Phenolic Compounds as Markers of Wine Quality and Authenticity
Abstract
1. Introduction
2. Classification of Phenolic Compounds of Wine
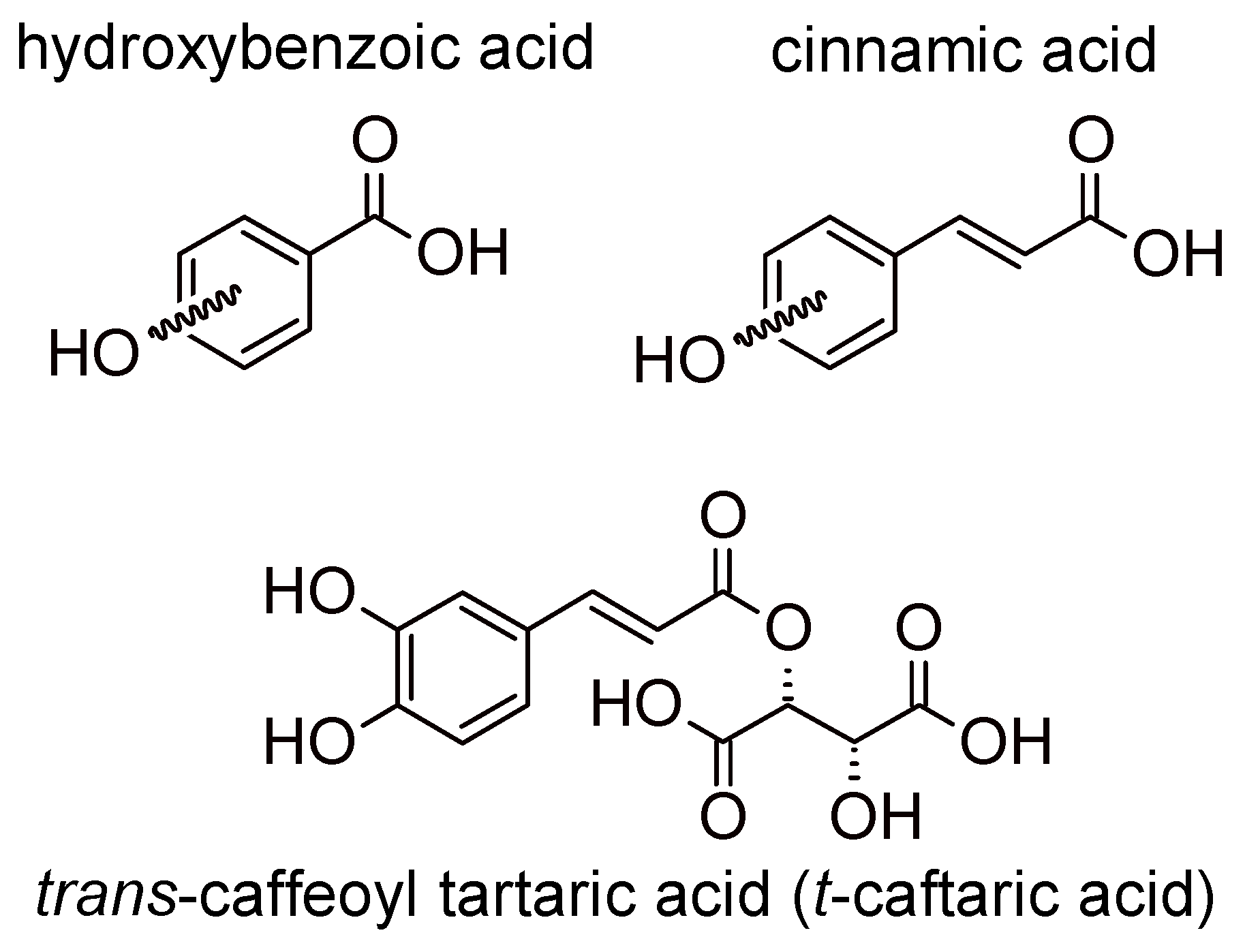
2.1. Phenolic Acids
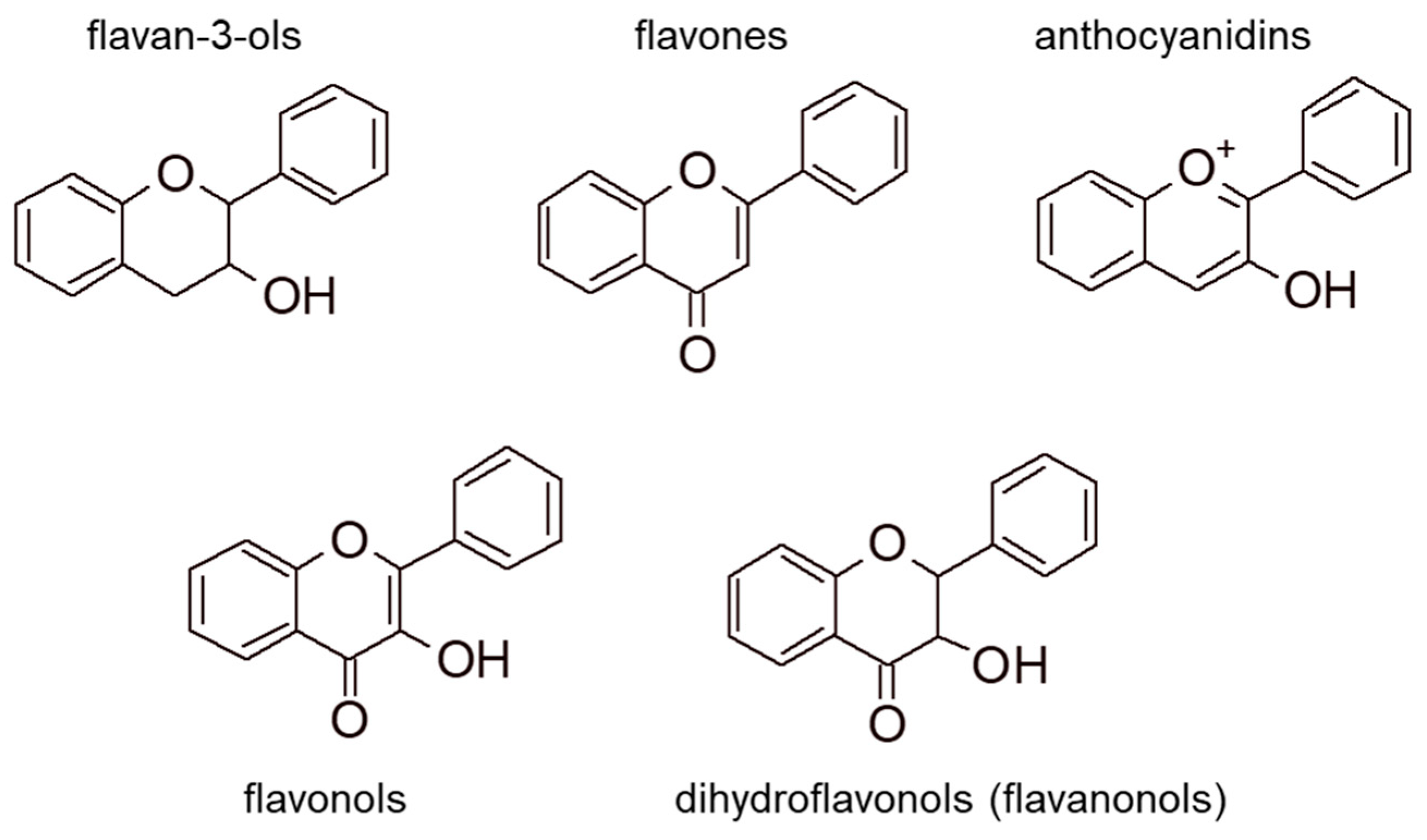
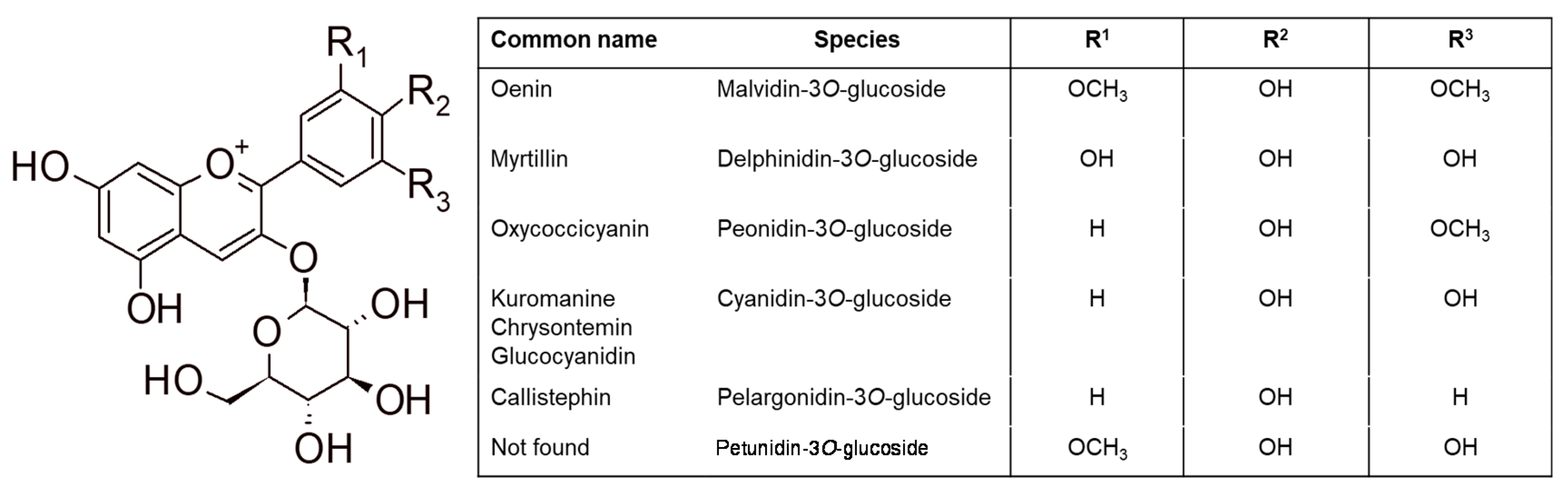
2.2. Flavonoids
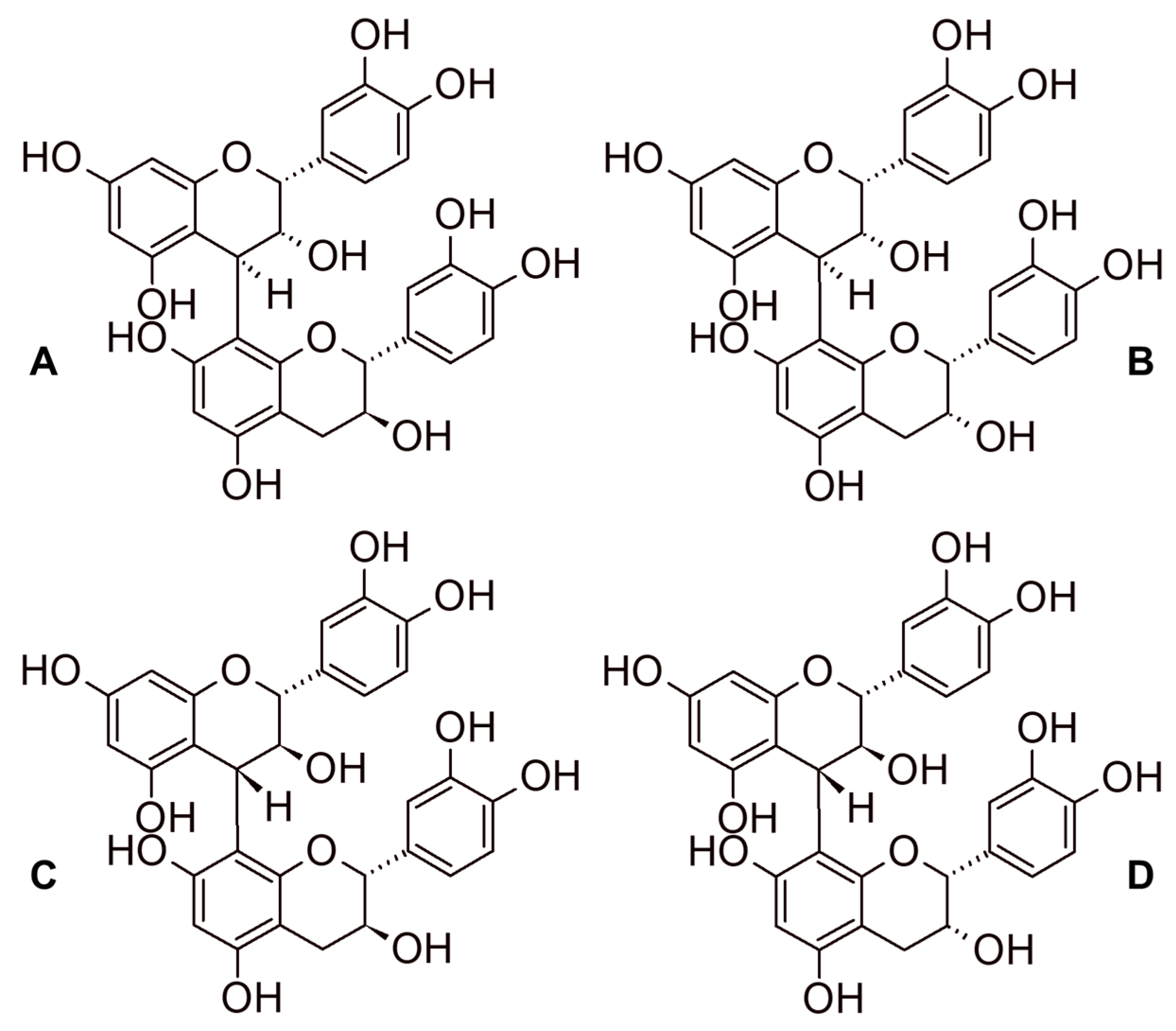
2.3. Tannins
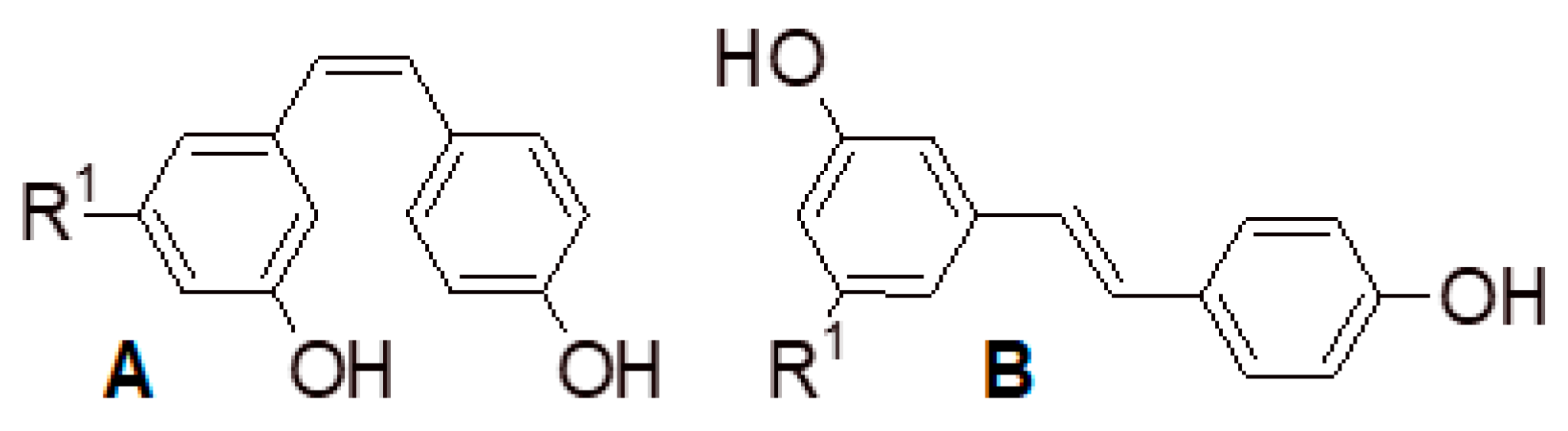
2.4. Stilbenes
3. Applications
3.1. Grape Variety
3.1.1. Grape Variety of White and Rosè Wine
3.1.2. Grape Variety of Red Wine
- (1)
- glucosides: Mv-3-glu > Pt-3-glu > Dp-3-glu > Pn-3-glu > Cy-3-glu;
- (2)
- 3-acetyl-glucosides: Mv-3-ace > Pt-3-ace > Pn-3-ace > Dp-3-ace > Cy-3-ace;
- (3)
- 3-p-coumaryl-glucosides: trans-Mv-3-cum > Dp-3-cum > Pn-3-cum > cis-Mv-3-cum > Cy-3-cum;
- (4)
- caffeoyl-3-glucosides: Mv-caf > Pn-caf.(Mv = malvidin; Pt = petunidin; Dp = delphinidin; Pn = peonidin; Cy = cyanidin)
3.1.3. Non Vitis Vinifera Grape Species
3.2. Geographical Origin and Phenolic Compounds
3.3. Winemaking
3.4. Aging
3.5. Vintage Year
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Geana, E.I.; Ciucure, C.T.; Apetrei, C.; Artem, V. Application of Spectroscopic UV-Vis and FT-IR Screening Techniques Coupled with Multivariate Statistical Analysis for Red Wine Authentication: Varietal and Vintage Year Discrimination. Molecules 2019, 24, 4166. [Google Scholar] [CrossRef]
- ICQRF. ICQRF Activity Report; ICQRF: Rome, Italy, 2019; pp. 1–73. Available online: https://www.politicheagricole.it (accessed on 30 September 2020).
- Reuters. Italian police put cork on ‘world’ best wine’ fakes. World News, 14 October 2020. [Google Scholar]
- OLAF. More than 1 m Litres of Counterfeit Wine and Alcoholic Beverages Seized under OLAF’s Lead, Press Release No. 22/2020. 2020. Available online: https://ec.europa.eu/anti-fraud/file/5190/download_en?token=HSWzFi7K (accessed on 30 September 2020).
- Stój, A.; Kapusta, I.; Domagała, D. Classification of Red Wines Produced from Zweigelt and Rondo Grape Varieties Based on the Analysis of Phenolic Compounds by UPLC-PDA-MS/MS. Molecules 2020, 25, 1342. [Google Scholar] [CrossRef] [PubMed]
- Costa, N.L.; Llobodanin, L.A.G.; Castro, I.A.; Barbosa, R. Using Support Vector Machines and neural networks to classify Merlot wines from South America. Inf. Process. Agric. 2019, 6, 265–278. [Google Scholar] [CrossRef]
