Description of Cumbeba (Tacinga inamoena) Waste Drying at Different Temperatures Using Diffusion Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Convective Drying of the Waste
2.3. Mathematical Modeling of Drying Kinetics
2.3.1. Diffusion Equation or Governing Equation
Model 1: Analytical Solution for the Diffusion Equation Considering Third-Kind or Convective Boundary Condition
Model 2: First-Kind or Prescribed Boundary Condition
Model 3: First Term of the Infinite Series
2.4. Computer Simulation
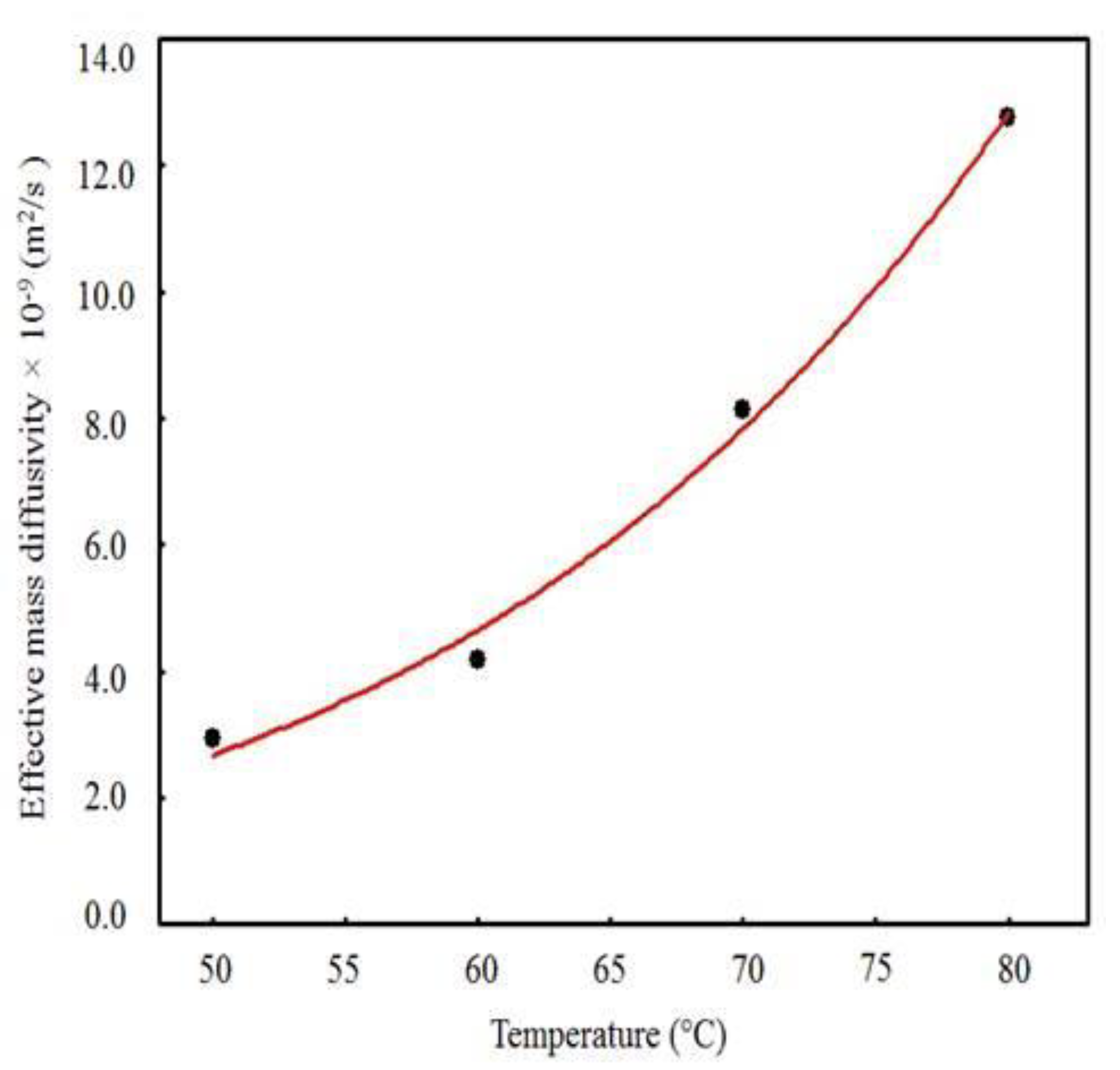
2.5. Arrhenius Equation
3. Results and Discussion
3.1. Results Obtained with Model 3
3.2. Results Obtained with Model 2
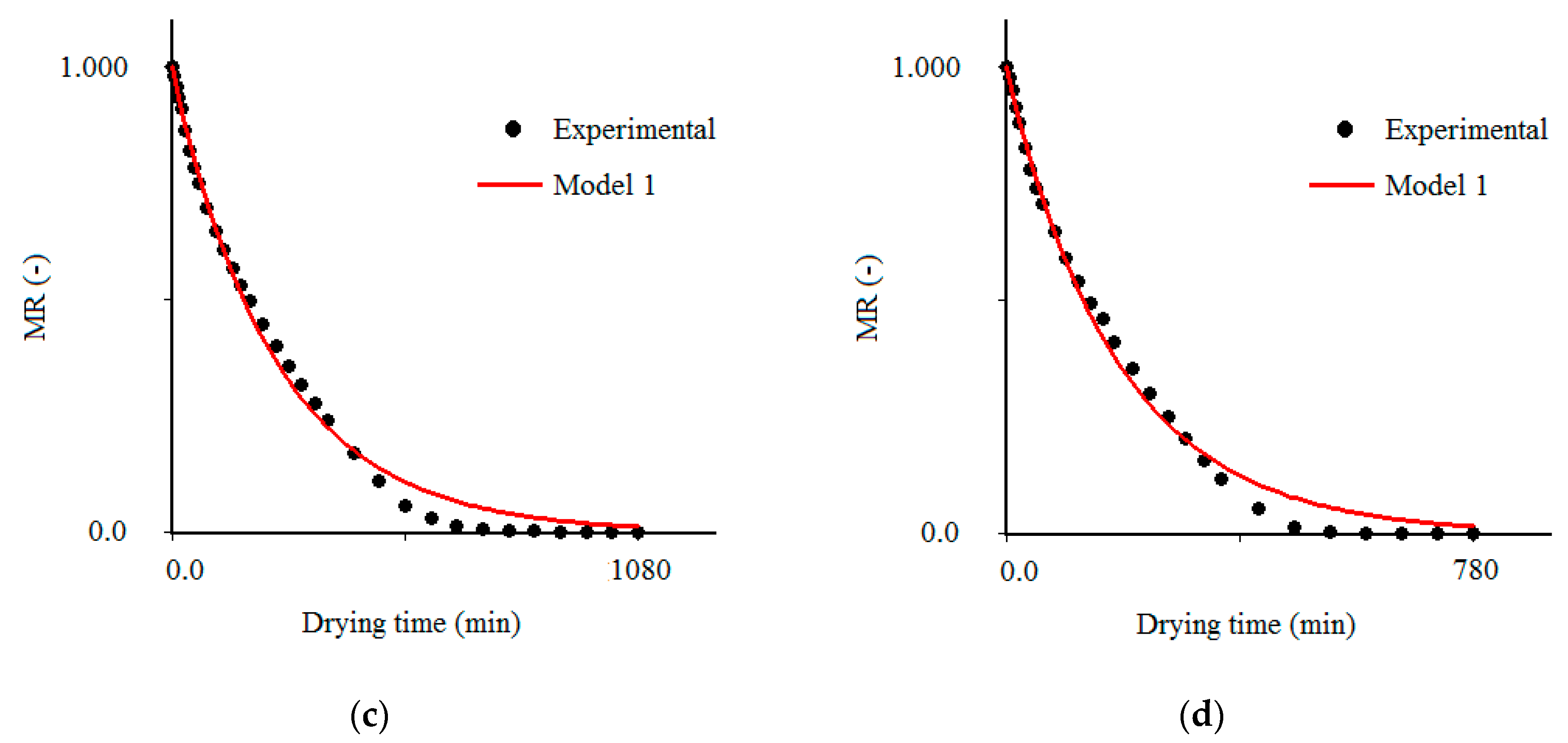
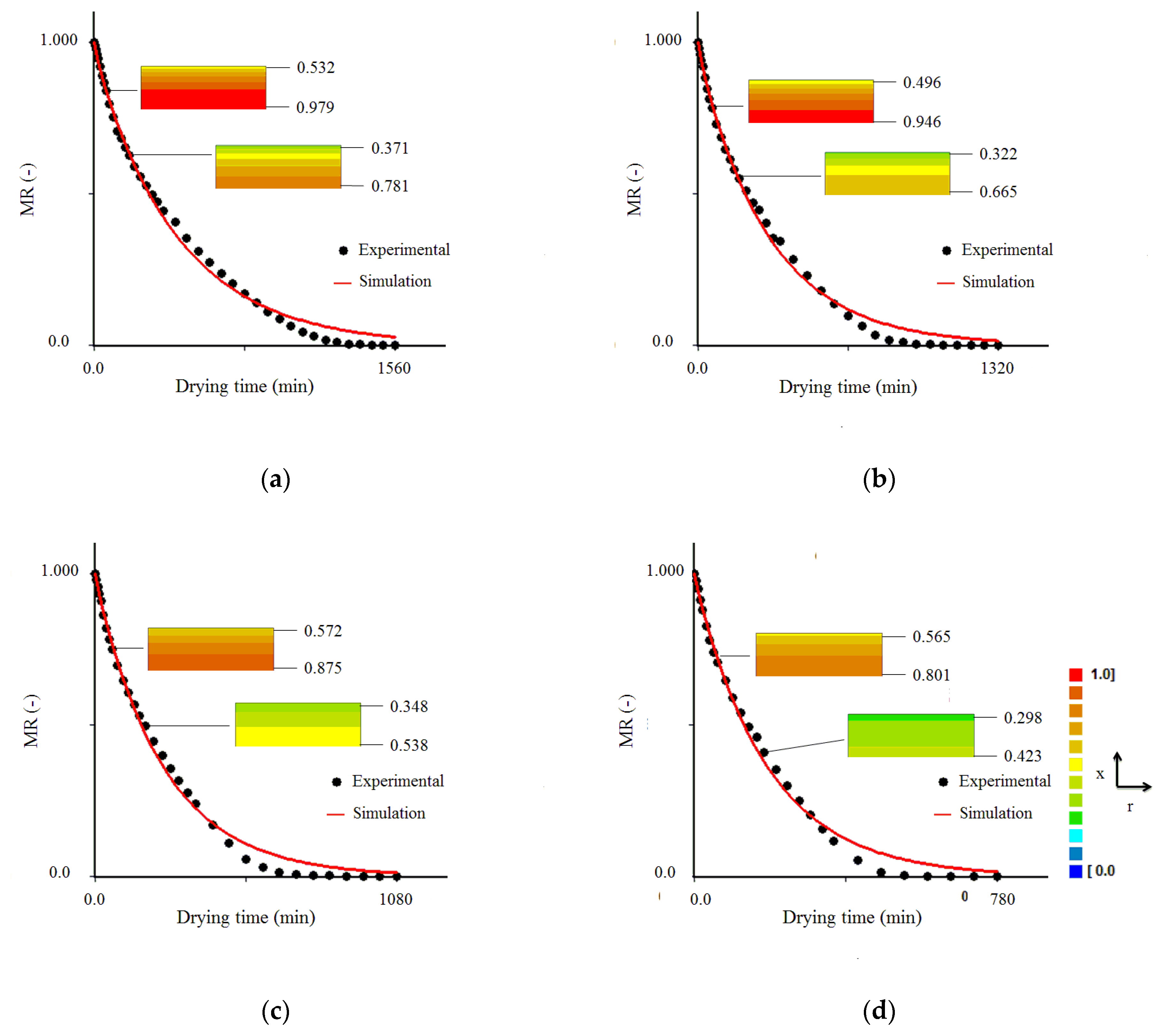
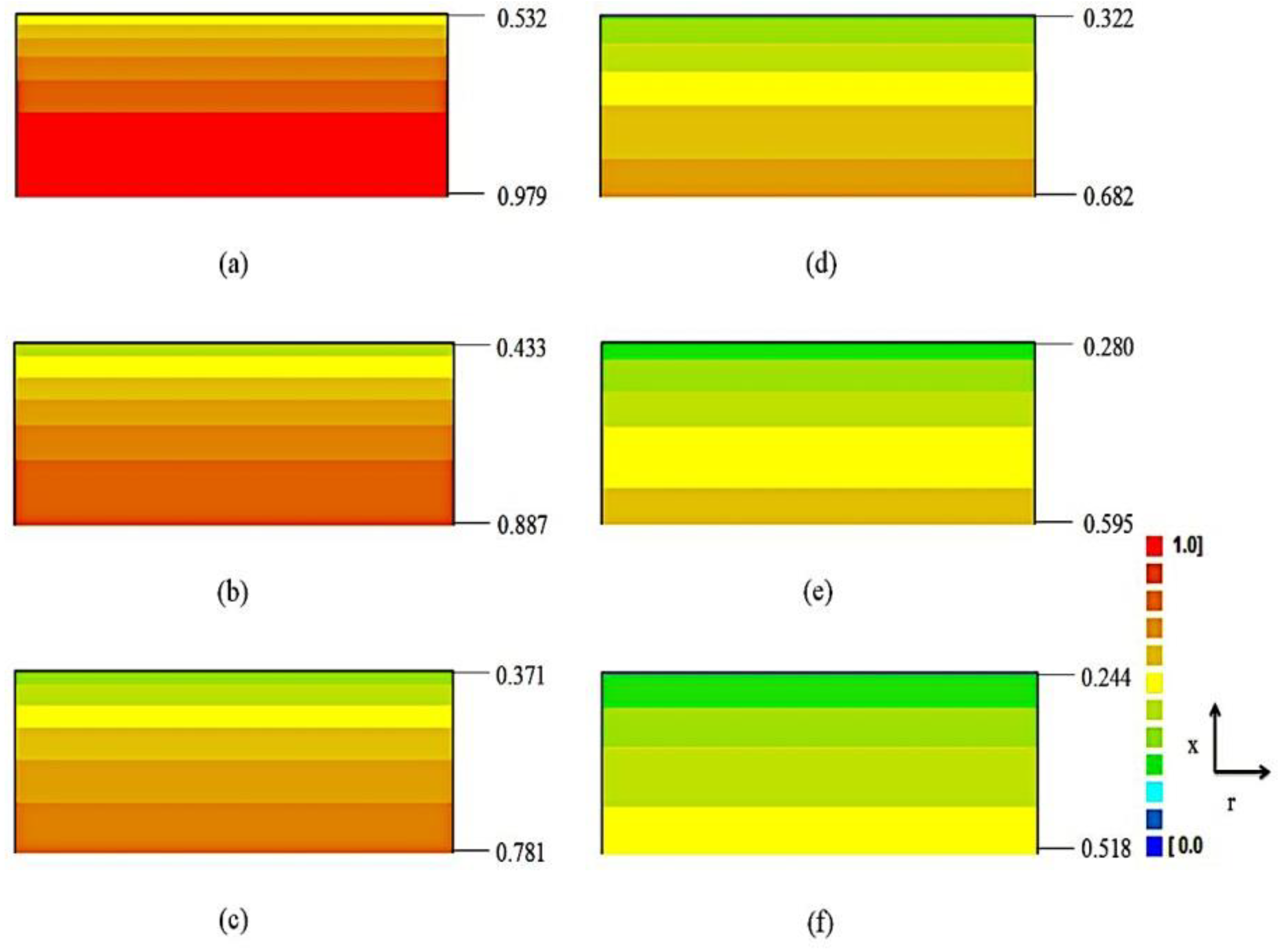
3.3. Results Obtained with the Model 1
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Souza, A.C.M.; Gamarra-Rojas, G.; Andrade, S.A.C.; Guerra, N.B. Características físicas, químicas e organolépticas de quipá (Tacinga inamoena, Cactaceae). Rev. Bras. Frutic. 2007, 29, 292–295. [Google Scholar] [CrossRef]
- Silva, S.; Primo, D.B.; Torres, L.; Martins, L.; Lima, A.; Silva, F. Features of Postharvest Physiology and Quality of Cactaceae Fruits from Brazilian Northeast. Acta Hortic. 2009, 811, 113–122. [Google Scholar] [CrossRef]
- Nascimento, V.T.D.; De Moura, N.P.; Vasconcelos, M.A.D.S.; Maciel, M.I.S.; De Albuquerque, U.P. Chemical characterization of native wild plants of dry seasonal forests of the semi-arid region of northeastern Brazil. Food Res. Int. 2011, 44, 2112–2119. [Google Scholar] [CrossRef]
- Dantas, R.; Shunemann, A.; Silva, S.; Melo, R.; Silva, R.; Souza, F. Quality and descriptive terminology of tacinga inamoena (k. Schum.). Fruits. Acta Hortic. 2015, 58, 143–149. [Google Scholar] [CrossRef]
- Dantas, R.; Silva, S.; Santos, L.; Dantas, A.; Lima, R.; Soares, L. Betalains and antioxidant activity in fruits of cactaceae from brazilian semiarid. Acta Hortic. 2015, 58, 151–157. [Google Scholar] [CrossRef]
- Formiga, A.D.S.; Da Costa, F.B.; Da Silva, M.S.; Pereira, E.M.; Brasil, Y.L. Aspectos físicos e químicos de frutos de Quipá (Tacinga inamoena). Rev. Verde Agroecol. Desenvolv. Sustentável 2016, 11, 25–29. [Google Scholar] [CrossRef][Green Version]
- Lima, R.K.B.; Sarmento, J.D.A.; Neta, T.R.; Morais, P.L.D.; Silva, G.G.; Sarmento, D.H.A. Caracterização dos frutos do pelo (Tacinga inamoena) e do mandacaru (Cereus jamacaru). In Coleção Agroecologia e Meio Ambiente no Semiárido: Produção Orgânica no Semiárido; Universidade Federal Rural do Semi-Árido (EDUFERSA): Mossoró, Brazil, 2016; Volume 3, pp. 335–344. ISBN 978-85-5757-063-4. [Google Scholar]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, technological and in vitro antioxidant properties of mango, guava, pineapple and passion fruit dietary fibre concentrate. Food Chem. 2012, 135, 1520–1526. [Google Scholar] [CrossRef]
