Influence of Processing Temperature on Membrane Performance and Characteristics of Process Streams Generated during Ultrafiltration of Skim Milk
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Ultrafiltration of Skim Milk
2.3. Characterisation of Process Streams
2.3.1. Composition of Feed, Retentate, and Permeate Samples
2.3.2. Viscosity and Particle Size Distribution of Feed and Retentate Samples
2.3.3. Protein Profile Analysis
2.3.4. Plasmin and Plasminogen-Derived Activity
2.4. Statistical Analysis
3. Results and Discussion
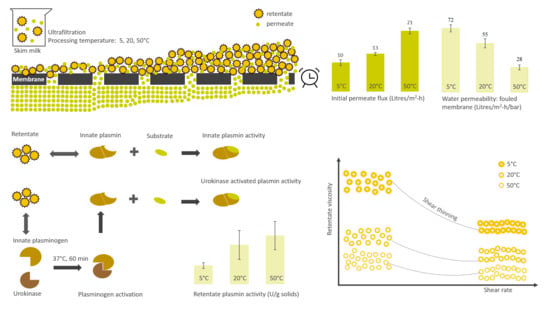
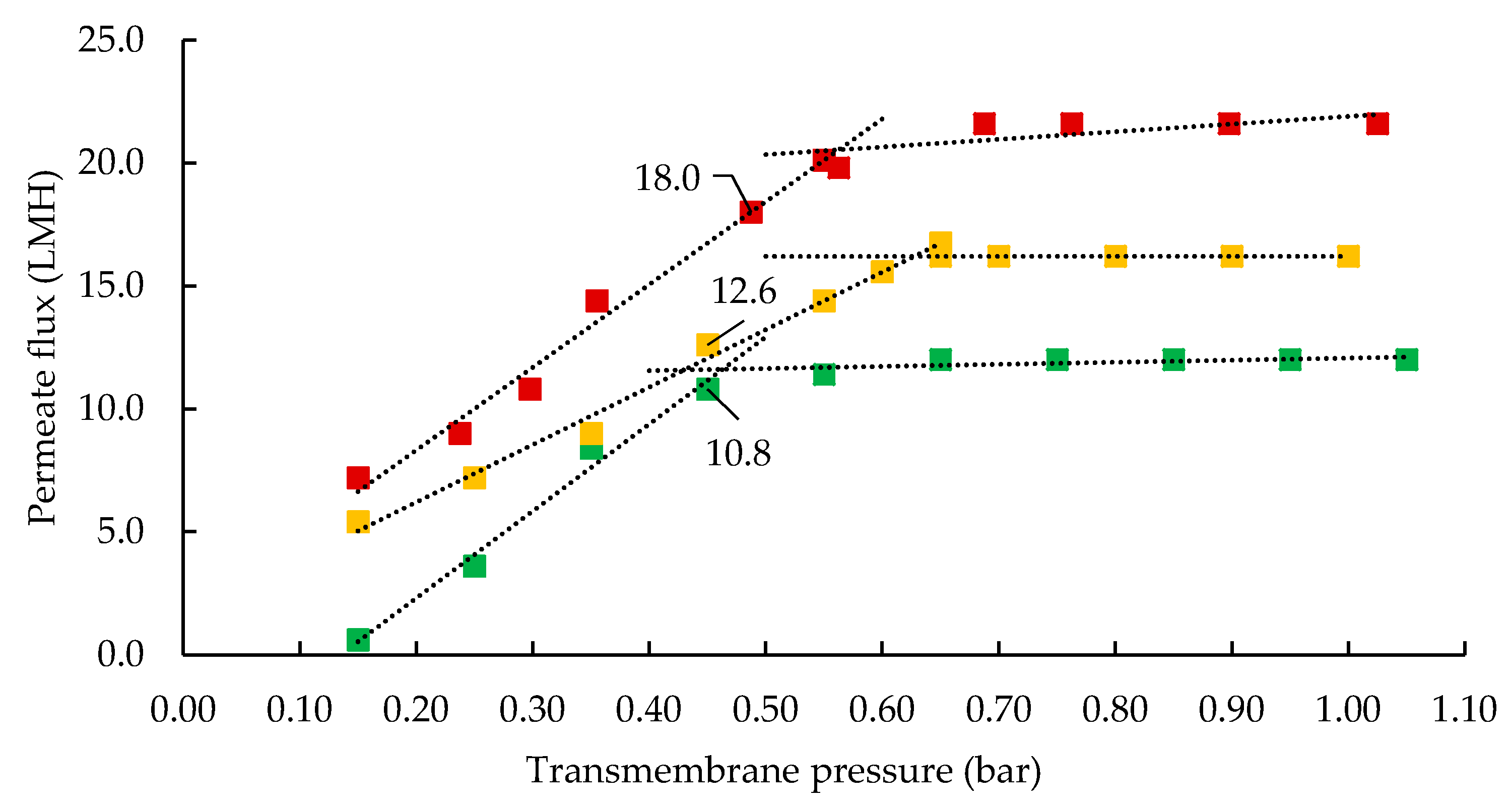
3.1. Influence of Processing Temperature on Membrane Performance
3.2. Composition of Retentate and Permeate Streams
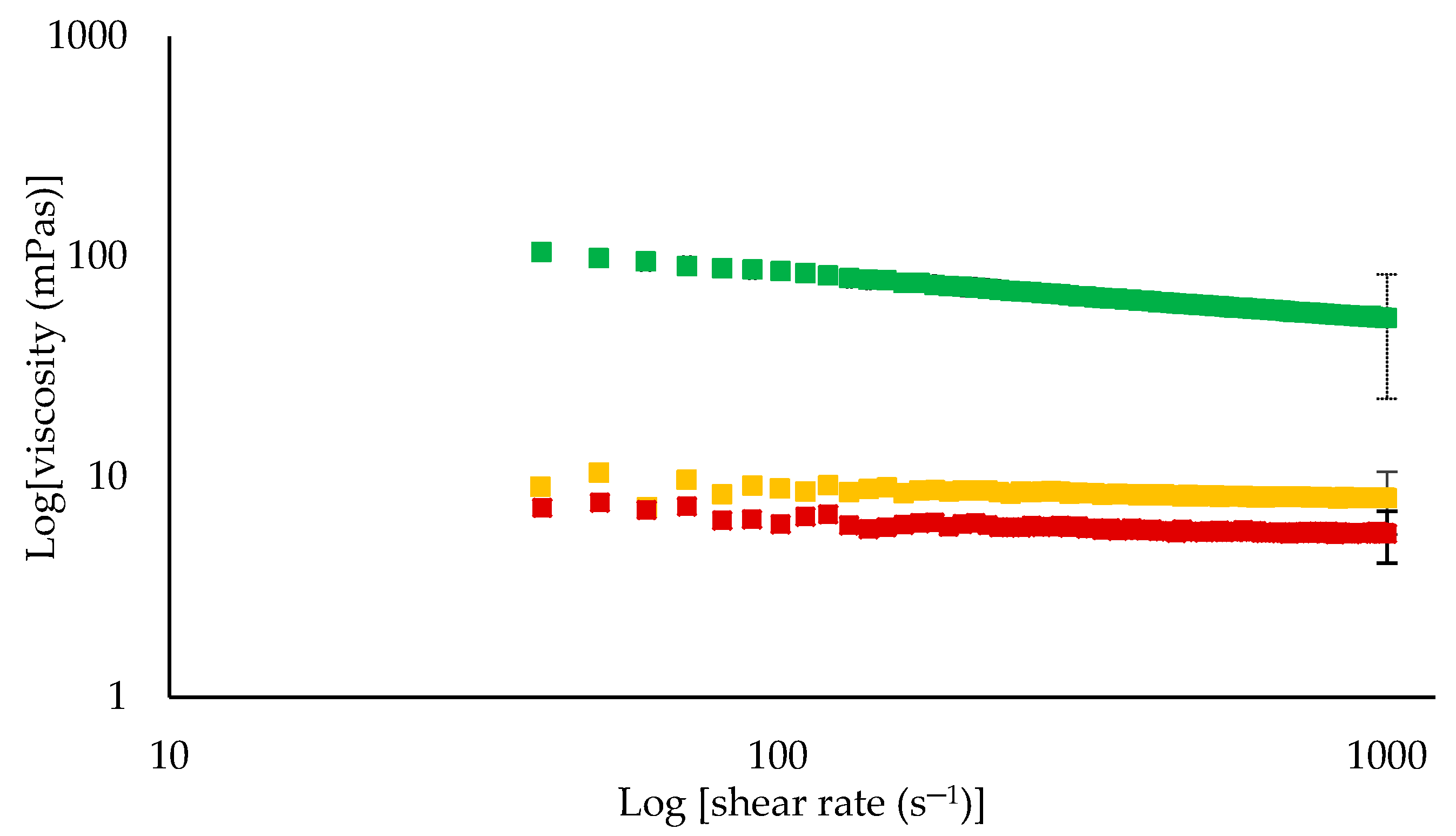
3.3. Physical Properties of Retentates
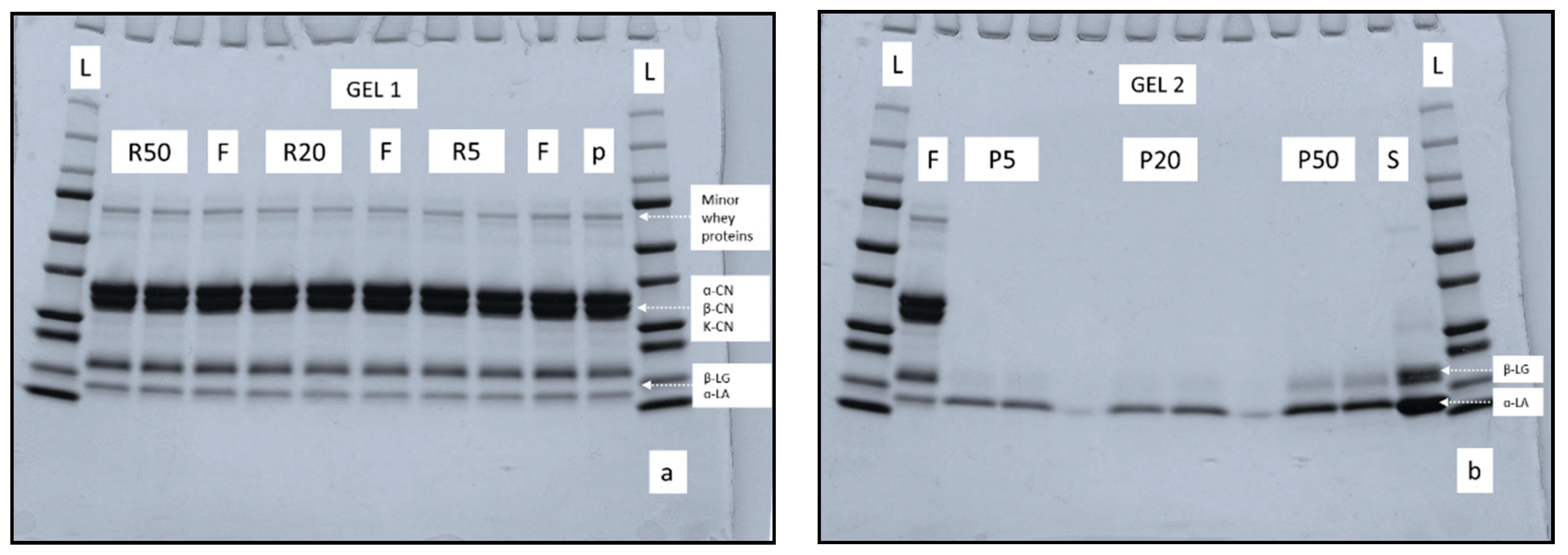
3.4. Protein Profile of Retentate and Permeate Samples
3.5. Plasmin- and Plasminogen-Derived Activity in Retentates
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rinaldoni, A.N.; Tarazaga, C.C.; Campderrós, M.E.; Padilla, A.P. Assessing performance of skim milk ultrafiltration by using technical parameters. J. Food Eng. 2009, 92, 226–232. [Google Scholar] [CrossRef]