- Anastasiadi, M.; Zira, A.; Magiatis, P.; Haroutounian, S.A.; Skaltsounis, A.L.; Mikros, E. 1H NMR-Based Metabonomics for the Classification of Greek Wines According to Variety, Region, and Vintage. Comparison with HPLC Data. J. Agric. Food Chem. 2009, 57, 11067–11074. [Google Scholar] [CrossRef] [PubMed]
- Kupsa, J.; Pavloušek, P.; Kumšta, M.; Lampíř, L. Phenolic profiles of Riesling wines originating from different terroirs of the Czech Republic. Mitt. Klosterneubg. 2017, 67, 182–193. [Google Scholar]
- Parpinello, G.P.; Ricci, A.; Arapitsas, P.; Curioni, A.; Moio, L.; Segade, S.R.; Ugliano, M.; Versari, A. Multivariate characterisation of Italian monovarietal red wines using MIR spectroscopy. OENO One 2019, 4, 741–751. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Milojkovic-Opsenica, D.; Morton, D.W.; Ristivojevic, P. Chemometric characterization of wines according to their HPTLC fingerprints. Eur. Food Res. Technol. 2017, 243, 659–667. [Google Scholar] [CrossRef]
- Sartor, S.; Malinovski, L.I.; Caliari, V.; Da Silva, A.L.; Bordignon-Luiz, M.T. Particularities of Syrah wines from different growing regions of Southern Brazil: Grapevine phenology and bioactive compounds. J. Food Sci. Technol. 2017, 54, 1414–1424. [Google Scholar] [CrossRef][Green Version]
- Li, S.; Zhu, B.; Reeves, M.J.; Duan, C. Phenolic Analysis and Theoretic Design for Chinese Commercial Wines’ Authentication. J. Food Sci. 2018, 83, 30–39. [Google Scholar] [CrossRef]
- Pavloušek, P.; Kumšta, M. Authentication of Riesling Wines from the Czech Republic on the Basis of the Non-flavonoid Phenolic Compounds. Czech J. Food Sci. 2013, 31, 474–482. [Google Scholar] [CrossRef]
- Pisano, P.L.; Silva, M.F.; Olivieri, A.C. Anthocyanins as markers for the classification of Argentinean wines according to botanical and geographical origin. Chemometric modeling of liquid chromatography–mass spectrometry data. Food Chem. 2015, 175, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Urvieta, R.; Buscema, F.; Bottini, R.; Coste, B.; Fontana, A. Phenolic and sensory profiles discriminate geographical indications for Malbec wines from different regions of Mendoza, Argentina. Food Chem. 2018, 265, 120–127. [Google Scholar] [CrossRef] [PubMed]
- OIV. International Standard for the Labelling of Wines; Organisation Internationale de la Vigne et du Vin: Paris, France, 2015; pp. 1–15. Available online: http://www.oiv.int/public/medias/4776/oiv-wine-labelling-standard-en-2015.pdf (accessed on 30 September 2020).