- Chaurasiya, R.S.; Hebbar, H.U. Extraction of bromelain from pineapple core and purification by RME and precipitation methods. Sep. Purif. Technol. 2013, 111, 90–97. [Google Scholar] [CrossRef]
- Arlene, A.A.; Prima, K.A.; Utama, L.; Anggraini, S.A. The Preliminary Study of the Dye Extraction from the Avocado Seed Using Ultrasonic Assisted Extraction. Procedia Chem. 2015, 16, 334–340. [Google Scholar] [CrossRef]
- Chel-Guerrero, L.; Barbosa-Martín, E.; Martínez-Antonio, A.; González-Mondragón, E.; Betancur-Ancona, D. Some physicochemical and rheological properties of starch isolated from avocado seeds. Int. J. Biol. Macromol. 2016, 86, 302–308. [Google Scholar] [CrossRef]
- Oba, C.; Ota, M.; Nomura, K.; Fujiwara, H.; Takito, J.; Sato, Y.; Ohizumi, Y.; Inomata, H. Extraction of nobiletin from Citrus Unshiu peels by supercritical fluid and its CRE-mediated transcriptional activity. Phytomedicine 2017, 27, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Ndayishimiye, J.; Lim, D.J.; Chun, B. Antioxidant and antimicrobial activity of oils obtained from a mixture of citrus by-products using a modified supercritical carbon dioxide. J. Ind. Eng. Chem. 2018, 57, 339–348. [Google Scholar] [CrossRef]
- Chimplee, S.; Klinkesorn, U. Thin-Layer Drying Model of Rambutan (Nephelium lappaceum L.) Kernel and Its Application in Fat Extraction Process. Int. J. Food Eng. 2015, 11, 243–253. [Google Scholar] [CrossRef]
- Avhad, M.; Marchetti, J. Mathematical modelling of the drying kinetics of Hass avocado seeds. Ind. Crops Prod. 2016, 91, 76–87. [Google Scholar] [CrossRef]
- Dos Santos, F.S.; De Figueirêdo, R.M.F.; Queiroz, A.J.D.M.; Santos, D.D.C. Drying kinetics and physical and chemical characterization of white-fleshed ‘pitaya’ peels. Rev. Bras. Eng. Agrícola Ambient. 2017, 21, 872–877. [Google Scholar] [CrossRef]
- Guimarães, R.; De Oliveira, D.E.C.; Resende, O.; Silva, J.D.S.; De Rezende, T.A.M.; Egea, M.B. Thermodynamic properties and drying kinetics of ‘okara’. Rev. Bras. Eng. Agrícola Ambient. 2018, 22, 418–423. [Google Scholar] [CrossRef]
- Nogueira, G.D.; Silva, P.B.; Duarte, C.R.; Barrozo, M.A.D.S. Analysis of a hybrid packed bed dryer assisted by infrared radiation for processing acerola (Malpighia emarginata D.C.) residue. Food Bioprod. Process. 2019, 114, 235–244. [Google Scholar] [CrossRef]
- Kumar, C.; Millar, G.J.; Karim, M.A.; Karim, M. Effective Diffusivity and Evaporative Cooling in Convective Drying of Food Material. Dry. Technol. 2014, 33, 227–237. [Google Scholar] [CrossRef]
- Perussello, C.A.; Kumar, C.; De Castilhos, F.; Karim, M. Heat and mass transfer modeling of the osmo-convective drying of yacon roots (Smallanthus sonchifolius). Appl. Therm. Eng. 2014, 63, 23–32. [Google Scholar] [CrossRef]
- Ju, H.-Y.; Law, C.-L.; Fang, X.-M.; Xiao, H.-W.; Liu, Y.-H.; Gao, Z.-J. Drying kinetics and evolution of the sample’s core temperature and moisture distribution of yam slices (Dioscorea alata L.) during convective hot-air drying. Dry. Technol. 2015, 34, 1297–1306. [Google Scholar] [CrossRef]