- Rupp, L.; Molitor, M.; Lucey, J. Effect of processing methods and protein content of the concentrate on the properties of milk protein concentrate with 80% protein. J. Dairy Sci. 2018, 101, 7702–7713. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Z.; Weeks, M.G.; Dunstan, D.E.; Martin, G.J. Alterations to the composition of casein micelles and retentate serum during ultrafiltration of skim milk at 10 and 40 C. Int. Dairy J. 2014, 35, 63–69. [Google Scholar] [CrossRef]
- Luo, X.; Ramchandran, L.; Vasiljevic, T. Lower ultrafiltration temperature improves membrane performance and emulsifying properties of milk protein concentrates. Dairy Sci. Technol. 2015, 95, 15–31. [Google Scholar] [CrossRef]
- Ng, K.S.; Dunstan, D.E.; Martin, G.J. Influence of processing temperature on flux decline during skim milk ultrafiltration. Sep. Purif. Technol. 2018, 195, 322–331. [Google Scholar] [CrossRef]
- Govindasamy-Lucey, S.; Jaeggi, J.; Johnson, M.; Wang, T.; Lucey, J. Use of cold ultrafiltered retentates for standardization of milks for pizza cheese: Impact on yield and functionality. Int. Dairy J. 2005, 15, 941–955. [Google Scholar] [CrossRef]
- Kapsimalis, D.; Zall, R. Ultrafiltration of skim milk at refrigerated temperatures. J. Dairy Sci. 1981, 64, 1945–1950. [Google Scholar] [CrossRef]
- Crowley, S.V.; O’Callaghan, T.F.; Kelly, A.L.; Fenelon, M.A.; O’Mahony, J.A. Use of ultrafiltration to prepare a novel permeate for application in the functionality testing of infant formula ingredients. Sep. Purif. Technol. 2015, 141, 294–300. [Google Scholar] [CrossRef]
