreak of Botulism Type e Associated with Eating a Beached Whale—Western Alaska, July 2002. Morb. Mortal. Wkly Rep. 2003, 52, 24.
- Telzak, E.E.; Bell, E.R.; Kautter, D.A.; Crowell, L.; Budnick, L.D.; Morse, D.L.; Schultz, S. An International Outbreak of Type E Botulism Due to Uneviscerated Fish. J. Infect. Dis. 1990. [Google Scholar] [CrossRef]
- Franciosa, G.; Pourshaban, M.; Gianfranceschi, M.; Gattuso, A.; Fenicia, L.; Ferrini, A.M.; Mannoni, V.; De Luca, G.; Aureli, P. Clostridium botulinum Spores and Toxin in Mascarpone Cheese and Other Milk Products. J. Food Prot. 1999. [Google Scholar] [CrossRef]
- Aureli, P.; Di Cunto, M.; Maffei, A.; De Chiara, G.; Franciosa, G.; Accorinti, L.; Gambardella, A.M.; Greco, D. An Outbreak in Italy of Botulism Associated with a Dessert Made with Mascarpone Cream Cheese. Eur. J. Epidemiol. 2000. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, N.H.; Hamza, D.A. Epidemiological Studies on Clostridium perfringens Food Poisoning in Retail Foods. Rev. Sci. Tech. 2017. [Google Scholar] [CrossRef] [PubMed]
- Trotz-Williams, L.A.; Mercer, N.J.; Walters, J.M.; Maki, A.M.; Johnson, R.P. Pork Implicated in a Shiga Toxin-Producing Escherichia coli O157:H7 Outbreak in Ontario, Canada. Can. J. Public Heal. 2012, 103, e322–e326. [Google Scholar] [CrossRef]
- Medina-Pradas, E.; Arroyo-López, F.N. Presence of Toxic Microbial Metabolites in Table Olives. Front. Microbiol. 2015. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2017. EFSA J. 2018. [Google Scholar] [CrossRef]
- Dong, Q.; Tu, K.; Guo, L.; Li, H.; Zhao, Y. Response Surface Model for Prediction of Growth Parameters from Spores of Clostridium sporogenes under Different Experimental Conditions. Food Microbiol. 2007. [Google Scholar] [CrossRef]
- Khanipour, E.; Flint, S.H.; McCarthy, O.J.; Golding, M.; Palmer, J.; Tamplin, M. Evaluation of the Effects of Sodium Chloride, Potassium Sorbate, Nisin and Lysozyme on the Probability of Growth of Clostridium sporogenes. Int. J. Food Sci. Technol. 2014. [Google Scholar] [CrossRef]
- Khanipour, E.; Flint, S.H.; Mccarthy, O.J.; Golding, M.; Palmer, J.; Ratkowsky, D.A.; Ross, T.; Tamplin, M. Modelling the Combined Effects of Salt, Sorbic Acid and Nisin on the Probability of Growth of Clostridium sporogenes in a Controlled Environment (Nutrient Broth). Food Control 2016, 62, 32–43. [Google Scholar] [CrossRef]