- Moran, M.; Higgins, T. Analytical heat diffusion theory. J. Frankl. Inst. 1970, 289, 406–408. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion, 1st ed.; Clarendon Press: Oxford, UK, 1992. [Google Scholar]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2001. [Google Scholar]
- Silva, W.P.; Silva, C.M.D.P.S.; Silva, D.D.P.S.E.; Neves, G.D.A.; De Lima, A.G.B. Mass and heat transfer study in solids of revolution via numerical simulations using finite volume method and generalized coordinates for the Cauchy boundary condition. Int. J. Heat Mass Transf. 2010, 53, 1183–1194. [Google Scholar] [CrossRef]
- Henríquez, C.; Cordova, A.; Almonacid, S.; Saavedra, J. Kinetic modeling of phenolic compound degradation during drum-drying of apple peel by-products. J. Food Eng. 2014, 143, 146–153. [Google Scholar] [CrossRef]
- Kara, C.; Doymaz, I. Thin Layer Drying Kinetics of By-Products from Pomegranate Juice Processing. J. Food Process. Preserv. 2014, 39, 480–487. [Google Scholar] [CrossRef]
- Goula, A.M.; Thymiatis, K.; Kaderides, K. Valorization of grape pomace: Drying behavior and ultrasound extraction of phenolics. Food Bioprod. Process. 2016, 100, 132–144. [Google Scholar] [CrossRef]
- Saavedra, J.; Córdova, A.; Navarro, R.; Díaz-Calderón, P.; Fuentealba, C.; Astudillo-Castro, C.; Toledo, L.; Enrione, J.; Galvez, L. Industrial avocado waste: Functional compounds preservation by convective drying process. J. Food Eng. 2017, 198, 81–90. [Google Scholar] [CrossRef]
- Wilkins, R.; Brusey, J.; Gaura, E. Modelling uncontrolled solar drying of mango waste. J. Food Eng. 2018, 237, 44–51. [Google Scholar] [CrossRef]
- Badaoui, O.; Hanini, S.; Djebli, A.; Haddad, B.; Benhamou, A. Experimental and modelling study of tomato pomace waste drying in a new solar greenhouse: Evaluation of new drying models. Renew. Energy 2019, 133, 144–155. [Google Scholar] [CrossRef]
- Da Silva, W.P.; Precker, J.W.; Silva, C.M.E.; Silva, D.D.E. Determination of the effective diffusivity via minimization of the objective function by scanning: Application to drying of cowpea. J. Food Eng. 2009, 95, 298–304. [Google Scholar] [CrossRef]
- Da Silva, W.P.; Precker, J.W.; Silva, C.M.E.; Gomes, J.P. Determination of effective diffusivity and convective mass transfer coefficient for cylindrical solids via analytical solution and inverse method: Application to the drying of rough rice. J. Food Eng. 2010, 98, 302–308. [Google Scholar] [CrossRef]
- Silva, W.P.; Farias, V.S.O.; Neves, G.D.A.; De Lima, A.G.B. Modeling of water transport in roof tiles by removal of moisture at isothermal conditions. Heat Mass Transf. 2011, 48, 809–821. [Google Scholar] [CrossRef]
- Silva, W.P.; Precker, J.W.; Gomes, J.P.; Nascimento, P.L. Diffusion models for the description of seedless grape drying using analytical and numerical solutions. Agric. Sci. 2012, 3, 545–556. [Google Scholar] [CrossRef][Green Version]