- Liu, D.Z.; Weeks, M.G.; Dunstan, D.E.; Martin, G.J. Temperature-dependent dynamics of bovine casein micelles in the range 10–40 C. Food Chem. 2013, 141, 4081–4086. [Google Scholar] [CrossRef]
- Dalgleish, D.G.; Corredig, M. The structure of the casein micelle of milk and its changes during processing. Annu. Rev. Food Sci. Technol. 2012, 3, 449–467. [Google Scholar] [CrossRef]
- Kelly, A.; O’Flaherty, F.; Fox, P. Indigenous proteolytic enzymes in milk: A brief overview of the present state of knowledge. Int. Dairy J. 2006, 16, 563–572. [Google Scholar] [CrossRef]
- Ismail, B.; Nielsen, S. Invited review: Plasmin protease in milk: Current knowledge and relevance to dairy industry. J. Dairy Sci. 2010, 93, 4999–5009. [Google Scholar] [CrossRef] [PubMed]
- Precetti, A.; Oria, M.; Nielsen, S. Presence in bovine milk of two protease inhibitors of the plasmin system. J. Dairy Sci. 1997, 80, 1490–1496. [Google Scholar] [CrossRef]
- Gazi, I.; Vilalva, I.C.; Huppertz, T. Plasmin activity and proteolysis in milk protein ingredients. Int. Dairy J. 2014, 38, 208–212. [Google Scholar] [CrossRef]
- Crowley, S.V.; Caldeo, V.; McCarthy, N.A.; Fenelon, M.A.; Kelly, A.L.; O’Mahony, J.A. Processing and protein-fractionation characteristics of different polymeric membranes during filtration of skim milk at refrigeration temperatures. Int. Dairy J. 2015, 48, 23–30. [Google Scholar] [CrossRef]