- Chira, K.; Pacella, N.; Jourdes, M.; Teissedre, P.L. Chemical and sensory evaluation of Bordeaux wines (Cabernet-Sauvignon and Merlot) and correlation with wine age. Food Chem. 2011, 126, 1971–1977. [Google Scholar] [CrossRef] [PubMed]
- Heras-Roger, J.; Díaz-Romero, C.; Darias-Martín, J. A comprehensive study of red wine properties according to variety. Food Chem. 2016, 196, 1224–1231. [Google Scholar] [CrossRef] [PubMed]
- Lukic, I.; Radek, S.; Budic-Leton, I.; Bubola, M.; Vrhovsek, U. Targeted UPLC-QqQ-MS/MS profiling of phenolic compounds for differentiation of monovarietal wines and corroboration of particular varietal typicity concepts. Food Chem. 2019, 300, 125251. [Google Scholar] [CrossRef] [PubMed]
- Proestos, C.; Tzachristas, A. Polyphenols in Wine Authenticity. In Scholarly Community Encyclopedia; MDPI: Basel, Switzerland, 2020. [Google Scholar]
- Kalogiouri, N.P.; Samanidou, V.F. Liquid chromatographic methods coupled to chemometrics: A short review to present the key workflow for the investigation of wine phenolic composition as it is affected by environmental factors. Environ. Sci. Pollut. Res. 2020, 1–15. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Vicario, A.; Guillén, D.A.; Hermosín-Gutiérrez, I.; Pérez-Coello, M.S. Phenolic characterization of minor red grape varieties grown in Castilla-La Mancha region in different vinification stages. Eur. Food Res. Technol. 2015, 240, 595–607. [Google Scholar] [CrossRef]
- Ragusa, A.; Centonze, C.; Grasso, M.E.; Latronico, M.F.; Mastrangelo, P.F.; Sparascio, F.; Fanizzi, F.P.; Maffia, M. A Comparative Study of Phenols in Apulian Italian Wines. Foods 2017, 6, 24. [Google Scholar] [CrossRef]
- Di Lecce, G.; Boselli, E.; D’Ignazi, G.; Frega, N.G. Evolution of phenolics and glutathione in Verdicchio wine obtained with maceration under reductive conditions. LWT 2013, 53, 54–60. [Google Scholar] [CrossRef]
- Boselli, E.; Di Lecce, G.; Alberti, F.; Frega, N.G. Nitrogen gas affects the quality and the phenolic profile of must obtained from vacuum-pressed white grapes. LWT 2010, 43, 1494–1500. [Google Scholar] [CrossRef]
- Berente, B.; De la Calle García, D.; Reichenbächer, M.; Danzer, K. Method development for the determination of anthocyanins in red wines by high-performance liquid chromatography and classification of German red wines by means of multivariate statistical methods. J. Chromatogr. A 2000, 871, 95–103. [Google Scholar] [CrossRef]
- Revilla, E.; Garcia-Beneytez, E.; Cabello, F.; Martin-Ortega, G.; Ryan, J. Value of high-performance liquid chromatographic analysis of anthocyanins in the differentiation of red grape cultivars and red wines made from them. J. Chromatogr. A 2001, 915, 53–60. [Google Scholar] [CrossRef]
- Castillo-Munoz, N.; Gomez-Alonso, S.; Garcia-Romero, E.; Hermosian-Gutierrez, I. Flavonol Profiles of Vitis vinifera Red Grapes and Their Single-Cultivar Wines. J. Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar] [CrossRef] [PubMed]
- Vergara, C.; Von Baer, D.; Mardones, C.; Gutierrez, L.; Hermosin-Gutierrez, I.; Castillo-Munoz, N. Flavonol profiles for varietal differentiation between Carmenere and Merlot wines produced in Chile: HPLC and chemometric analysis. J. Chil. Chem. Soc. 2011, 56, 827–832. [Google Scholar] [CrossRef][Green Version]
- Mattivi, F.; Vrhovsek, U.; Masuero, D.; Trainotti, D. Differences in the amount and structure of extractable skin and seed tannins amongst red grape varieties. Aust. J. Grape Wine Res. 2008, 15, 27–35. [Google Scholar] [CrossRef]
- Chira, K.; Jourdes, M.; Teissedre, P. Cabernet sauvignon red wine astringency quality control by tannin characterization and polymerization during storage. Eur. Food Res. Technol. 2012, 234, 253–261. [Google Scholar] [CrossRef]
- Basalekou, M.; Kallithraka, S.; Tarantilis, P.A.; Kotseridis, Y.; Pappas, C. Ellagitannins in wines: Future prospects in methods of analysis using FT-IR spectroscopy. LWT 2019, 101, 48–53. [Google Scholar] [CrossRef]
- Motta, S.; Guaita, M.; Cassino, C.; Bosso, A. Relationship between polyphenolic content, antioxidant properties and oxygen consumption rate of different tannins in a model wine solution. Food Chem. 2020, 313, 126045. [Google Scholar] [CrossRef]
- Sun, B.; Leandro, C.; da Silva, J.M.R.; Spranger, I. Separation of Grape and Wine Proanthocyanidins According to Their Degree of Polymerization. J. Agric. Food Chem. 1998, 46, 1390–1396. [Google Scholar] [CrossRef]
- Llaudy, M.; Canals, R.; Canals, J.M.; Rozéz, N.; Arola, L.; Zamora, F. New method for evaluating astringency in red wine. J. Agric. Food Chem. 2004, 52, 742–746. [Google Scholar] [CrossRef]
- Mazza, G. Anthocyanins in grapes and grapes products. Crit. Rev. Food Sci. Nutr. 1995, 35, 341–371. [Google Scholar] [CrossRef] [PubMed]
- Baron, M.; Sochor, J.; Tomaskova, L.; Prusova, B.; Kumsta, M. Study on Antioxidant Components in Rosé Wine Originating from the Wine Growing Region of Moravia, Czech Republic. Erwerbs-Obstbau 2017, 59, 253–262. [Google Scholar] [CrossRef]
- Martelo-Vidal, M.J.; Vázquez, M. Determination of polyphenolic compounds of red wines by UV–VIS–NIR spectroscopy and chemometrics tools. Food Chem. 2014, 158, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, A.; Centonze, C.; Grasso, M.E.; Latronico, M.F.; Mastrangelo, P.F.; Sparascio, F.; Maffia, M. HPLC Analysis of Phenols in Negroamaro and Primitivo Red Wines from Salento. Foods 2019, 8, 45. [Google Scholar] [CrossRef]
- Lampir, L.; Pavlousek, P. Influence of Locality on Content of Phenolic Compounds in White Wines. Czech J. Food Sci. 2013, 31, 619–626. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; González-Centeno, M.R.; Chira, K.; Jourdes, M.; Teissedre, P.L. Phenolic Compounds of Grapes and Wines: Key Compounds and Implications in Sensory Perception. In Winemaking-Stabilization, Aging Chemistry and Biochemistry; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Lampir, L. Varietal Differentiation of White Wines on the Basis of Phenolic Compounds profile. Czech J. Food Sci. 2013, 31, 172–179. [Google Scholar] [CrossRef]
- Magdas, D.A.; Pirnau, A.; Feher, I.; Guyon, F.; Cozar, B.I. Alternative approach of applying 1H NMR in conjunction with chemometrics for wine classification. LWT 2019, 109, 422–428. [Google Scholar] [CrossRef]
- Ali, K.; Maltese, F.; Toepfer, R.; Choi, Y.H.; Verpoorte, R. Metabolic characterization of Palatinate German white wines according to sensory attributes, varieties, and vintages using NMR spectroscopy and multivariate data analyses. J. Biomol. NMR 2011, 49, 255–266. [Google Scholar] [CrossRef]
- Godelmann, R.; Fang, F.; Humpfer, E.; Schütz, B.; Bansbach, M.; Schäfer, H.; Spraul, M. Targeted and Nontargeted Wine Analysis by 1H NMR Spectroscopy Combined with Multivariate Statistical Analysis. Differentiation of Important Parameters: Grape Variety, Geographical Origin, Year of Vintage. J. Agric. Food Chem. 2013, 61, 5610–5619. [Google Scholar] [CrossRef]
- Boselli, E.; Minardi, M.; Giomo, A.; Frega, N.G. Phenolic composition and quality of white d.o.c. wines from Marche (Italy). Anal. Chim. Acta 2006, 563, 93–100. [Google Scholar] [CrossRef]
- La Notte, E.; Liuzzi, V.A.; Esti, M. I componenti polifenolici del vino—Nota II: Gli antociani in relazione a differenti sistemi di vinificazione. Vigne Vini 1992, 10, 49–55. [Google Scholar]
- Storchi, P.; Baldi, M.; Faviere, V.; Giannetti, F.; Leprini, M.; Peduto, F. Valutazione del comportamento produttivo e della componente fenolica in vitigni a bacca nera coltivati in Toscana. Quaderni di Scienze Viticole ed Enologiche 2008, 30, 3–21. [Google Scholar]
- Wenzel, K.; Dittrich, H.H.; Heimfarth, M. Die Zusammensetzung der Anthocyane in den Beeren verschiedener Rebsorten. Vitis 1987, 26, 65–78. [Google Scholar]