- Khanipour, E.; Flint, S.H.; Mccarthy, O.J.; Palmer, J.; Golding, M.; Ratkowsky, D.A.; Ross, T.; Tamplin, M. Modelling the Combined Effect of Salt, Sorbic Acid and Nisin on the Probability of Growth of Clostridium sporogenes in High Moisture Processed Cheese Analogue. Int. Dairy J. 2016, 57, 62–71. [Google Scholar] [CrossRef]
- Huang, L.; Li, C.; Hwang, C. International Journal of Food Microbiology Growth/No Growth Boundary of Clostridium perfringens from Spores in Cooked Meat: A Logistic Analysis. Int. J. Food Microbiol. 2018, 266, 257–266. [Google Scholar] [CrossRef]
- Lund, B.M.; Graham, A.F.; Franklin, J.G. The Effect of Acid pH on the Probability of Growth of Proteolytic Strains of Clostridium Botulinum. Int. J. Food Microbial. 1987, 4, 215–226. [Google Scholar] [CrossRef]
- Montville, T.J. Interaction of pH and NaCl on Culture Density of Clostridium botulinum 62A. Appl. Environ. Microbiol. 1983, 46, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Montville, T.J. Quantitation of pH- and Salt-Tolerant Subpopulations from Clostridium botulinum. Appl. Environ. Microbiol. 1984, 47, 28–30. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.M.; Young-Perkins, K.E.; Merson, R.L. Factors Influencing Clostridium botulinum Spore Germination, Outgrowth, and Toxin Formation in Acidified Media. Appl. Environ. Microbiol. 1988, 54, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Jobin, M.P.; Clavel, T.; Carlin, F.; Schmitt, P. Acid Tolerance Response Is Low-PH and Late-Stationary Growth Phase Inducible in Bacillus cereus TZ415. Int. J. Food Microbiol. 2002. [Google Scholar] [CrossRef]
- Derman, Y.; Söderholm, H.; Lindström, M.; Korkeala, H. Role of Csp Genes in NaCl, PH, and Ethanol Stress Response and Motility in Clostridium botulinum ATCC 3502. Food Microbiol. 2015. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, F.; Valero, A. Predictive Microbiology in Foods; SpringerBriefs in Food, Health, and Nutrition: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Bollerslev, A.M.; Nauta, M.; Hansen, T.B.; Aabo, S. A Risk Modelling Approach for Setting Microbiological Limits Using Enterococci as Indicator for Growth Potential of Salmonella in Pork. Int. J. Food Microbiol. 2017. [Google Scholar] [CrossRef]
- Ikawa, J.Y.; Genigeorgis, C. Probability of Growth and Toxin Production by Nonproteolytic Clostridium botulinum in Rockfish Fillets Stored under Modified Atmospheres. Int. J. Food Microbiol. 1987, 4, 167–181. [Google Scholar] [CrossRef]
- Zhao, L.; Montville, T.J.; Schaffner, D.W. Time-to-Detection, Percent-Growth-Positive and Maximum Growth Rate Models for Clostridium botulinum 56A at Multiple Temperatures. Int. J. Food Microbiol. 2002, 77, 187–197. [Google Scholar] [CrossRef]