- International IDF Standard. Milk, Determination of Nitrogen Content (Kjeldahl Method) and Calculation of Crude Protein Content, 20A; International Dairy Federation: Schaerbeek, Belgium, 1986. [Google Scholar]
- International IDF Standard. Milk, Cream and Evaporated Milk–Total Solids, 21 B; International Dairy Federation: Schaerbeek, Belgium, 1987. [Google Scholar]
- Crowley, S.V.; Kelly, A.L.; O’Mahony, J.A.; Lucey, J.A. Colloidal properties of protein complexes formed in β-casein concentrate solutions as influenced by heating and cooling in the presence of different solutes. Colloids Surf. B 2019, 174, 343–351. [Google Scholar] [CrossRef]
- Mun, D.; Hsieh, T.T.; Tiu, C.; Sutherland, B. Steady shear viscosity of ultrafiltered whole and skim milk retentates before and during fermentation. J. Texture Stud. 1999, 30, 549–561. [Google Scholar] [CrossRef]
- Beliciu, C.; Moraru, C. Effect of solvent and temperature on the size distribution of casein micelles measured by dynamic light scattering. J. Dairy Sci. 2009, 92, 1829–1839. [Google Scholar] [CrossRef]
- Richardson, B.; Pearce, K. Determination of plasmin in dairy products. N. Z. J. Dairy Sci. 1981, 16, 209–220. [Google Scholar]
- Saint-Denis, T.; Humbert, G.; Gaillard, J.-L. Enzymatic assays for native plasmin, plasminogen and plasminogen activators in bovine milk. J. Dairy Res. 2001, 68, 437–449. [Google Scholar] [CrossRef]