- Gonzalez-Neves, G.; Favre, G.; Piccardo, D.; Gil, G. Anthocyanin profile of young red wines of Tannat, Syrah and Merlot made using maceration enzymes and cold soak. Int. J. Food Sci. 2016, 51, 260–267. [Google Scholar] [CrossRef]
- García-Beneytez, E.; Cabello, F.; Revilla, E. Analysis of Grape and Wine Anthocyanins by HPLC-MS. J. Agric. Food Chem. 2003, 51, 5622–5629. [Google Scholar] [CrossRef]
- Muccillo, L.; Gambuti, A.; Frusciante, L.; Iorizzo, M.; Moio, L.; Raieta, K.; Rinaldi, A.; Colantuoni, V.; Aversano, R. Biochemical features of native red wines and genetic diversity of the corresponding grape varieties from Campania region. Food Chem. 2014, 143, 506–513. [Google Scholar] [CrossRef]
- Kumšta, M.; Pavloušek, P.; Kárník, P. Use of Anthocyanin Profiles When Differentiating Individual Varietal Wines and Terroirs. Food Technol. Biotechnol. 2014, 52, 383–390. [Google Scholar] [CrossRef]
- Ferrandino, A.; Guidoni, S.; Mannini, F. Grape Quality Parameters and Polyphenolic Content of Different ‘Barbera’ and ‘Nebbiolo’ (Vitis vinifera L.) Clones as Influenced by Environmental Conditions—Preliminary Results. Acta Hortic. 2007, 754, 437–442. [Google Scholar] [CrossRef]
- Río Segade, S.; Pace, C.; Torchio, F.; Giacosa, S.; Gerbi, V.; Rolle, L. Impact of maceration enzymes on skin softening and relationship with anthocyanin extraction in wine grapes with different anthocyanin profiles. Food Res. Int. 2015, 71, 50–57. [Google Scholar] [CrossRef]
- Giacosa, S.; Ossola, C.; Botto, R.; Río Segade, S.; Paissoni, M.A.; Pollon, M.; Gerbi, V.; Rolle, L. Impact of specific inactive dry yeast application on grape skin mechanical properties, phenolic compounds extractability, and wine composition. Food Res. Int. 2019, 116, 1084–1093. [Google Scholar] [CrossRef]
- Arapitsas, P.; Ugliano, M.; Marangon, M.; Piombino, P.; Rolle, L.; Gerbi, G.; Versari, A.; Mattivi, F. Use of Untargeted Liquid Chromatography-Mass Spectrometry Metabolome To Discriminate Italian Monovarietal Red Wines, Produced in Their Different Terroirs. J. Agric. Food Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Geana, E.I.; Popescu, R.; Costinel, D.; Dinca, O.R.; Ionete, R.E.; Stefanescu, I.; Artem, V.; Bala, C. Classification of red wines using suitable markers coupled with multivariate statistic analysis. Food Chem. 2016, 192, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Mayr, C.M.; De Rosso, M.; Dalla Vedova, A.; Flamini, R. High-Resolution Mass Spectrometry Identification of Secondary Metabolites in Four Red Grape Varieties Potentially Useful as Traceability Markers of Wines. Beverages 2018, 4, 74. [Google Scholar] [CrossRef]
- De Rosso, M.; Mayr, C.M.; Girardi, G.; Dalla Vedova, A.; Flamini, R. High-resolution mass spectrometry metabolomics of grape chemical markers to reveal use of not-allowed varieties in the production of Amarone and Recioto wines. Metabolomics 2018, 14, 124. [Google Scholar] [CrossRef]
- Pisano, P.L.; Silva, M.F.; Olivieri, A.C. Exploration of liquid chromatographic-diode array data for Argentinean wines by extended multivariate curve resolution. Chemometr. Intell. Lab. 2014, 132, 1–7. [Google Scholar] [CrossRef]
- Fanzone, M.; Zamora, F.; Jofre, V.; Assof, M.; Gomez-Cordovesc, C.; Pena-Neirad, A. Phenolic characterisation of red wine from different grape varieties cultivated in Mendoza province (Argentina). J. Sci. Food Agric. 2012, 92, 704–718. [Google Scholar] [CrossRef]
- Monagas, M.; Gomez-Cordoves, C.; Bartolome, B.; Laureano, O.; da Silva, J.R.M. Monomeric, Oligomeric, and Polymeric Flavan-3-ol Composition of Wines and Grapes from Vitis vinifera L. Cv. Graciano, Tempranillo, and Cabernet Sauvignon. J. Agric. Food Chem. 2003, 51, 6475–6481. [Google Scholar] [CrossRef]
- Minnaar, P.P.; Booyse, M. Differentiation among Young and Market-ready Cabernet Sauvignon, Pinotage and Shiraz Wines: Application of Canonical Discriminant Analysis using Flavonoid and Non-Flavonoid Compositional Data. S. Afr. J. Enol. Vitic. 2011, 32, 251–261. [Google Scholar] [CrossRef][Green Version]
- Gris, E.F.; Mattivi, F.; Ferreira, E.A.; Vrhovsek, U.; Pedrosa, R.C.; Bordignon-Luiz, M.T. Proanthocyanidin profile and antioxidant capacity of Brazilian Vitis vinifera red wines. Food Chem. 2011, 126, 213–220. [Google Scholar] [CrossRef]
- Sun, X.; Li, L.; Ma, T.; Liu, X.; Huang, W.; Zhan, J. Profiles of Phenolic Acids and Flavan-3-ols for Select Chinese Red Wines: A Comparison and Differentiation According to Geographic Origin and Grape Variety. J. Food Sci. 2015, 80, C2170–C2179. [Google Scholar] [CrossRef]
- Pajović-Šćepanović, R.; Wendelin, S.; Eder, R. Phenolic composition and varietal discrimination of Montenegrin red wines (Vitis vinifera var. Vranac, Kratošija, and Cabernet Sauvignon). Eur. Food Res. Technol. 2018, 244, 2243–2254. [Google Scholar] [CrossRef]
- Salvatore, E.; Cocchi, M.; Marchetti, A.; Marini, F.; De Juan, A. Determination of phenolic compounds and authentication of PDO Lambrusco wines by HPLC-DAD and chemometric techniques. Anal. Chim. Acta 2013, 761, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Magdas, D.A.; Pinzaru, S.C.; Guyon, F.; Feher, I.; Cozar, B.I. Application of SERS technique in white wines discrimination. Food Control 2018, 92, 30–36. [Google Scholar] [CrossRef]
- Magdas, D.A.; Guyon, F.; Feher, I.; Pinzaru, S.C. Wine discrimination based on chemometric analysis of untargeted markers using FT-Raman spectroscopy. Food Control 2018, 85, 385–391. [Google Scholar] [CrossRef]
- Magdas, D.A.; Cozar, B.I.; Feher, I.; Guyon, F.; Dehelean, A.; Pinzaru, S.C. Testing the limits of FT-Raman spectroscopy for wine authentication: Cultivar, geographical origin, vintage and terroir effect influence. Sci. Rep. 2019, 27, 19954. [Google Scholar] [CrossRef] [PubMed]
- Longo, E.; Merkytė, V.; Rossetti, F.; Teissedre, P.L.; Jourdes, M.; Boselli, E. Relative abundances of novel cyclic prodelphinidins in wine depending on the grape variety. Mass Spectrom. 2018, 53, 1116–1125. [Google Scholar] [CrossRef]
- Longo, E.; Rossetti, F.; Jouin, A.; Teissedre, P.; Jourdes, M.; Boselli, E. Distribution of crown hexameric procyanidin and its tetrameric and pentameric congeners in red and white wines. Food Chem. 2019, 299, 125125. [Google Scholar] [CrossRef]
- Zeng, L.; Pons-Mercadé, P.; Richard, T.; Krisa, S.; Teissedre, P.L.; Jourdes, M. Crown procyanidin tetramer: A procyanidin with an unusual cyclic skeleton with a potent protective effect against amyloid-β-induced toxicity. Molecules 2019, 24, 1915. [Google Scholar] [CrossRef]
- Favre, G.; González-Neves, G.; Piccardo, D.; Gómez-Alonso, S.; Pérez-Navarro, J.; Hermosín-Gutiérrez, I. New acylated flavonols identified in Vitis vinifera grapes and wines. Food Res. Int. 2018, 112, 98–107. [Google Scholar] [CrossRef]
- Baldi, A.; Romani, A.; Mulinacci, N.; Vincieri, F.F.; Casetta, B. HPLC/MS Application to Anthocyanins of Vitis vinifera L. J. Agric. Food Chem. 1995, 43, 2104–2109. [Google Scholar] [CrossRef]
- Mattivi, F.; Cova, G.; Dalla Serra, A.; Soligo, S. Recupero, Conservazione e Valorizzazione del Germoplasma Viticolo Veneto; Veneto Agricoltura: Legnaro, Italy, 2004; pp. 17–19. [Google Scholar]
- Boselli, E.; Giomo, A.; Minardi, M.; Frega, N.G. Characterization of phenolics in Lacrima di Morro d’Alba wine and role on its sensory attributes. Eur. Food Res. Technol. 2008, 227, 709–720. [Google Scholar] [CrossRef]
- Xing, R.R.; Li, S.Y.; He, F.; Yang, Z.; Duan, C.Q.; Li, Z.; Wang, J.; Pan, Q.H. Mass spectrometric and enzymatic evidence confirm the existence of anthocyanidin 3,5-O-diglucosides in cabernet sauvignon (Vitis vinifera L.) grape berries. J. Agric. Food Chem. 2015, 63, 3251–3260. [Google Scholar] [CrossRef] [PubMed]
- OIV. Maximum Acceptable Limits of Various Substances Contained in Wine; OENO, OIV-MA-C1-01; OIV: Paris, France, 2015; pp. 1–4. Available online: http://www.oiv.int/public/medias/3741/e-code-annex-maximum-acceptable-limits.pdf (accessed on 30 September 2020).