- Ghabraie, M.; Vu, K.D.; Tnani, S.; Lacroix, M. Antibacterial Effects of 16 Formulations and Irradiation against Clostridium sporogenes in a Sausage Model. Food Control 2016, 63, 21–27. [Google Scholar] [CrossRef]
- Beerens, H. Bifidobacteria as Indicators of Faecal Contamination in Meat and Meat Products: Detection, Determination of Origin and Comparison with Escherichia coli. Int. J. Food Microbiol. 1998. [Google Scholar] [CrossRef]
- Agresti, A. Building and Applying Logistic Regression Models. In An Introduction to Categorical Data Analysis; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Daelman, J.; Vermeulen, A.; Willemyns, T.; Ongenaert, R.; Jacxsens, L.; Uyttendaele, M.; Devlieghere, F. Growth/No Growth Models for Heat-Treated Psychrotrophic Bacillus cereus Spores under Cold Storage. Int. J. Food Microbiol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ponce, A. Production and Characterization of Pure Clostridium Spore Suspensions. J. Appl. Microbiol. 2009, 106, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.H.; Dunn, M.L.; Ogden, L.V.; Jefferies, L.K.; Eggett, D.L.; Steele, F.M. Conditions Associated with Clostridium Sporogenes Growth as a Surrogate for Clostridium botulinum in Nonthermally Processed Canned Butter. J. Dairy Sci. 2013. [Google Scholar] [CrossRef]
- Crosthwait, C.D. Adaptation to Growth at Low pH by Clostridium Sporogenes. Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 1979. [Google Scholar]
- Whiting, R.C.; Call, J.E. Time of Growth Model for Proteolytic Clostridium botulinum. Food Microbiol. 1993. [Google Scholar] [CrossRef]
- Stewart, C.M.; Cole, M.B.; Legan, J.D.; Slade, L.; Vandeven, M.H.; Schaffner, D.W. Modeling the Growth Boundary of Staphylococcus aureus for Risk Assessment Purposes. J. Food Prot. 2001. [Google Scholar] [CrossRef]
- Graham, A.F.; Mason, D.R.; Peck, M.W. Predictive Model of the Effect of Temperature, pH and Sodium Chloride on Growth from Spores of Non-Proteolytic Clostridium botulinum. Int. J. Food Microbiol. 1996. [Google Scholar] [CrossRef]
- Couvert, O.; Divanac’h, M.L.; Lochardet, A.; Thuault, D.; Huchet, V. Modelling the Effect of Oxygen Concentration on Bacterial Growth Rates. Food Microbiol. 2019. [Google Scholar] [CrossRef]
- Skandamis, P.N.; Stopforth, J.D.; Kendall, P.; Belk, K.E.; Scanga, J.; Smith, G.C.; Sofos, J.N. Modeling the Effect of Inoculum Size and Acid Adaptation on Growth/No Growth Interface of Escherichia coli O157:H7. Int. J. Food Microbiol. 2007, 120, 237–249. [Google Scholar] [CrossRef]