- De Lima, A.G.B.; Delgado, J.M.P.Q.; De Oliveira, V.A.B.; De Melo, J.C.S.; Silva, C.J.E. Porous Materials Drying Model Based on the Thermodynamics of Irreversible Processes: Background and Application. In From Creep Damage Mechanics to Homogenization Methods; Springer Science and Business Media LLC.: Berlin/Heidelberg, Germany, 2014; Volume 48, pp. 1–23. [Google Scholar]
- Johann, G.; De Menezes, M.L.; Pereira, N.C.; Da Silva, E.A. Comparing models to Neumann and Dirichlet conditions in grape seed drying. Appl. Therm. Eng. 2016, 93, 865–871. [Google Scholar] [CrossRef]
- Nascimento, E.M.D.; Mulet, A.; Ascheri, J.L.R.; De Carvalho, C.W.P.; Cárcel, J.A. Effects of high-intensity ultrasound on drying kinetics and antioxidant properties of passion fruit peel. J. Food Eng. 2016, 170, 108–118. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis. Association of Official Analytical Chemists; Method 934.01, 16th ed.; AOAC: Arlington, Virginia, USA, 1997. [Google Scholar]
- Silva, W.P.; Silva, C.M.D.P.S.; Lins, M.A.A.; Da Costa, W.S. Optimal Removal of Experimental Points to Determine Apparent Thermal Diffusivity of Canned Products. Int. J. Food Eng. 2014, 10, 223–231. [Google Scholar] [CrossRef]
- Zhao, P.; Zhong, L.; Zhu, R.; Zhao, Y.; Luo, Z.; Yang, X. Drying characteristics and kinetics of Shengli lignite using different drying methods. Energy Convers. Manag. 2016, 120, 330–337. [Google Scholar] [CrossRef]
- Galaz, P.; Valdenegro, M.; Ramírez, C.; Nuñez, H.; Almonacid, S.; Simpson, R. Effect of drum drying temperature on drying kinetic and polyphenol contents in pomegranate peel. J. Food Eng. 2017, 208, 19–27. [Google Scholar] [CrossRef]
- Erbay, Z.; Icier, F. A Review of Thin Layer Drying of Foods: Theory, Modeling, and Experimental Results. Crit. Rev. Food Sci. Nutr. 2010, 50, 441–464. [Google Scholar] [CrossRef]
- Kucuk, H.; Midilli, A.; Kilic, A.; Dincer, I. A Review on Thin-Layer Drying-Curve Equations. Dry. Technol. 2014, 32, 757–773. [Google Scholar] [CrossRef]
- Bevington, P.R.; Robinson, D.K. Data Reduction and Error Analysis for the Physical Sciences, 2rd ed.; WCB/McGraw-Hill: Boston, MA, USA, 1992. [Google Scholar]
- Taylor, J.R. An Introduction to Error Analysis, 2nd ed.; University Science Books: Sausalito, CA, USA, 1997. [Google Scholar]
- Guiné, R.; Henrriques, F.; Barroca, M.J. Mass Transfer Coefficients for the Drying of Pumpkin (Cucurbita moschata) and Dried Product Quality. Food Bioprocess Technol. 2012, 5, 176–183. [Google Scholar] [CrossRef]
- Tzempelikos, D.A.; Mitrakos, D.; Vouros, A.; Bardakas, A.V.; Filios, A.; Margaris, D.P. Numerical modeling of heat and mass transfer during convective drying of cylindrical quince slices. J. Food Eng. 2015, 156, 10–21. [Google Scholar] [CrossRef]
- Pilatti, D.; Johann, G.; Palú, F.; Da Silva, E.A. Evaluation of a concentrated parameters mathematical model applied to drying of yerba mate leaves with variable mass transfer coefficient. Appl. Therm. Eng. 2016, 105, 483–489. [Google Scholar] [CrossRef]