- Nixdorf, S.L.; Hermosín-Gutiérrez, I. Brazilian red wines made from the hybrid grape cultivar Isabel: Phenolic composition and antioxidant capacity. Anal. Chim. Acta 2010, 659, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Xu, T.; Qin, M.; Zhuang, X.; Fang, Y.; Zhang, Z. Phenolic characterization of young wines made from spine grape (Vitis davidii Foex) grown in Chongyi County (China). Food Res. Int. 2012, 49, 664–671. [Google Scholar] [CrossRef]
- Li, S.; He, F.; Zhu, B.; Wang, J.; Duan, C. Comparison of phenolic and chromatic characteristics of dry red wines made from native Chinese grape species and Vitis vinifera. Int. J. Food Prop. 2017, 20, 2134–2146. [Google Scholar] [CrossRef]
- Burns, J.; Mullen, W.; Landrault, N.; Teissedre, P.L.; Lean, M.E.J.; Crozier, A. Variations in the Profile and Content of Anthocyanins in Wines Made from Cabernet Sauvignon and Hybrid Grapes. J. Agric. Food Chem. 2002, 50, 4096–4102. [Google Scholar] [CrossRef]
- Gougeon, L.; Da Costa, G.; Le Mao, I.; Ma, W.; Teissedre, P.L.; Guyon, F.; Richard, T. Wine Analysis and Authenticity Using 1H-NMR Metabolomics Data: Application to Chinese Wines. Food Anal. Methods 2018, 11, 3425–3434. [Google Scholar] [CrossRef]
- Giaccio, M.; Vicentini, A. Determination of the geographical origin of wines by means of the mineral content and the stable isotope ratios: A review. J. Commod. Sci. Technol. Qual. 2008, 47, 267–284. [Google Scholar]
- Kumšta, M.; Pavloušek, P.; Kupsa, J. Phenolic Profile in Czech White Wines from Different Terroirs. Food Sci. Biotechnol. 2012, 21, 1593–1601. [Google Scholar] [CrossRef]
- Rocchetti, G.; Gatti, M.; Bavaresco, L.; Lucini, L. Untargeted metabolomics to investigate the phenolic composition of Chardonnay wines from different origins. J. Food Compost. Anal. 2018, 71, 87–93. [Google Scholar] [CrossRef]
- Bellomarino, S.A.; Conlan, X.A.; Parker, R.M.; Barnett, N.W.; Adams, M.J. Geographical classification of some Australian wines by discriminant analysis using HPLC with UV and chemiluminescence detection. Talanta 2009, 80, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Lambert, M.; Meudec, E.; Verbaere, A.; Mazerolles, G.; Wirth, J.; Masson, G.; Cheynier, V.; Sommerer, N. A High-Throughput UHPLC-QqQ-MS Method for Polyphenol Profiling in Rosé Wines. Molecules 2015, 20, 7890–7914. [Google Scholar] [CrossRef] [PubMed]
- Radovanovic, B.C.; Radovanovic, A.N.; Souquet, J. Phenolic profile and free radical-scavenging activity of Cabernet Sauvignon wines of different geographical origins from the Balkan region. J. Sci. Food Agric. 2010, 90, 2455–2461. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Zhang, Z.W. Free Radical-scavenging Activity and Anthocyanin Profiles of Cabernet Sauvignon and Merlot Wines from Four Wine Grapegrowing Regions in China. S. Afr. J. Enol. Vitic. 2019, 40, 1–10. [Google Scholar] [CrossRef]
- Radovanovic, B.; Radovanovic, A. Free Radical Scavenging Activity and Anthocyanin Profile of Cabernet Sauvignon Wines from the Balkan Region. Molecules 2010, 15, 4213–4226. [Google Scholar] [CrossRef]
- Acevedo, F.J.; Jimenez, J.; Maldonado, S.; Dominguez, E.; Narvaez, A. Classification of Wines Produced in Specific Regions by UV-Visible Spectroscopy Combined with Support Vector Machines. J. Agric. Food Chem. 2007, 55, 6842–6849. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A Review of the Effect of Winemaking Techniques on Phenolic Extraction in Red Wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar]
- Bosch-Fusté, J.; Sartini, E.; Flores-Rubio, C.; Caixach, J.; López-Tamames, E.; Buxaderas, S. Viability of total phenol index value as quality marker of sparkling wines, “cavas”. Food Chem. 2009, 114, 782–790. [Google Scholar] [CrossRef]
- Izquierdo-Llopart, A.; Saurina, J. Characterization of Sparkling Wines According to Polyphenolic Profiles Obtained by HPLC-UV/Vis and Principal Component Analysis. Foods 2019, 8, 22. [Google Scholar] [CrossRef]
- Dupas de Matos, A.; Longo, E.; Chiotti, D.; Pedri, U.; Eisenstecken, D.; Sanoll, C.; Robatscher, P.; Boselli, E. Pinot Blanc: Impact of the Winemaking Variables on the Evolution of the Phenolic, Volatile and Sensory Profiles. Foods 2020, 9, 499. [Google Scholar] [CrossRef]
- Suriano, S.; Alba, V.; Tarricone, L.; Di Gennaro, D. Maceration with stems contact fermentation: Effect on proanthocyanidins compounds and color in Primitivo red wines. Food Chem. 2015, 177, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Merkytė, V.; Dupas de Matos, A.; Longo, E.; Boselli, E. Cyclic proanthocyanidins in Pinot Noir wine. Ital. J. Food Sci. 2020, 32, 337–351. [Google Scholar]
- Lorenzo, C.; Pardo, F.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Effect of Red Grapes Co-winemaking in Polyphenols and Colour of Wines. J. Agric. Food Chem. 2005, 53, 7609–7616. [Google Scholar] [CrossRef] [PubMed]
- Loizzo, M.R.; Bonesi, M.; Di Lecce, G.; Boselli, E.; Tundis, R.; Pugliesen, A.; Menichini, F.; Frega, N.G. Phenolics, Aroma Profile, and In Vitro Antioxidant Activity of Italian Dessert Passito Wine from Saracena (Italy). J. Food Sci. 2013, 78, C704–C708. [Google Scholar] [CrossRef]
- Matejicek, D.; Mikes, O.; Klejdus, B.; Sterbova, D.; Kuban, V. Changes in contents of phenolic compounds during maturing of barrique red wines. Food Chem. 2005, 90, 791–800. [Google Scholar] [CrossRef]
- Arapitsas, P.; Guella, G.; Mattivi, F. The impact of SO2 on wine flavanols and indoles in relation to wine style and age. Sci. Rep. 2018, 8, 858–870. [Google Scholar] [CrossRef]
- Sanz, M.; Fernández de Simón, B.; Esteruelas, E.; Munoza, A.M.; Cadahía, E.; Hernández, M.T.; Estrella, I.; Martinez, J. Polyphenols in red wine aged in acacia (Robinia pseudoacacia) and oak (Quercus petraea) wood barrels. Anal. Chim. Acta 2012, 732, 83–90. [Google Scholar] [CrossRef]
- Alañón, M.E.; Schumacher, R.; Castro-Vázquez, L.; Díaz-Maroto, M.C.; Hermosín-Gutiérrez, I.; Pérez-Coello, M.S. Enological potential of chestnut wood for aging Tempranillo wines Part II: Phenolic compounds and chromatic characteristics. Food. Res. Int. 2013, 51, 536–543. [Google Scholar] [CrossRef]
- Chinnici, F.; Natali, N.; Bellachioma, A.; Versari, A.; Riponi, C. Changes in phenolic composition of red wines aged in cherry wood. LWT 2015, 60, 977–984. [Google Scholar] [CrossRef]
- Del Alamo, M.; Nevares, I.; Gallego, L.; Martina, C.; Merino, S. Aging markers from bottled red wine aged with chips, staves and barrels. Anal. Chim. Acta 2008, 621, 86–99. [Google Scholar] [CrossRef]
- Ortega-Heras, M.; Pérez-Magariño, S.; Cano-Mozo, E.; González-San José, M.L. Differences in the phenolic composition and sensory profile between red wines aged in oak barrels and wines aged with oak chips. LWT 2010, 43, 1533–1541. [Google Scholar] [CrossRef]
- Baiano, A.; De Gianni, A.; Mentana, A.; Quinto, M.; Centonze, D.; Del Nobile, M.A. Effects of the treatment with oak chips on color-related phenolics, volatile composition, and sensory profile of red wines: The case of Aglianico and Montepulciano. Eur. Food. Res. Technol. 2016, 242, 745–767. [Google Scholar] [CrossRef]
- Basalekou, M.; Pappas, C.; Tarantilis, P.; Kotseridis, Y.; Kallithraka, S. Wine authentication with Fourier Transform Infrared Spectroscopy: A feasibility study on variety, type of barrel wood and ageing time classification. Int. J. Food Sci. 2017, 52, 1307–1313. [Google Scholar] [CrossRef]
- Bellomarino, S.A.; Parker, R.M.; Conlan, X.A.; Barnett, N.W.; Adams, M.J. Partial least squares and principal components analysis of wine vintage by high performance liquid chromatography with chemiluminescence detection. Anal. Chim. Acta 2010, 678, 34–38. [Google Scholar] [CrossRef]
- Schwarz, M.; Hofmann, G.; Winterhalter, P. Investigations on Anthocyanins in Wines from Vitis vinifera cv. Pinotage: Factors Influencing the Formation of Pinotin A and Its Correlation with Wine Age. J. Agric. Food Chem. 2004, 52, 498–504. [Google Scholar] [CrossRef]
- Revilla, E.; Garcia-Beneytez, E.; Cabello, F. Anthocyanin fingerprint of clones of Tempranillo grapes and wines made with them. Aust. J. Grape Wine Res. 2009, 15, 70–78. [Google Scholar] [CrossRef]
- Arapitsas, P.; Perenzoni, D.; Nicolini, G.; Mattivi, F. Study of Sangiovese Wines Pigment Profile by UHPLC-MS/MS. J. Agric. Food Chem. 2012, 60, 10461–10471. [Google Scholar] [CrossRef]
- Eder, R.; Beyer, B.; Patzl-Fischerleitner, E.; Wendelin, S.; Hann, S. Determination of pyranoanthocyanine and malvidin-3-glucoside content in red wine of different vintages via LC-MS/ESI. Mitt. Klosterneubg. 2014, 64, 183–192. [Google Scholar]
| Grape Variety | Type of Wine | Chemical Markers | Role of Chemical Markers | Analytical Method | Statistical Method | References |
|---|---|---|---|---|---|---|
| Aurelius, Chardonnay, Müller Thurgau, Moravian Muscat, Hibernal, Malverina, Merzling | W | p-coutaric acid, caftaric acid, protocatechuic and syringic acid; (+)-catechin, (−)-epicatechin | Different set of specific phenolic acids (absolute concentrations) | HPLC-DAD | CVA | [42] |