- Vermeulen, A.; Gysemans, K.P.M.; Bernaerts, K.; Geeraerd, H.; Debevere, J.; Devlieghere, F.; Van Impe, J.F. Modelling the Influence of the Inoculation Level on the Growth/No Growth Interface of Listeria monocytogenes as a Function of pH, aw and Acetic Acid. Int. J. Food Microbiol. 2009, 135, 83–89. [Google Scholar] [CrossRef]
- International Olive Oil Council. Trade Standard Applying to Table Olives. RES-2/91-IV/04; International Olive Oil Council: Madrid, Spain, 2004. [Google Scholar]
- Gallardo-Guerrero, L.; Gandul-Rojas, B.; Moreno-Baquero, J.M.; López-López, A.; Bautista-Gallego, J.; Garrido-Fernández, A. Pigment, Physicochemical, and Microbiological Changes Related to the Freshness of Cracked Table Olives. J. Agric. Food Chem. 2013. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Bellido, M.Á.; Valero, A.; Pradas, E.M.; Gil, V.R.; Rodríguez-Gómez, F.; Posada-Izquierdo, G.D.; Rincón, F.; Possas, A.; García-Gimeno, R.M.; Arroyo-López, F.N. A Probabilistic Decision-Making Scoring System for Quality and Safety Management in Aloreña de Málaga Table Olive Processing. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
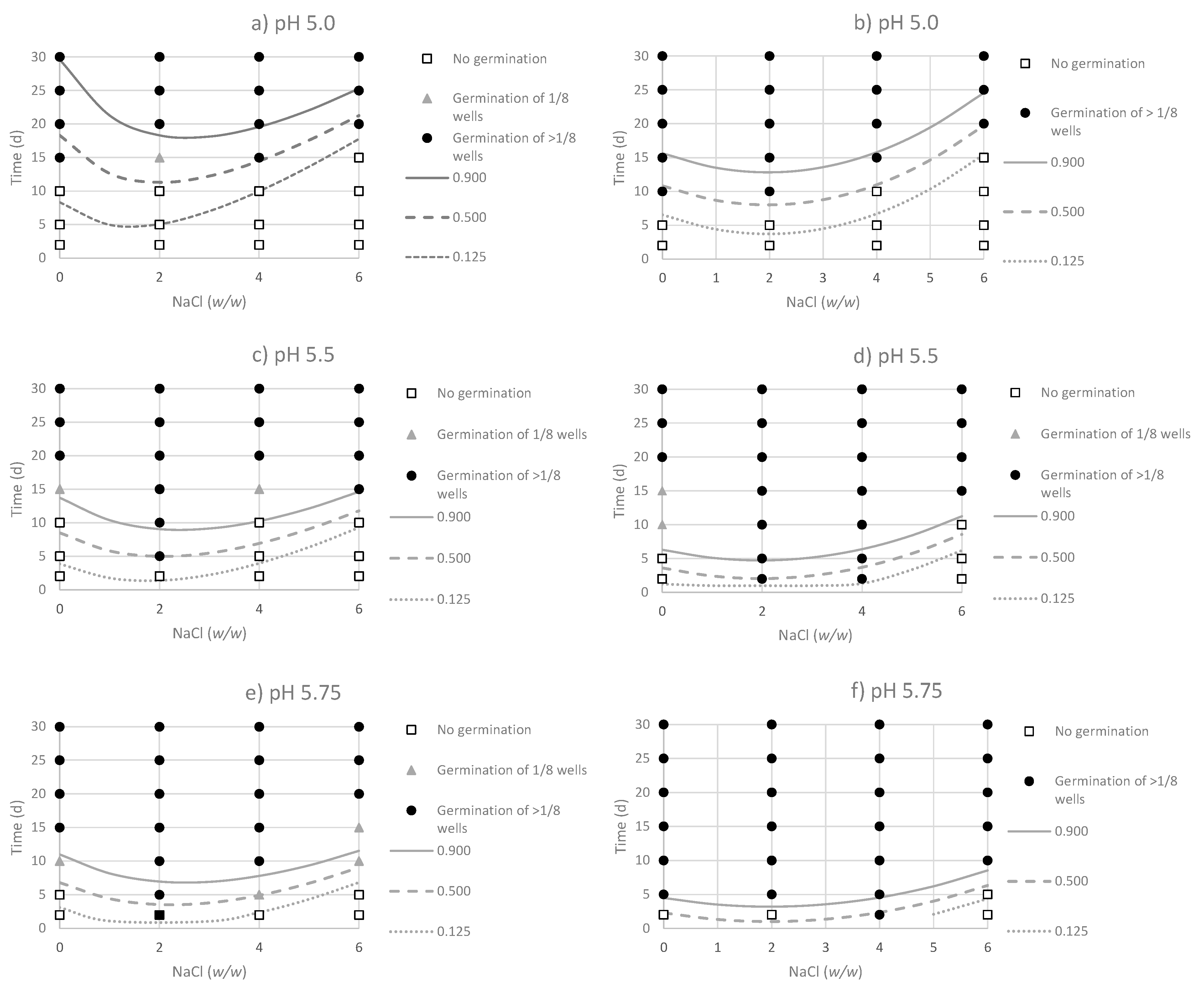

| pH | ||||||||
|---|---|---|---|---|---|---|---|---|
| NaCl (%, w/w) | 4.00 | 4.50 | 5.00 | 5.50 | 5.75 | 6.00 | 6.50 | 7.00 |
| 0 | ||||||||
| 2 | ||||||||
| 4 | ||||||||
| 6 | ||||||||
| Coefficient | Estimate | S.E. | Wald | df | P-Value | Lower C.I (95%) | Upper C.I (95%) | |
|---|---|---|---|---|---|---|---|---|
| Acid-Adapted C. sporogenes Strains | time | −3.175 | 0.527 | 36.289 | 1 | <0.001 | −4.208 | −2.142 |
| pH | 20.898 | 8.639 | 5.851 | 1 | 0.016 | 3.965 | 37.831 | |
| NaCl | 1.291 | 0.410 | 9.903 | 1 | 0.002 | 0.487 | 2.095 | |
| Time × pH | 0.726 | 0.113 | 41.182 | 1 | <0.001 | 0.504 | 0.948 | |
| pH2 | −1.616 | 0.714 | 5.126 | 1 | 0.024 | −3.016 | −0.217 | |
| NaCl2 | −0.328 | 0.075 | 19.331 | 1 | <0.001 | −0.474 | −0.182 | |
| constant | −68.996 | 25.954 | 7.067 | 1 | 0.008 | − | − | |
| Non-Adapted C. sporogenes Strains | time | −3.610 | 0.382 | 89.462 | 1 | <0.001 | −4.358 | −2.862 |
| NaCl | −6.566 | 1.620 | 16.424 | 1 | <0.001 | −9.741 | −3.390 | |
| Time × NaCl | 0.059 | 0.014 | 17.743 | 1 | <0.001 | 0.032 | 0.087 | |
| NaCl2 | −0.343 | 0.062 | 30.180 | 1 | <0.001 | −0.466 | −0.221 | |
| ln(pH) × time | 2.364 | 0.248 | 91.015 | 1 | <0.001 | 1.878 | 2.850 | |
| ln(pH) × NaCl | 4.514 | 0.943 | 22.927 | 1 | <0.001 | 2.666 | 6.362 | |
| constant | −3.565 | 0.555 | 41.218 | 1 | <0.001 | − | − |