- Silva, W.P.; Amaral, D.S.D.; Duarte, M.E.M.; Mata, M.E.; Silva, C.M.E.; Pinheiro, R.M.; Pessoa, T. Description of the osmotic dehydration and convective drying of coconut (Cocos nucifera L.) pieces: A three-dimensional approach. J. Food Eng. 2013, 115, 121–131. [Google Scholar] [CrossRef]
- Da Silva, W.P.; Silva, C.M.E.; Gomes, J.P. Drying description of cylindrical pieces of bananas in different temperatures using diffusion models. J. Food Eng. 2013, 117, 417–424. [Google Scholar] [CrossRef]
- Silva, W.P.; Silva, C.M.D.P.; Da Silva, L.D.; Farias, V.S.O. Drying of Clay Slabs during the Falling Rate Period: Optimization and Simulation of the Process Using Diffusion Models. J. Mater. Sci. Res. 2013, 2, 1–13. [Google Scholar] [CrossRef]
- Da Silva, W.P.; Silva, C.M.E.; Lins, M.A.; Gomes, J.P. Osmotic dehydration of pineapple (Ananas comosus) pieces in cubical shape described by diffusion models. LWT 2014, 55, 1–8. [Google Scholar] [CrossRef]
- Silva, W.P.; Silva, C.M.D.P.S.; Aires, J.E.D.F.; Júnior, A.F.D.S. Osmotic dehydration and convective drying of coconut slices: Experimental determination and description using one-dimensional diffusion model. J. Saudi Soc. Agric. Sci. 2014, 13, 162–168. [Google Scholar] [CrossRef]
- Pinheiro, R.M.M.; Silva, W.P.; Miranda, D.S.D.A.; Silva, C.M.D.P.; Pessoa, T.; De Medeiros, M.S. Description of Osmotic Dehydration of Melon Cubes using a Three-dimensional Diffusion Model: An Algorithm to Determine the Effective Diffusivity. Int. J. Food Eng. 2017, 13, 1–13. [Google Scholar] [CrossRef]
- Çengel, Y.A.; Ghajar, A.J. Transfer of Mass and Heat, 4th ed.; AMGH Publisher LTDA: Porto Alegre, Brazil, 2012. [Google Scholar]
- Chen, Q.; Bi, J.; Wu, X.; Yi, J.; Zhou, L.; Zhou, Y. Drying kinetics and quality attributes of jujube (Zizyphus jujuba Miller) slices dried by hot-air and short- and medium-wave infrared radiation. LWT 2015, 64, 759–766. [Google Scholar] [CrossRef]
- Abbasi, E.; Azizpour, M. Evaluation of physicochemical properties of foam mat dried sour cherry powder. LWT 2016, 68, 105–110. [Google Scholar] [CrossRef]
- Ozcelik, M.; Ambros, S.; Heigl, A.; Dachmann, E.; Kulozik, U. Impact of hydrocolloid addition and microwave processing condition on drying behavior of foamed raspberry puree. J. Food Eng. 2019, 240, 83–91. [Google Scholar] [CrossRef]
- Roberts, J.S.; Kidd, D.R.; Padilla-Zakour, O. Drying kinetics of grape seeds. J. Food Eng. 2008, 89, 460–465. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; Miranda, M.; Díaz, L.P.; Lopez, L.; Rodriguez, K.; Di Scala, K. Effective moisture diffusivity determination and mathematical modelling of the drying curves of the olive-waste cake. Bioresour. Technol. 2010, 101, 7265–7270. [Google Scholar] [CrossRef]
- Perussello, C.A.; Mariani, V.C.; do Amarante, Á.C.C. Numerical and experimental analysis of the heat and mass transfer during okara drying. Appl. Therm. Eng. 2012, 48, 325–331. [Google Scholar] [CrossRef]