| Chardonnay, Riesling, Sauvignon Blanc | W | Shikimic acid, ferulic acid, trans-caffeic acid, epicatechin | The most significant loadings in the NMR spectrum | NMR | LDA | [43] |
| Chardonnay, Muscat, Cabernet Sauvignon, Cienna, Dolcetto, Durif, Merlot, Petit Verdot, Pinot Noir, Primitivo, Syrah, Zinfandel | W, R | Caffeic acid, galic acid, rutin, trans-resveratrol | Different content of caffeic acid, gallic acid and rutin in wine samples; lack of trans-resveratrol in Chardonnay and Muscat | HPTLC | HCA, SVD, PCA, ANN | [10] |
| Müller-Thurgau, Riesling | W | Amino and organic acids, phenolic acids, flavonoids and stilbenes | High concentration of quercetin, kaempferol, resveratrol in Müller-Thurgau; high (+)-catechin, (−)-epicatechin, caftarate and coutarate in Riesling | NMR | PLS | [44] |
| Lemberger, Pinot Blanc, Pinot Gris, Müller-Thurgau, Riesling, Gewürztraminer, Pinot Noir | W, R | Phenolic compounds (quercetin, catechin, resveratrol, gallate) | Specific fingerprints due to variety, origin, vintage, physiological state, and technological treatment | NMR | CV, LDA, MANOVA, MC, NCM, PCA, | [45] |
| Passerina, Verdicchio | W | Various phenolic compounds | High concentration of tyrosol, quercetin and glucuronide in Passerina; and hydroxytyrosol, caffeic, caftaric, coumaric and 2-S-glutathionylcaftaric acids (including their esters) in Verdicchio | HPLC–DAD | PCA | [46] |
| Bianco d’Alessano, Chardonnay, Falanghina, Fiano, Malvasia, Moscato, Negroamaro, Verdecca, Malvasia Nera, Primitivo, Susumaniello | W, Rs, R | Gallic acid, syringic acid, luteolin, quercitin, hydroxytyrosol, trans-resveratrol | Specific set of absolute concentrations of phenolics | HPLC-DAD | GDA, PCA | [23] |
| Blaufränkisch, Blauer Portugieser, Pinot Noir, Sankt Laurent, Zweigeltrebe | Rs | Caftaric acid, coumaric acid, ferrulic acid, catechin, malvidin-3-O-glucoside, epicatechin, cis- and trans-resveratrol | Specific set of absolute concentrations of phenolics | HPLC-DAD | Box and Whisker Plot | [37] |
| Cabernet Sauvignon, Feteasca Neagra, Mamaia, Merlot, Pinot Noir | R | Anthocyanins, anthocyanins ratios, phenolic and | Different contents (expressed in malvidin-3-O-glucoside) of acylated malvidin and acylated malvidin/malvidin in combination with acids in different grape varieties | HPLC-PDA, NMR | LDA, PCA | [58] |
| Aspiran, Bonarda, Cabernet Sauvignon, Malbec, Merlot, Sangiovese, Syrah, Tempranillo | R | Malvidin-3-O-glucoside, malvidin-3-(6-O-acetylglucoside), malvidin-3-O-glucoside-4-vinylguaiacol | Absolute concentration (not specified according grape variety) | HPLC-MS | MCR-ALS, D-UPLS | [14] |
| Aglianico (Ag), Cannonau (Ca), Corvina, Montepulciano, Nebbiolo (Nb), Nerello (Nr), Primitivo (Pr), Raboso, Sagrantino, Sangiovese (Sa), Teroldego (Te) | R | Hydroxycinnamates (Ca), Anthocyanins (Te), flavan-3-ols (Ag, Sa, Nb, Nr), flavonols (Sa), amino acids (Pr) | Specific classes of markers through grape varieties | UHPLC-QTOF MS | ANOVA, PCA | [56] |
| Corvina Veronese, Negroamaro, Primitivo, Raboso Piave | R | Ratios of anthocyanins, various phenolic compounds, volatile profile | High concentration of disubstituted compounds and lower acyl derivatives in Corvina and Negroamaro; high content of trisubstituted flavonoids in Primitivo; and anthocyanic and non-anthocyanic acyl derivatives in Raboso | UHPLC-Q/TOF | HCA, PCA, Tukey test | [59] |
| Amarone, Recioto, Primitivo | R | Ratios laricitrin, delphinidin, and petunidin | Ratios have identified the use of 10% Primitivo in wine blends | UHPLC-Q/TOF | HCA, Heat maps, Tukey test | [60] |
| Aspiran, Bonarda, Cabernet Sauvignon, Malbec, Merlot, Sangiovese, Syrah, Tempranillo | R | Phenolic acids and anthocyanins | Differentiation of Malbec; less effective for geographical origin | HPLC-DAD | MCR–ALS | [61] |
| Baboso, Castellana, Listán Negro, Listán Prieto, Merlot, Negramoll, Ruby Cabernet, Syrah, Tintilla, Vijariego. | R | Flavonols and anthocyanins | Lower or higher absolute concentrations | HPLC-DAD | ANOVA, PCA, Pearson coefficient | [18] |
| Bonarda, Cabernet Sauvignon, Malbec, Merlot, Syrah, Tempranillo | R | Phenolic acids, flavan-3-ols, anthocyanins | Specific set of the absolute concentrations of phenolics | HPLC-MS | ANOVA, HSD | [62] |
| Cabernet Sauvignon, Graciano, Tempranillo | R | Flavan-3-ols | Absolute concentrations of phenolics | HPLC-DAD/ESI-MS | - | [63] |
| Cabernet Sauvignon, Pinotage, Syrah | R | Flavan-3-ols and phenolic acids | Specific set of the absolute concentrations of phenolics | HPLC-DAD | Multiple linear regression analysis | [64] |
| Cabernet Sauvignon, Cabernet Franc, Carménère, Merlot, Syrah | R | Flavan-3-ols and phenolic acids | Absolute concentrations of phenolics | HPLC-DAD/PDA | PCA | [65] |
| Cabernet Franc, Merlot, Sangiovese, Syrah | R | Flavan-3-ols, tannins | Lower or higher absolute concentrations | HPLC-DAD-MS | ANOVA, PCA, Tukey test | [64] |
| Carménère, Merlot | R | Ratio of total quercetin/total myricetin and concentration of myricetin | Different ratio contents | HPLC-DAD-ESI-MSn | DA, PCA | [29] |
| Chardonnay, Graševina, Malvazija Istarska, Maraština Muscat Blanc; Pošip, Cabernet Sauvignon, Merlot, Plavac mali, Teran | W, R | Phenolic acids, flavonoids, tannins and stilbenes | Specific concentrations for each wine; the content of cis-piceid—discriminant for white wines, peonidin 3-(6″-acetyl)-glucoside and taxifolin—for red wines | UHPLC-QqQ- MS/MS | ANOVA, LSA, SLDA | [19] |
| Rías Baixas, Ribeira Sacra | R | Syringic acid, malvin, oenin, (+)-catechin, (−)-epicatechin, quercetin, trans-resveratrol | Specific absolute concentrations; high amounts of trans-resveratrol in Ribeira Sacra and malvin in Rías Baixas | HPLC-DAD | LDA, PCA, SIMCA, SVM | [38] |
| Vranac, Kratošija, Cabernet Sauvignon | R | Phenolic acids, flavonoids, tannins and stilbenes | Vranac—high content of anthocyanins; Kratošija—high content of hydroxycinnamic acids; Cabernet Sauvignon—high content of flavan-3-ols and low content of stilbenes | HPLC-DAD | ANOVA, LSD | [67] |
| Negroamaro, Primitivo | R | Gallic acid, syringic acid, catechin, quercetin, hydroxytyrosol, trans-resveratrol | Significant differences of amounts of syringic acid and hydroxytyrosol in Primitivo, trans-resveratrol in Negroamaro | HPLC-DAD | MVA, PCA, OPLS-DA, SIMCA | [39] |
| Lambrusco Sorbara, Lambrusco Salamino di Santa Croce, Lambrusco Grasparossa di Castelvetro | R | Caffeic acid, galic acid, p-coumaric acid, syringic acid, catechin, miricetin, quercitin | p-coumaric and caffeic acids describes Sorbara; gallic acid—Salamino; myricetin and quercitin—Grasparossa | HPLC-DAD | PCA, PLS | [68] |
| Vilana, Dafni, Kotsifali, Mandilari | W, R | Spectral regions from 1800 to 1500 cm−1 and from 1300 to 900 cm−1 | Different fingerprints (band intensity) | FT-IR | LDA | [32] |
| Cabernet Sauvignon, Feteasca Neagra, Mamaia, Merlot, Pinot Noir | R | Phenolics compounds in the 250–600 nm region | Different fingerprints (band intensity) | UV-Vis | LDA, PCA, PLS-DA | [1] |
| Chardonnay, Feteasca Regala, Sauvignon Blanc | W | Signals at 1245, 1575 and 1581 cm−1 | Different fingerprints (band intensity) | SERS | LDA | [69] |
| Feteasca Regala, Sauvignon Blanc | W | Mainly phenolic acids at −767, −543, −530, −653, 1608 and −881 cm−1 | Different fingerprints (band intensity) | FT-Raman | SLDA | [70] |
| Sangiovese, Nebbiolo, Aglianico, Nerello Mascalese, Primitivo, Raboso, Cannonau, Teroldego, Sagrantino, Montepulciano, Corvina | R | Tannins | Different fingerprints (band intensity) | MIR | LDA, PCA, SIMCA, SVM | [9] |
| Chardonnay, Pinot Gris, Riesling, Sauvignon | W | Flavonoids, tannins, stilbenes | Different fingerprints (band intensity) | FT-Raman | LDA | [71] |
| Chardonnay, Gewürztraminer, Sauvignon Blanc, Lagrein, Cabernet Franc, Cabernet Sauvignon, Merlot, Pinot Nero | W, R | Ratios of cyclic prodelphinidins and the sum of cyclic and non-cyclic prodelphinidins | High or low ratios according to variety | HPLC-DAD-HRMS/MS | PCA | [72] |
| Chardonnay, Gewürztraminer, Sauvignon Blanc, Lagrein, Cabernet Franc, Cabernet Sauvignon, Merlot, Pinot Nero | W, R | Ratios of cyclic procyanidins and the sum of cyclic and non-cyclic procyanidins | High or low ratios according to variety | HPLC-DAD-HRMS/MS | PCA | [73] |
| Grape Variety | Chemical Markers and Their Role | References |
|---|---|---|
| Tempranillo | Mv-3-glu > Pt-3-glu > Dp-3-glu > Pn-3-glu > Cy-3-glu, Mv-3-ace > Pt-3-ace > Pn-3-ace > Dp-3-ace > Cy-3-ace, trans-Mv-3-cum > Dp-3-cum > Pn-3-cum > cis-Mv-3-cum > Cy-3-cum, Mv-caf > Pn-caf | [22] |
| Tortosi | All present, except: Cy-3-glu, Pt-3-ace, Pn-3-ace, Dp-3-cum, Pn-caf | |
| Bobal | Low concentrations of anthocyanins, Pt-3-ace, Pn-3-ace and Pn-caf were not present | |