| Goodness of Fit/Predictive Power | Acid-Adapted C. sporogenes Strains | Non-Adapted C. sporogenes Strains |
|---|---|---|
| Coefficient | Coefficient | |
| −2lnL 1 | 119.848 | 195.89 |
| AIC 2 | 133.848 | 209.89 |
| Hosmer-Lemeshow (df = 8) | 2.065 | 6.044 |
| p-value | 0.979 | 0.642 |
| Nagelkerke R2 | 0.950 | 0.921 |
| ACID-ADAPTED C. SPOROGENES STRAINS | ||||
|---|---|---|---|---|
| Training | Estimated Probability | Total | Correct Prediction (%) | |
| Observed response | No germination | Germination | ||
| No germination | 292 | 32 | 324 | 90.12 |
| Germination | 1 | 683 | 684 | 99.85 |
| Total | 293 | 715 | 1008 | 96.83 |
| Validation | Estimated Probability | Total | Correct Prediction (%) | |
| Observed response | No germination | Germination | ||
| No germination | 92 | 12 | 104 | 88.46 |
| Germination | 0 | 232 | 232 | 100.00 |
| Total | 92 | 244 | 336 | 96.43 |
| NON-ADAPTED C. SPOROGENES STRAINS | ||||
| Training | Estimated Probability | Total | Correct Prediction (%) | |
| Observed response | No germination | Germination | ||
| No germination | 311 | 55 | 366 | 84.97 |
| Germination | 0 | 642 | 642 | 100.00 |
| Total | 311 | 697 | 1008 | 94.54 |
| Validation | Estimated Probability | Total | Correct Prediction (%) | |
| Observed response | No germination | Germination | ||
| No germination | 89 | 26 | 115 | 77.39 |
| Germination | 0 | 221 | 221 | 100.00 |
| Total | 89 | 247 | 336 | 92.26 |
| Non-Adapted Strains | Acid-Adapted Strains | ||||||
|---|---|---|---|---|---|---|---|
| pH | NaCl (%) | Germination | Dataset | pH | NaCl (%) | Germination | Dataset |
| 5.0 | 0.0 | 5/8 | Training | 5.0 | 0.0 | 6/8 | Training |
| 5.0 | 2.0 | 2/8 | Validation | 5.0 | 2.0 | 7/8 | Validation |
| 5.0 | 4.0 | 3/8 | Training | 5.0 | 4.0 | 5/8 | Training |
| 5.0 | 6.0 | 4/8 | Training | 5.0 | 6.0 | 7/8 | Training |
| 5.5 | 6.0 | 5/8 | Training | 5.5 | 0.0 | 7/8 | Validation |
| 5.75 | 0.0 | 5/8 | Training | ||||
| 5.75 | 4.0 | 5/8 | Training | ||||
| 5.75 | 6.0 | 4/8 | Validation | ||||
| Non-Adapted C. sporogenes Strains | Adapted C. sporogenes Strains | ||||||
|---|---|---|---|---|---|---|---|
| pH | NaCl (%) | Obst_brine | Predt_model | pH | NaCl (%) | Obst_brine | Predt_model |
| 5.0 | 2 | 8 | 5.07 | 5.0 | 2 | 6 | 3.72 |
| 5.0 | 4 | >13 | 9.96 | 5.0 | 4 | 10 | 6.70 |
| 5.0 | 6 | >13 | >13 | 5.0 | 6 | >13 | >13 |
| 5.5 | 2 | 3 | 1.37 | 5.5 | 2 | 2 | 1 |
| 5.5 | 4 | 8 | 3.93 | 5.5 | 4 | 1 | 1.34 |
| 5.5 | 6 | >13 | 9.26 | 5.5 | 6 | >13 | 6.18 |