- Bezerra, C.V.; Da Silva, L.H.M.; Corrêa, D.F.; Rodrigues, A.M. A modeling study for moisture diffusivities and moisture transfer coefficients in drying of passion fruit peel. Int. J. Heat Mass Transf. 2015, 85, 750–755. [Google Scholar] [CrossRef]
- Koukouch, A.; Idlimam, A.; Asbik, M.; Sarh, B.; Izrar, B.; Bostyn, S.; Bah, A.; Ansari, O.; Zegaoui, O.; Amine, A. Experimental determination of the effective moisture diffusivity and activation energy during convective solar drying of olive pomace waste. Renew. Energy 2017, 101, 565–574. [Google Scholar] [CrossRef]
- Munhoz, C.L.; Sanjinez-Argandoña, E.J.; Campagnolli, R.; Macedo, M.L.R. Drying of the kernel and fresh and osmotically dehydrated bocaiuva pulps. Acta Sci. Technol. 2013, 36, 165–170. [Google Scholar] [CrossRef]
- Corrêa, J.; Rasia, M.; Mulet, A.; Cárcel, J.A. Influence of ultrasound application on both the osmotic pretreatment and subsequent convective drying of pineapple (Ananas comosus). Innov. Food Sci. Emerg. Technol. 2017, 41, 284–291. [Google Scholar] [CrossRef]
- Silva, W.P.; Nunes, J.S.; Gomes, J.P.; De Araújo, A.C.; Silva, C.M.D.P. Description of jambolan (Syzygium cumini (L.)) anthocyanin extraction kinetics at different stirring frequencies of the medium using diffusion models. Heat Mass Transf. 2018, 54, 3275–3285. [Google Scholar] [CrossRef]
- Da Silva, W.P.; Rodrigues, A.F.; Silva, C.M.D.E.; De Castro, D.S.; Gomes, J.P. Comparison between continuous and intermittent drying of whole bananas using empirical and diffusion models to describe the processes. J. Food Eng. 2015, 166, 230–236. [Google Scholar] [CrossRef]
- Fernando, W.; Low, H.; Ahmad, A. Dependence of the effective diffusion coefficient of moisture with thickness and temperature in convective drying of sliced materials. A study on slices of banana, cassava and pumpkin. J. Food Eng. 2011, 102, 310–316. [Google Scholar] [CrossRef]
- Da Silva, W.P.; Silva, C.M.D.P.; Farias, V.S.O.; Lima, A.G.B. Effect of the geometry on the description of the water absorption by composite materials using diffusion models. Mater. Werkst. 2011, 42, 747–752. [Google Scholar] [CrossRef]
- Milczarek, R.; Dai, A.A.; Otoni, C.G.; McHugh, T.H. Effect of shrinkage on isothermal drying behavior of 2-phase olive mill waste. J. Food Eng. 2011, 103, 434–441. [Google Scholar] [CrossRef]
- Ruiz-López, I.I.; Ruiz-Espinosa, H.; Arellanes-Lozada, P.; Barcenas-Pozos, M.; García-Alvarado, M. Analytical model for variable moisture diffusivity estimation and drying simulation of shrinkable food products. J. Food Eng. 2012, 108, 427–435. [Google Scholar] [CrossRef]
- Zhan, J.-F.; Gu, J.-Y.; Cai, Y.-C. Analysis of moisture diffusivity of larch timber during convective drying condition by using Crank’s method and Dincer’s method. J. For. Res. 2007, 18, 199–202. [Google Scholar] [CrossRef]
- Thuwapanichayanan, R.; Prachayawarakorn, S.; Kunwisawa, J.; Soponronnarit, S. Determination of effective moisture diffusivity and assessment of quality attributes of banana slices during drying. LWT 2011, 44, 1502–1510. [Google Scholar] [CrossRef]