| Moravia Agria | Cy-3-ace, Mv-3-ace, cis-Mv-3-cum, Pn-3-cum, trans-Mv-3-cum and Mv-caf only | |
| Moravia Dulce | Dp-3-glu, Pt-3-glu, Pn-3-glu, Mv-3-glu, Cy-3-ace, Mv-3-ace, cis-Mv-3-cum, Pn-3-cum, trans-Mv-3-cum only | |
| Rojal | Pn-3-glu, Mv-3-glu, Cy-3-ace only | |
| Cabernet Sauvignon | Mv-3-glu, Mv-3-ace, Mv-3-cum, Dp-3-glu, Pt-3-glu and Pn-3-glu | [27] |
| Garnacha | Highest concentration of Mv-3-glu and low concentrations of other anthocyanins | |
| Graciano | Mv-3-glu > Pn-3-ace > Mv-3-cum > Pt-3-glu > Dp-3-glu | |
| Merlot | Mv-3-glu > Mv-3-ace > Pn-3-glu > Mv-3-cum > Dp-3-glu > Pt-3-glu | |
| Tempranillo | Higher content of 3-O-glucosides (except Mv-3-glu) than Cabernet Sauvignon | |
| Syrah | Pn-3-glu > Pt-3-glu > Dp-3-glu | [50] |
| Tannat | Pt-3-glu > Dp-3-glu > Pn-3-glu | |
| Alicante Bouschet, Cabernet Sauvignon, Merlot, Monastrel Bouschet | Various concentrations of Dp-3-ace, Cy-3-ace and Pt-3-ace; Pn-3-glu is a major anthocyanin only for Bouschet varieties | [51] |
| Bobal, Garnacha, Petit Bouschet, Tempranillo | Not present: Dp-3-ace, Cy-3-ace and Pt-3-ace; Pn-3-glu is a major anthocyanin | |
| Cabernet Sauvignon, Lingua di Femmina, Merlot, Piedirosso | Specific absolute concentrations distribution: Mv-3-glu > Mv-3-ace > Mv-3-cum | [52] |
| Aglianico del Taburno, Aglianico del Vulture, Aglianico di Taurasi | Specific absolute concentrations distribution: Mv-3-glu > Mv-3-cum > Mv-3-ace | |
| Blaufränkisch, Blauer Portugieser, Saint Laurent | Specific set of absolute concentrations: Dp-3-glu, Mv-3-glu, Dp-3-ace for each variety | [53] |
| Nebbiolo | Mv-3-glu > Pn-3-glu (Pn-3-glu is half of Mv-3-glu concentration) | [56] |
| Grape Species | Type of Wine | Chemical Markers | Role of Chemical Marker | Analytical Method | Statistical Method | References |
|---|---|---|---|---|---|---|
| Hybrid grape Isabel (Vitis vinifera × Vitis labrusca) | R | 3,5-diglucosidic anthocyanins | The presence in the hybrid grape wine | HPLC-DAD-ESI-MSn | - | [81] |
| Hybrid grape Rondo (Vitis amurensis × Vitis vinifera) | R | 3,5-diglucosidic anthocyanins, gallic acid, trans-piceid and cis-piceid | High concentrations in Rondo | UPLC-PDA-MS/MS | HCA | [5] |
| Spine grape (Vitis davidii Foex) | R, W | Malvidin-3,5-diglucoside, syringetin-3-O-glucoside, dihydroquercetin-3-hexoside and coutaric acid | High concentrations in Spine grapes wine | HPLC-DAD/ESI-MS | ANOVA, Duncan’s multiple range tests | [82] |
| Vitis amurensis, Vitis davidii | R | 3,5-diglucosidic anthocyanins, phenolic acids, kaempferol-3-O-glucoside, quercetin-3-O-rhamnoside | Higher concentrations in Vitis davidii | HPLC-QqQ-MS/MS | PCA | [83] |
| Hybrid grapes: Baco (V. Vinifera × V. Labrusca × V. Riparia × V. Rupestris × V. aestivalis), Seybel (V. Vinifera × V. Rupestris × V. lincecu), Clinton (V. Labrusca × V. riparia), Jacquez (V. Aestivalis × V. Cinerea × V. Vinifera), Othello (V. Labrusca × V. Riparia × V. Vinifera) | R | Proportion of mono-glucoside and acetylated anthocyanins | The ratio lower than three indicates hybrid grape wine | LC-MS-MS | - | [84] |
| Beihong (Vitis vinifera × Vitis amurensis) | R | Mainly phenolic and amino acids | Specific fingerprints | NMR | PCA | [85] |
| Grape Variety | Wine Origin | Chemical Markers | Role of Chemical Markers | Analytical Method | Statistical Method | References |
|---|---|---|---|---|---|---|
| Riesling | Czech Republic | Gallic acid, caffeic acid, caftartic acid, p-coutaric acid, ferulic acid ethylester, p-coumaric acid ethylester, (+)-catechin, (−)-epicatechin | Different absolute concentrations of phenolics in comparison with Riesling from other origins from the literature | HPLC-DAD | ANOVA, CVA, LSD | [87] |
| Riesling | Czech Republic | Protocatechuic acid, p-hydroxybenzoic acid, caftaric acid, p-coutaric acid, trans-resveratrol, cis-resveratrol | Specific absolute concentration of each phenolic through five regions | HPLC-DAD | ANOVA, CDA, LSD, PCA | [13] |
| Riesling | Czech Republic | p-coutaric acid, trans-resveratrol, cis-resveratrol, (+)-catechin, (−)-epicatechin | Specific absolute concentration of each phenolic through five regions | HPLC-DAD | ANOVA, CVA, LSD, PCA | [8] |
| Aurelius, Chardonnay, Müller Thurgau, Moravian Muscat, Hibernal, Malverina, Merzling | Czech Republic | Protocatechuic acid, p-hydroxybenzoic acid, caftaric acid, cis-piceid, (+)-catechin and (–)-epicatechin | Specific set of absolute concentrations of each phenolic through two regions | HPLC-DAD | ANOVA | [40] |
| Chardonnay, Feteasca Regala, Sauvignon Blanc | France and Romania | Mainly phenolic acids at 655, 703, 755, 834, 973, and 1601 cm−1 | Different fingerprints (band intensity) | SERS | LDA | [69] |
| Feteasca Regala, Sauvignon Blanc | France and Romania | Mainly phenolic acids at −709, −887, −740, −721, −503, and −628 cm−1 | Different fingerprints (band intensity) | FT-Raman | SLDA | [70] |
| Chardonnay, Pinot Gris, Riesling, Sauvignon | France and Romania | Mainly phenolic acids at −451, 1453, −455, 503, 1407, 1428, and 1457 cm−1 | Different fingerprints (band intensity) | FT-Raman | LDA | [81] |
| Chardonnay, Pinot Gris, Riesling, Sauvignon Blanc | France and Romania | Gallic acid, ferulic acid, cis-caftaric acid, quercitin | Different fingerprints (peak intensity) | NMR | LDA | [43] |
| Chardonnay, Cabernet Sauvignon | Australia | Cinnamic acid, tartaric acid, myricetin | Specific absolute concentrations (not presented in the article) | HPLC-DAD/MS | LDA, PCA | [89] |
| Chardonnay | Australia, France, Israel, Italy | Mainly flavonols, flavan-3-ols, flavones, flavanones | Specific absolute concentrations for each region and country | UHPLC-ESI/QTOF-MS | ANOVA, OPLS-DA | [88] |
| Bobal, Cabernet Sauvignon, Garnacha, Merlot, Tempranillo | France and Spain | Flavonols | Specific molar percentage of eight flavonols through the samples | LC-DAD-ESI-MSn | Student-Newman-Keuls test | [28] |
| Cabernet Sauvignon, Cabernet Franc, Carménère Merlot, Syrah | China | Flavan-3-ols and phenolic acids | Significant differences in the absolute concentrations | HPLC-DAD/PDA | PCA | [66] |
| Cabernet Sauvignon | China | Gallic acid, (+)-catechin, (−)-epicatechin, procyanidin B1, procyanidin B2, procyanidin C1 | Specific absolute concentrations | HPLC-QqQ-MS/MS | PCA, PLS-DA, OPLS-DA | [12] |
| Cabernet Sauvignon | Bulgaria, Croatia, Macedonia, Montenegro, Serbia | Catechin, quercetin | Specific absolute concentrations through the samples from different countries | HPLC-DAD/FL | ANOVA | [91] |
| Syrah | Brazil | Catechin | Notable differences in absolute concentrations through the terroirs | HPLC-DAD | ANOVA, PCA, Tukey test | [11] |
| Merlot | Argentina, Brazil, Chile, Uruguay | Anthocyanins | Specific absolute concentration | HPLC-DAD/MS | SVM | [6] |
| Cabernet Sauvignon, Merlot | China | Percentage of malvidin-3-O-glucoside and its derivatives to the total content of anthocyanins | Specific percentage through the terroirs | HPLC-MS/MS | Tukey test | [92] |
| Teran, Plavac mali, Merlot, Cabernet Sauvignon | Croatia | Anthocyanins | Specific absolute concentrations | UPLC-QqQ-MS/MS | ANOVA, LSA, SLDA | [19] |
| Aspiran, Bonarda, Cabernet Sauvignon, Malbec, Merlot, Sangiovese, Syrah, Tempranillo | Argentina | Malvidin-3-O-glucoside and its derivatives | Specific absolute concentrations through the terroirs (not presented in the article) | HPLC-MS | MCR-ALS, D-UPLS | [14] |
| Cabernet Sauvignon | Bulgaria, Croatia, Macedonia, Montenegro, Serbia | Anthocyanins | Specific absolute concentrations | HPLC-DAD, DPPH | - | [93] |
| Blaufränkisch, Blauer Portugieser, Saint Laurent | Czech Republic | Delphinidin-3-O-glucoside, malvidin-3-O-glucoside, delphinidin-3-O-p-coumarylglucoside | Different absolute concentrations of anthocyanins in comparison with studied wines from other origins from the literature | HPLC-DAD | ANOVA, CDA, LSD, PCA | [53] |
| Various monovarietal and blended wines | Spain | Hydroxycinnamic acids (for white wines), anthocyanin (for red wines) | Not specified | UV-Vis | SVM | [94] |
| Malbec | Argentina | (+)-Catechin, caftaric acid and quercetin-3-O-glucoside | Specific absolute concentrations through six regions (lowest content was in Rivadavia) | HPLC-DAD | DA | [15] |
| Rías Baixas, Ribeira Sacra | Spain | Syringic acid, malvin, oenin, (+)-catechin, (−)-epicatechin, quercetin, trans-resveratrol | Different absolute concentrations (not presented in the article) | HPLC-DAD | LDA, PCA, SIMCA, SVM | [35] |
| Lemberger, Pinot Blanc, Pinot Gris, Müller-Thurgau, Riesling, Gewürztraminer, Pinot Noir | Germany | Phenolic and amino acids | Specific fingerprint | NMR | CV, LDA, MANOVA, MC, NCM, PCA, | [43] |
| Moschofilero, Asyrtiko, Agiorgitiko, Mandilaria, | Greece | Gallic acid, trans-caffeic, (−)-epicatechin | Both W and R wines from Santorini had twice more of gallic acid and lower concentrations of trans-caffeic, (−)-epicatechin than wines from Nemea | NMR | t test | [7] |