| 6.0 | 4 | 1 | 1.18 | 6.0 | 4 | 1 | 1 |
| 6.0 | 6 | 3 | 4.93 | 6.0 | 6 | 3 | 3.30 |
| Microorganisms | T (°C) | pH | NaCl (%) | Growth | Obs. Time (Days) | Reference | p1 (non-Adapted) | p (Acid-Adapted) |
|---|---|---|---|---|---|---|---|---|
| C. botulinum proteolytic | 30 | 4.7 | 2.5 | No | >42 | FSA (UK) 2 | Yes (0.97) (fs) | Yes (0.99) (fs) |
| C. botulinum proteolytic | 30 | 4.8 | 1.5 | Yes | 8.96 | FSA (UK) | Yes (0.13) (c) | Yes (0.13) (c) |
| C. botulinum proteolytic | 30 | 5.6 | 5.5 | Yes | >42 | FSA (UK) | Yes (0.99) (c) | Yes (1.00) (c) |
| C. botulinum proteolytic | 30 | 6.3 | 5.5 | Yes | 11.08 | FSA (UK) | Yes (0.99) (c) | Yes (1.00) (c) |
| C. botulinum proteolytic | 30 | 5.2 | 1.5 | No | >42 | FSA (UK) | Yes (0.99) (fs) | Yes (1.00) (fs) |
| C. botulinum proteolytic | 30 | 5.3 | 4.5 | Yes | 12.96 | FSA (UK) | Yes (0.83) (c) | Yes (0.98) (c) |
| C. botulinum proteolytic | 30 | 5.1 | 3.5 | Yes | 11.00 | FSA (UK) | Yes (0.48) (c) | Yes (0.86) (c) |
| C. botulinum proteolytic | 30 | 7 | 5.5 | Yes | 21.17 | FSA (UK) | Yes (1.00) (c) | Yes (1.00) (c) |
| C. botulinum proteolytic | 25 | 4.7 | 0.5 | Yes | 11.88 | FSA (UK) | No (0.07) (fs) | No (0.04) (fs) |
| C. botulinum proteolytic | 25 | 5.9 | 3.5 | Yes | 8.31 | FSA (UK) | Yes (0.97) (c) | Yes (0.99) (c) |
| C. botulinum proteolytic | 25 | 5.7 | 4.5 | Yes | 14.05 | FSA (UK) | Yes (0.99) (c) | Yes (0.99) (c) |
| C. botulinum proteolytic | 25 | 5.6 | 5.5 | Yes | 29.02 | FSA (UK) | Yes (1.00) (c) | Yes (1.00) (c) |
| C. botulinum proteolytic | 25 | 7 | 4.5 | Yes | 8.31 | FSA (UK) | Yes (1.00) (c) | Yes (1.00) (c) |
| C. botulinum proteolytic | 35 | 4.7 | 0.5 | No | >42 | FSA (UK) | Yes (0.46) (fs) | Yes (0.98) (fs) |
| C. botulinum proteolytic 3 | 30 | 7 | 0.6 | Yes | 1.00 | Juneja et al. 1999 4 | Yes (0.20) (c) | Yes (0.65) (c) |
| C. sporogenes | 37 | 7 | 4 | Yes | >42 | 18 | Yes (1.00) (c) | Yes (1.00) (c) |
| C. sporogenes | 37 | 5.5 | 4 | Yes | >42 | 18 | Yes (1.00) (c) | Yes (1.00) (c) |
| C. sporogenes 5 | 30 | 5.5 | 2 | Yes | 1.00 | 16 | No (0.10) (fd) | Yes (0.30) (c) |
| C. sporogenes 5 | 29.6 | 5.6 | 6 | Yes | 1.00 | 16 | No (0.00) (fd) | No (0.00) (fd) |
| C. botulinum proteolytic 6 | 30 | 6.8 | 2 | Yes | 14.00 | 21 | Yes (1.00) (c) | Yes (1.00) (c) |
| C. botulinum proteolytic 6 | 30 | 5.1 | 2 | Yes | 14.00 | 21 | Yes (0.78) (c) | Yes (0.96) (c) |
| C. botulinum proteolytic 6 | 30 | 4.9 | 2 | Yes | 14.00 | 21 | Yes (0.56) (c) | Yes (0.84) (c) |
| C. botulinum proteolytic 6 | 30 | 4.9 | 2 | No | 14.00 | 21 | Yes (0.41) (fs) | Yes (0.65) (fs) |