- Irigoyen, R.M.T.; Goñi, S.; Giner, S.A. Drying–toasting kinetics of presoaked soybean. A mathematical model considering variable diffusivity, shrinkage and coupled heat transfe. J. Food Eng. 2014, 142, 70–79. [Google Scholar] [CrossRef]
- Nicoleti, J.F.; Silveira, V., Jr.; Telis-Romero, J.; Telis, V. Influence of Drying Conditions on Ascorbic Acid during Convective Drying of Whole Persimmons. Dry. Technol. 2007, 25, 891–899. [Google Scholar] [CrossRef]
- Silva, W.P.; Precker, J.W.; Silva, D.D.P.S.E.; Silva, C.D.E.; De Lima, A.G.B. Numerical simulation of diffusive processes in solids of revolution via the finite volume method and generalized coordinates. Int. J. Heat Mass Transf. 2009, 52, 4976–4985. [Google Scholar] [CrossRef]
- Da Silva, W.P.; Hamawand, I.; Silva, C.M.D.P.S. A liquid diffusion model to describe drying of whole bananas using boundary-fitted coordinates. J. Food Eng. 2014, 137, 32–38. [Google Scholar] [CrossRef]
- Aprajeeta, J.; Gopirajah, R.; Anandharamakrishnan, C. Shrinkage and porosity effects on heat and mass transfer during potato drying. J. Food Eng. 2015, 144, 119–128. [Google Scholar] [CrossRef]
- Llave, Y.; Takemori, K.; Fukuoka, M.; Takemori, T.; Tomita, H.; Sakai, N. Mathematical modeling of shrinkage deformation in eggplant undergoing simultaneous heat and mass transfer during convection-oven roasting. J. Food Eng. 2016, 178, 124–136. [Google Scholar] [CrossRef]
- Ahmad-Qasem, M.H.; Barrajón-Catalán, E.; Micol, V.; Cárcel, J.A.; Garcia-Perez, J. Influence of air temperature on drying kinetics and antioxidant potential of olive pomace. J. Food Eng. 2013, 119, 516–524. [Google Scholar] [CrossRef]
- Aregawi, W.; Defraeye, T.; Saneinejad, S.; Vontobel, P.; Lehmann, E.; Carmeliet, J.; Verboven, P.; Derome, D.; Nicolaï, B. Understanding forced convective drying of apple tissue: Combining neutron radiography and numerical modelling. Innov. Food Sci. Emerg. Technol. 2014, 24, 97–105. [Google Scholar] [CrossRef]
- Franco, T.S.; Perussello, C.A.; Ellendersen, L.D.S.N.; Masson, M.L. Foam mat drying of yacon juice: Experimental analysis and computer simulation. J. Food Eng. 2015, 158, 48–57. [Google Scholar] [CrossRef]
- Defraeye, T.; Nicolaï, B.; Mannes, D.; Aregawi, W.; Verboven, P.; Derome, D. Probing inside fruit slices during convective drying by quantitative neutron imaging. J. Food Eng. 2016, 178, 198–202. [Google Scholar] [CrossRef]
- Dehghannya, J.; Pourahmad, M.; Ghanbarzadeh, B.; Ghaffari, H. Heat and mass transfer modeling during foam-mat drying of lime juice as affected by different ovalbumin concentrations. J. Food Eng. 2018, 238, 164–177. [Google Scholar] [CrossRef]
- Dehghannya, J.; Pourahmad, M.; Ghanbarzadeh, B.; Ghaffari, H. Heat and mass transfer enhancement during foam-mat drying process of lime juice: Impact of convective hot air temperature. Int. J. Therm. Sci. 2019, 135, 30–43. [Google Scholar] [CrossRef]