| Grape Variety | Winemaking Method | Chemical Markers | Role of Chemical Markers | Analytical Method | Statistical Method | References |
|---|---|---|---|---|---|---|
| Not reported | Cava sparkling wine | Phenolics with absorbance at 280 nm | Decrease of hydroxycinnamic acids in sparkling wine | HPLC-DAD/ESI–TOFMS | MANOVA | [96] |
| Chardonnay (Cd), Macabeu, Xarel·lo, Parellada, Pinot Noir (PN), Garnacha (Gn), Trepat (Tp) | Cava sparkling wine | Phenolic acids | High amounts Cd, PN, Gn and Tp low—in Macabeu, Xarel·lo and Parellada | HPLC-DAD | PCA | [97] |
| Pinot Blanc | Prefermentative cold maceration with pectolytic enzyme | Trans-caftaric acid and astilbin | Higher concentrations in wines with cold maceration | HPLC-DAD/FLD, HPLC-MS | PCA | [98] |
| Primitivo | Destemmed 100%, 75%, 50% of grapes cluster and stem contact for all time fermentation | Anthocyanins | Concentration increasing when less stems are used | HPLC-DAD | ANOVA, Duncan multiple comparison test, PCA | [99] |
| Merlot, Syrah, Tannat | Traditional maceration; addition of maceration enzymes; cold soak | Anthocyanins | Higher concentration during cold soak | HPLC-DAD | Tukey test | [50] |
| Pinot Noir | Thermal maceration; treatment after a stuck fermentation; fermentation with 20% whole grape clusters; 100% raisin grapes | Ratios of cyclic and non-cyclic proanthocyanidins | Higher concentration of proanthocyanidins with raisin grapes | HPLC-HRMS/MS | PCA | [100] |
| Cabernet Sauvignon, Merlot, Monastrell | Wine blending | Groups of phenolics at 520 nm and 620 nm, petunidin-3-O-glucoside and peonidin-3-O-glucoside | Specific fingerprints through different blends | HPLC-DAD, UV-Vis spectrometry | DA | [101] |
| Grape Variety | Aging Time | Aging Method | Chemical Markers | Role of Chemical Markers | Analytical Method | Statistical Method | References |
|---|---|---|---|---|---|---|---|
| Chardonnay, Pinot Gris, Verdicchio, Amarone, Sagrantino, Sangiovese, Tannat | 1986–2016 | In bottles | Sulfonated indoles for white wines, sulfonated, monomeric, and oligomeric flavan-3-ols for red wines | Specific absolute concentrations through different varieties and vintages | UHPLC-MS/MS | One-way ANOVA, Tukey test | [104] |
| Blend of Cabernet Sauvignon and Merlot | 200 days | Medium toasted and highly toasted barrels from oak | Ellagic acid | Higher concentration in wine from oak barrels | HPLC-DAD | - | [103] |
| Syrah | 2, 4, 6, 9, and 12 months | Toasted acacia and French oak barrels | 2,4-dihydroxybenzoic acid and flavonoids | Present only in wine aged in acacia wood | LC–DAD/ESI-MS | - | [105] |
| Tempranillo | 3 and 6 months in barrels; 25 days with chips | Non-toasted and in medium toasted chestnut barrels and chestnut chips | Benzoic acids, anthocyanins | Specific absolute concentrations of increasing on the aging and distributing differently due to aging method | LC–DAD/ESI-MS | PCA | [106] |
| Blend of Sangiovese and Merlot | 4 months | Oak and cherry wood barriques, steel tanks | Flavanones | Present only in cherry barriques | HPLC-DAD | Post-hoc, LSD, PCA | [107] |
| Tinta del País | 6 and 12 months | Traditional barrels, oak chips and oak staves (American, French and Hungarian), stainless-steel tanks | Epicatechin, phenolic acids, anthocyanins | Specific absolute concentrations of phenolics influenced by aging time, type and wood | HPLC-DAD | LDA, PCA | [108] |
| Mencia, Tinta del País | 3, 6, 9, and 12 months | Oak barrels and oak chips (American and French) | Anthocyanins | Specific set of absolute concentrations (not presented in the article) | HPLC | DA | [110] |
| Aglianico, Montepulciano | 12 months | With and without oak chips | Anthocyanins, tannins | Polymerization of these markers when oak chips are used | HPLC–DAD/ ESI–MS/MS | t test, PCA | [110] |
| Vilana, Dafni, Kotsifali, Mandilari | 3, 6, 9, and 12 months | Medium toasted barrels (French oak, American oak, acacia and chestnut), stainless steel | Spectral regions from 1800 to 1500 cm−1 and from 1300 to 900 cm−1 | Different fingerprints according to aging time and type | FT-IR | LDA | [111] |
| Vilana, Dafni, Kotsifali, Mandilari | 3, 6, and 9 months | Tanks with oak sticks and barrels (French oak, American oak, acacia, and chestnut) | Ellagitannins | Content decrement: chestnut > French oak > American oak > chips > acacia | FT-IR | PLS | [32] |
| Grape Variety | Vintage | Chemical Markers | Role of Chemical Markers | Analytical Method | Statistical Method | References |
|---|---|---|---|---|---|---|
| Müller-Thurgau, Riesling | 2006, 2007 | Amino, organic and phenolic acids | High concentration of different acids through vintages | NMR | PLS | [44] |
| Moschofilero, Asyrtiko, Agiorgitiko, Mandilaria, | 2005, 2006 | Gallic acid, trans-caffeic acid, p-coumaric acid, syringic acid, ferulic acid, (+)-catechin, (−)-epicatechin, quercetin, kaempferol, trans-resveratrol | Lower concentration of polyphenols of samples from 2005 | NMR | t test | [7] |
| Lemberger, Pinot Blanc, Pinot Gris, Müller-Thurgau, Riesling, Gewürztraminer, Pinot Noir | 2008, 2009 | Phenolic and amino acids | Individual fingerprint of samples | NMR | CV, LDA, MANOVA, MC, NCM, PCA, | [45] |
| Chardonnay, Feteasca Regala, Sauvignon Blanc | 2011–2015 | Malic and tartaric acids | Different fingerprints (band intensity) | SERS | LDA | [69] |
| Feteasca Regala, Sauvignon Blanc | 2011–2015 | Mainly phenolic acids at −767, −543, −530, −653, 1608 and −881 cm−1 | Different fingerprints (band intensity) | FT-Raman | SLDA | [70] |
| Chardonnay, Pinot Gris, Riesling, Sauvignon | 2012–2016 | Caffeic, caftaric, ferulic acids | Different fingerprints (band intensity) | FT-Raman | LDA | [71] |
| Pinotage | 1996–2002 | Caffeic acid, malvidin-3-O-glucoside (MvGl), pinotin A | Increased ratio of caffeic acid/MvGl through time and pinotin A content | HPLC-MD | SD | [113] |
| Tempranillo | 2000–2002 | Delphinidin-3-O-glucoside (DpGl), petunidin-3-O-glucoside (PtGl), glucoside, malvidin-3-O-p-coumarylglucoside (MvGlCm), malvidin-3-O-glucoside (MvGl) | Increment of DpGl, PtGl and malvidin- MvGlCm and decrement of MvGl through time | HPLC | ANOVA, HCA, PCA | [114] |
| Sangiovese | 2008–2010 | Anthocyanins | Decrement of anthocyanins and increment of pinotin A through time | UHPLC-DAD-MS/MS | PLS | [115] |
| Cabernet Sauvignon (CS), Merlot (M) | Range 1978–2005 (for CS) and 1979–2003 (for M) | Anthocyanins, tannins | The specific sum of concentrations of phenolic classes through the samples | HPLC–DAD | ANOVA, PCA | [17] |
| Varietal red wines | 2000–2010 | Monomeric anthocyanins, malvidin-3-O-glucoside; pyranoanthocyanins are not effective | Decrement of monomeric anthocyanins | LC-ESI-MS | t-test, PCA | [116] |
| Cabernet Franc (CF), Merlot (M), Sangiovese (Sg), Syrah (Sr) | 2006, 2007 | Flavan-3-ols, tannins | Lower or higher concentrations (2006 < 2007 in CF, M; 2006 > 2007 in Sg, Sr) | HPLC-DAD-MS | ANOVA, PCA, Tukey test | [65] |
| Cabernet Sauvignon | 1971–2003 | Phenolic acids, flavonoids and resveratrol | Decrement and increment of concentrations of specific phenolics during the timeline | HPLC-MS | PCA, PLSR | [112] |
| Cabernet Sauvignon | 2003–2015 | (+)-catechin, (−)-epicatechin, malvidin-3-O-glucoside, malvidin- 3-O-acetylglucoside | Specific set of absolute concentrations (not presented in the article) | HPLC-QqQ-MS/MS | PCA, PLS-DA, OPLS-DA | [12] |
| Cabernet Sauvignon, Feteasca Neagra, Mamaia, Merlot, Pinot Noir | 2009–2017 | Phenolics and other chemical compounds in the 1600–900 cm−1 spectral region | Different fingerprints | FT-IR | LDA, PCA, PLS-DA | [1] |
| Cabernet Sauvignon, Feteasca Neagra, Mamaia, Merlot, Pinot Noir | 2009–2014 | Mainly delphinidin-3-O-glucoside, peonidin-3-O-glucoside, malvidin-3-O-acetylglucoside, malvidin-3-O-p-coumarylglucoside, peonidin-3-O-(6-p-coumaroyl)glucoside | Specific set of concentrations and ratios of anthocyanins (expressed in mg/L of malvidin-3-O-glucoside) together with NMR fingerprint | HPLC-PDA, NMR | LDA, PCA | [58] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merkytė, V.; Longo, E.; Windisch, G.; Boselli, E. Phenolic Compounds as Markers of Wine Quality and Authenticity. Foods 2020, 9, 1785. https://doi.org/10.3390/foods9121785
Merkytė V, Longo E, Windisch G, Boselli E. Phenolic Compounds as Markers of Wine Quality and Authenticity. Foods. 2020; 9(12):1785. https://doi.org/10.3390/foods9121785
Chicago/Turabian StyleMerkytė, Vakarė, Edoardo Longo, Giulia Windisch, and Emanuele Boselli. 2020. "Phenolic Compounds as Markers of Wine Quality and Authenticity" Foods 9, no. 12: 1785. https://doi.org/10.3390/foods9121785
APA StyleMerkytė, V., Longo, E., Windisch, G., & Boselli, E. (2020). Phenolic Compounds as Markers of Wine Quality and Authenticity. Foods, 9(12), 1785. https://doi.org/10.3390/foods9121785