| C. botulinum proteolytic 6 | 30 | 4.8 | 2 | No | 14.00 | 21 | Yes (0.26) (fs) | Yes (0.35) (fs) |
| C. botulinum proteolytic 6 | 30 | 4.7 | 2 | No | 14.00 | 21 | No (0.07) (c) | No (0.10) (c) |
| C. botulinum proteolytic | 30 | 5.5 | 4 | No | >42 | 30 | Yes (1.00) (fs) | Yes (1.00) (fs) |
| C. botulinum proteolytic | 30 | 5.5 | 2 | Yes | >42 | 30 | Yes (1.00) (c) | Yes (1.00) (c) |
| C. botulinum proteolytic | 30 | 6 | 4 | Yes | >42 | 30 | Yes (1.00) (c) | Yes (1.00) (c) |
| C. botulinum proteolytic 7 | 30 | 5.5 | 3 | Yes | 3 | 23 | Yes (0.19) (c) | Yes (0.61) (c) |
| C. botulinum proteolytic | 30 | 5.0 | 0 | Yes | 30 | 22 | Yes (0.91) (c) | Yes (1.00) (c) |
| C. botulinum proteolytic | 30 | 5.0 | 3 | No | 30 | 22 | Yes (1.00) (fs) | Yes (1.00) (fs) |
| C. botulinum proteolytic | 30 | 5.5 | 4 | Yes | 30 | 22 | Yes (1.00) (c) | Yes (1.00) (c) |
| TOTAL (c/fs/fd) 8 | 68.75%/25%/6.25% | 71.88%/25%/3.12% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valero, A.; Olague, E.; Medina-Pradas, E.; Garrido-Fernández, A.; Romero-Gil, V.; Cantalejo, M.J.; García-Gimeno, R.M.; Pérez-Rodríguez, F.; Posada-Izquierdo, G.D.; Arroyo-López, F.N. Influence of Acid Adaptation on the Probability of Germination of Clostridium sporogenes Spores Against pH, NaCl and Time. Foods 2020, 9, 127. https://doi.org/10.3390/foods9020127
Valero A, Olague E, Medina-Pradas E, Garrido-Fernández A, Romero-Gil V, Cantalejo MJ, García-Gimeno RM, Pérez-Rodríguez F, Posada-Izquierdo GD, Arroyo-López FN. Influence of Acid Adaptation on the Probability of Germination of Clostridium sporogenes Spores Against pH, NaCl and Time. Foods. 2020; 9(2):127. https://doi.org/10.3390/foods9020127
Chicago/Turabian StyleValero, Antonio, Elena Olague, Eduardo Medina-Pradas, Antonio Garrido-Fernández, Verónica Romero-Gil, María Jesús Cantalejo, Rosa María García-Gimeno, Fernando Pérez-Rodríguez, Guiomar Denisse Posada-Izquierdo, and Francisco Noé Arroyo-López. 2020. "Influence of Acid Adaptation on the Probability of Germination of Clostridium sporogenes Spores Against pH, NaCl and Time" Foods 9, no. 2: 127. https://doi.org/10.3390/foods9020127
APA StyleValero, A., Olague, E., Medina-Pradas, E., Garrido-Fernández, A., Romero-Gil, V., Cantalejo, M. J., García-Gimeno, R. M., Pérez-Rodríguez, F., Posada-Izquierdo, G. D., & Arroyo-López, F. N. (2020). Influence of Acid Adaptation on the Probability of Germination of Clostridium sporogenes Spores Against pH, NaCl and Time. Foods, 9(2), 127. https://doi.org/10.3390/foods9020127