- Madamba, P.S.; Driscoll, R.H.; Buckle, K.A. The thin-layer drying characteristics of garlic slices. J. Food Eng. 1996, 29, 75–97. [Google Scholar] [CrossRef]
- Zogzas, N.P.; Maroulis, Z.B.; Marinos-Kouris, D. Moisture Diffusivity Data Compilation in Foodstuffs. Dry. Technol. 1996, 14, 2225–2253. [Google Scholar] [CrossRef]
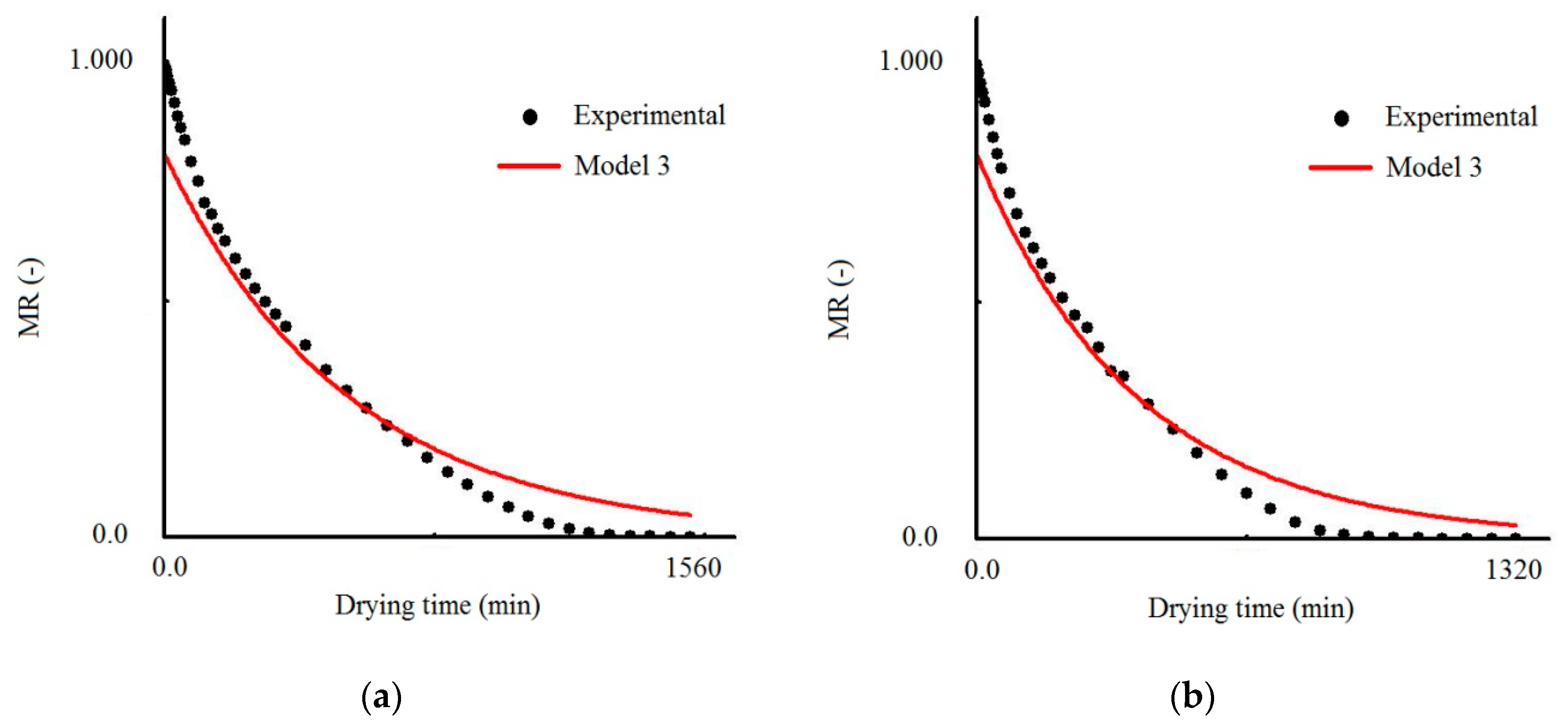
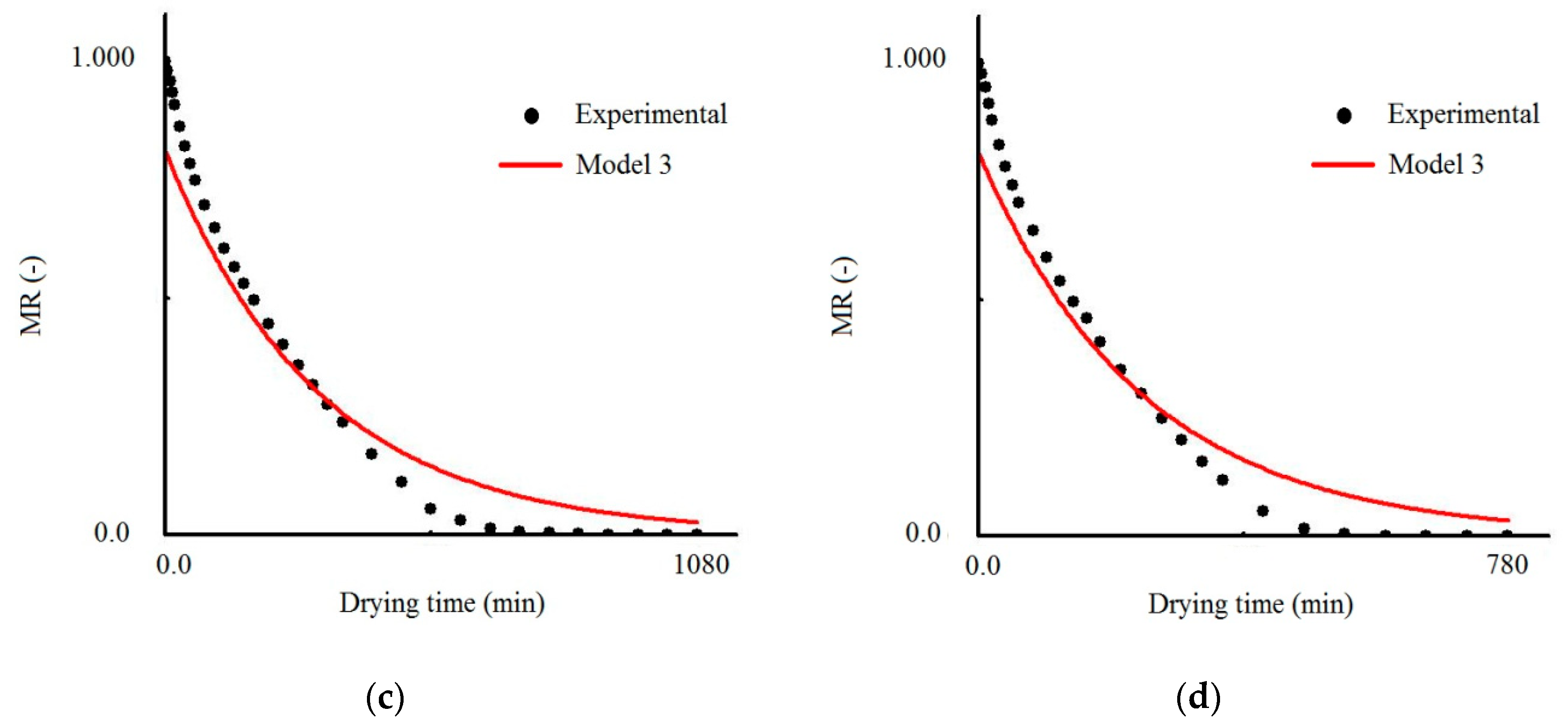
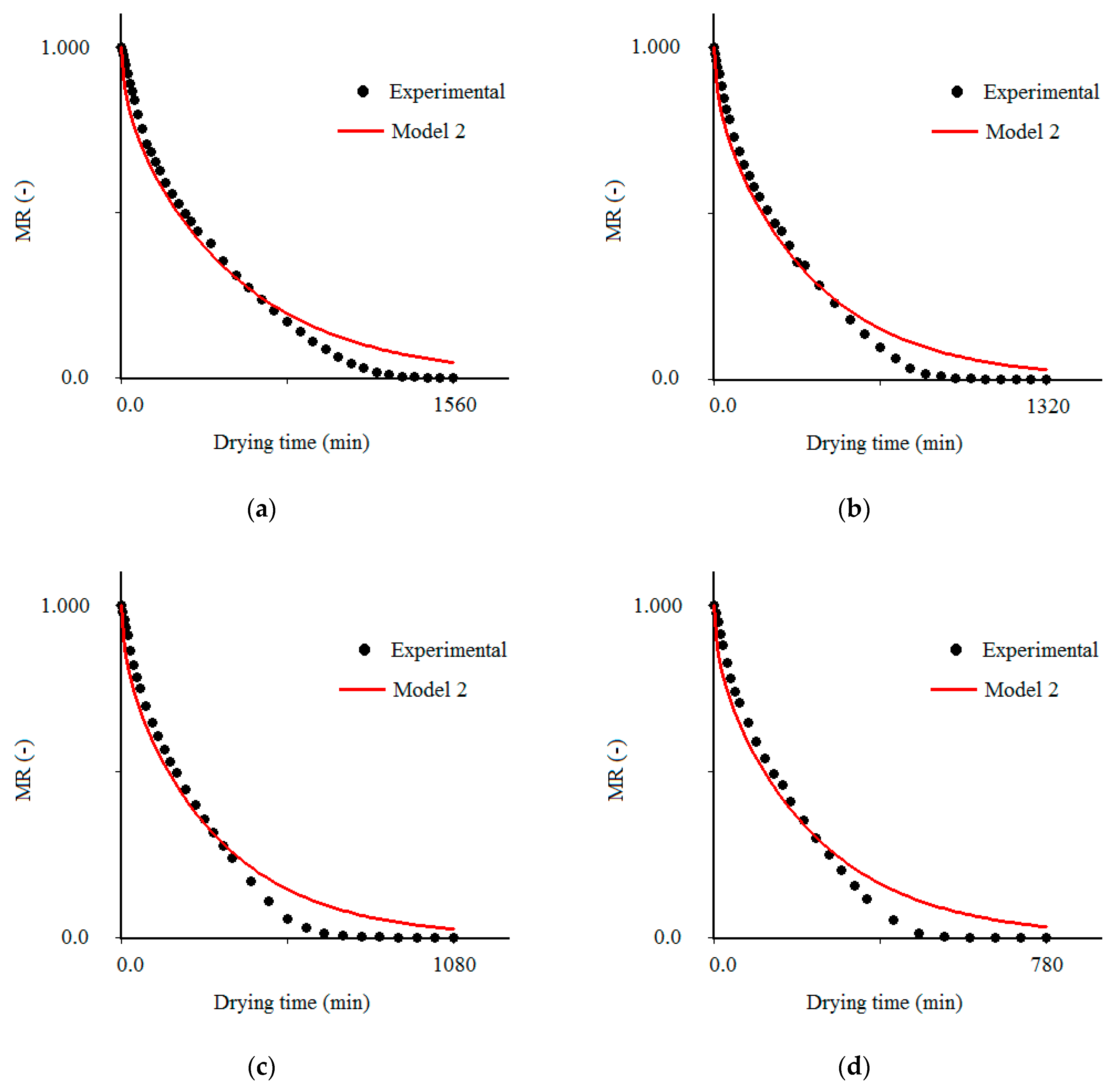
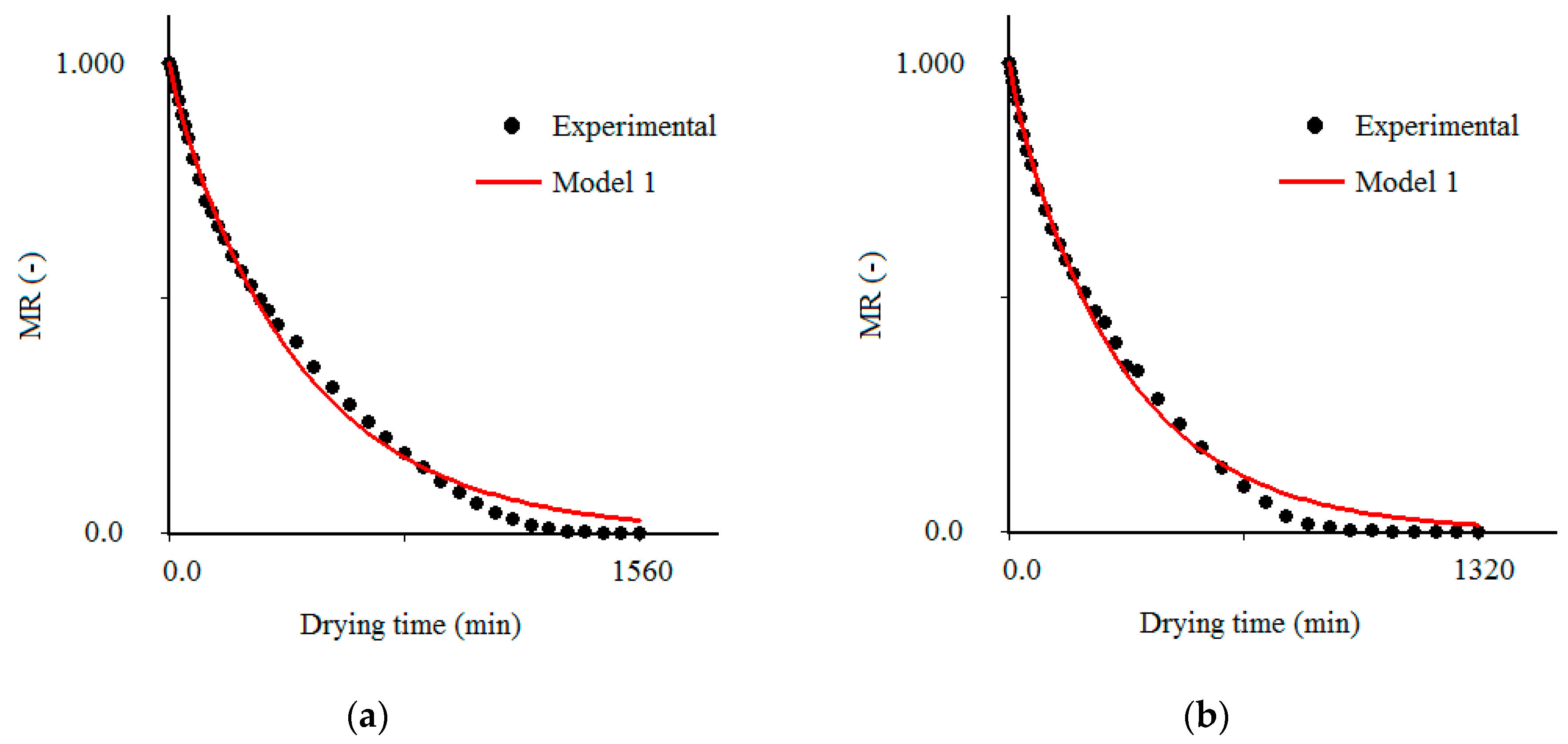
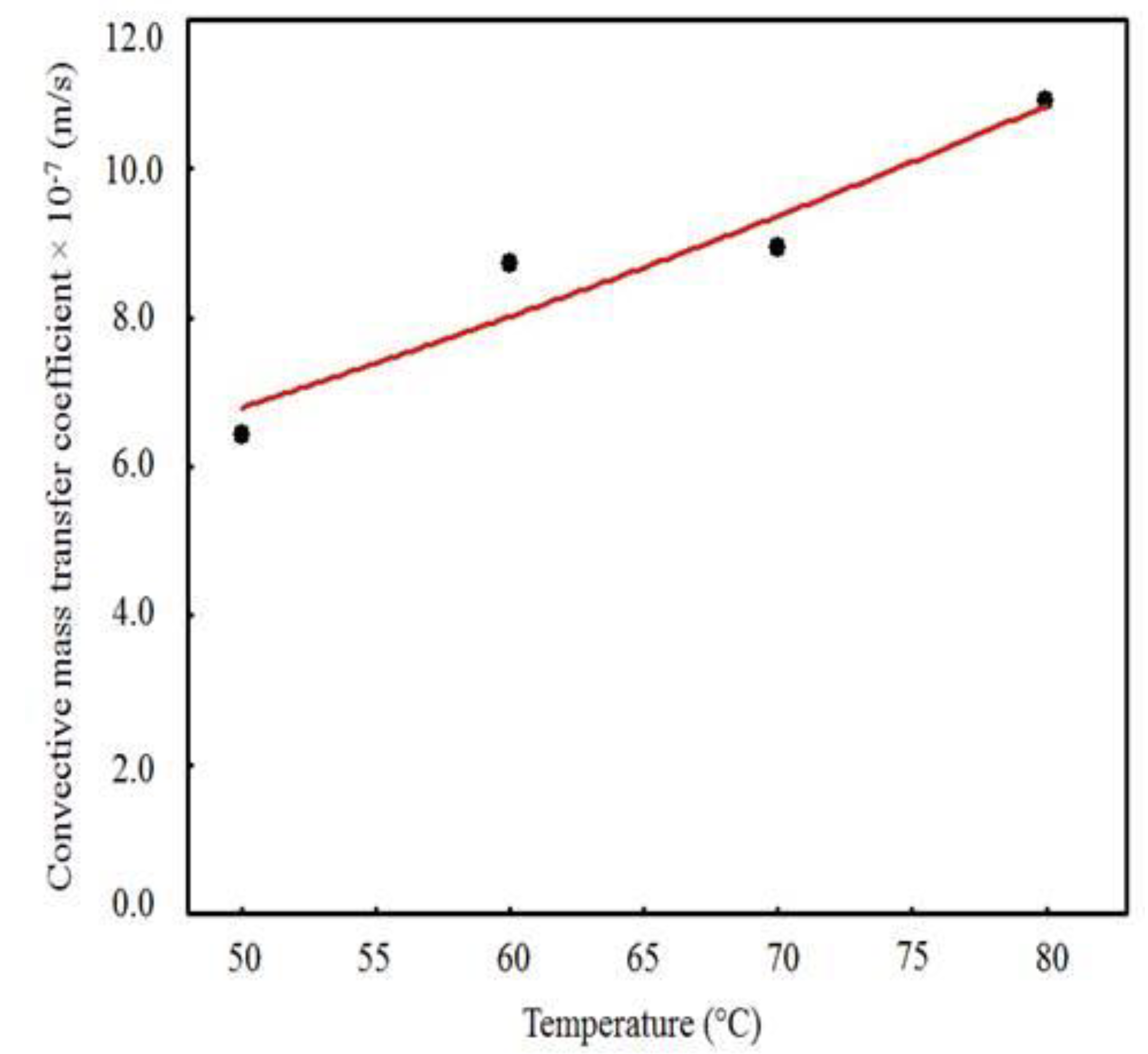
| Temperature (°C) | Effective Mass Diffusivity (Def) (m2/s) | R2 | χ2 × 10−1 |
|---|---|---|---|
| 50 | 1.1303 × 10−9 | 0.9380 | 3.1172 |
| 60 | 1.5615 × 10−9 | 0.9442 | 2.5671 |
| 70 | 1.9665 × 10−10 | 0.9386 | 2.5427 |
| 80 | 2.5440 × 10−10 | 0.9324 | 2.2856 |
| Temperature (°C) | Effective Mass Diffusivity (Def) (m2/s) | R2 | χ2 × 10−1 |
|---|---|---|---|
| 50 | 1.1285 × 10−9 | 0.9959 | 1.2094 |
| 60 | 1.5603 × 10−9 | 0.9954 | 1.0265 |
| 70 | 1.9615 × 10−9 | 0.9934 | 1.1395 |
| 80 | 2.5368 × 10−9 | 0.9921 | 1.0645 |
| Temperature (°C) | Effective Mass Diffusivity (Def) (m2/s) | Convective Mass Transfer Coefficients (h) (m/s) | Biot Number (Bi) (-) | R2 (-) | χ2 × 10−2 (-) |
|---|---|---|---|---|---|
| 50 | 2.9285 × 10−9 | 6.4362 × 10−7 | 2.00 | 0.9962 | 2.2826 |
| 60 | 4.1695 × 10−9 | 8.7273 × 10−7 | 2.00 | 0.9962 | 2.1171 |
| 70 | 8.1395 × 10−9 | 8.9445 × 10−7 | 1.05 | 0.9957 | 2.1434 |
| 80 | 1.2754 × 10−8 | 1.0912 × 10−6 | 0.82 | 0.9951 | 1.9738 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, J.P.L.; Silva, W.P.; Queiroz, A.J.M.; Figueirêdo, R.M.F.; Gomes, J.P.; Melo, B.A.; Santos, D.C.; Lima, T.L.B.; Branco, R.R.C.; Hamawand, I.; et al. Description of Cumbeba (Tacinga inamoena) Waste Drying at Different Temperatures Using Diffusion Models. Foods 2020, 9, 1818. https://doi.org/10.3390/foods9121818
Ferreira JPL, Silva WP, Queiroz AJM, Figueirêdo RMF, Gomes JP, Melo BA, Santos DC, Lima TLB, Branco RRC, Hamawand I, et al. Description of Cumbeba (Tacinga inamoena) Waste Drying at Different Temperatures Using Diffusion Models. Foods. 2020; 9(12):1818. https://doi.org/10.3390/foods9121818
Chicago/Turabian StyleFerreira, João P. L., Wilton P. Silva, Alexandre J. M. Queiroz, Rossana M. F. Figueirêdo, Josivanda P. Gomes, Bruno A. Melo, Dyego C. Santos, Thalis L. B. Lima, Rodolfo R. C. Branco, Ihsan Hamawand, and et al. 2020. "Description of Cumbeba (Tacinga inamoena) Waste Drying at Different Temperatures Using Diffusion Models" Foods 9, no. 12: 1818. https://doi.org/10.3390/foods9121818
APA StyleFerreira, J. P. L., Silva, W. P., Queiroz, A. J. M., Figueirêdo, R. M. F., Gomes, J. P., Melo, B. A., Santos, D. C., Lima, T. L. B., Branco, R. R. C., Hamawand, I., & Lima, A. G. B. (2020). Description of Cumbeba (Tacinga inamoena) Waste Drying at Different Temperatures Using Diffusion Models. Foods, 9(12), 1818. https://doi.org/10.3390/foods9121818