- Ng, K.S.; Haribabu, M.; Harvie, D.J.; Dunstan, D.E.; Martin, G.J. Mechanisms of flux decline in skim milk ultrafiltration: A review. J. Membr. Sci. 2017, 523, 144–162. [Google Scholar] [CrossRef]
- Bégoin, L.; Rabiller-Baudry, M.; Chaufer, B.; Faille, C.; Blanpain-Avet, P.; Bénézech, T.; Doneva, T. Methodology of analysis of a spiral-wound module. Application to PES membrane for ultrafiltration of skimmed milk. Desalination 2006, 192, 40–53. [Google Scholar] [CrossRef]
- Bastian, E.; Collinge, S.; Ernstrom, C. Ultrafiltration: Partitioning of milk constituents into permeate and retentate. J. Dairy Sci. 1991, 74, 2423–2434. [Google Scholar] [CrossRef]
- Pompei, C.; Resmini, P.; Peri, C. SKIM milk protein recovery and purification BY ultrafiltration influence of temperature on permeation rate and retention. J. Food Sci. 1973, 38, 867–870. [Google Scholar] [CrossRef]
- St-Gelais, D.; Haché, S.; Gros-Louis, M. Combined effects of temperature, acidification, and diafiltration on composition of skim milk retentate and permeate. J. Dairy Sci. 1992, 75, 1167–1172. [Google Scholar] [CrossRef]
- Broyard, C.; Gaucheron, F. Modifications of structures and functions of caseins: A scientific and technological challenge. Dairy Sci. Technol. 2015, 95, 831–862. [Google Scholar] [CrossRef]
- Dzurec, D.J., Jr.; Zall, R.R. Effect of heating, cooling, and storing milk on casein and whey proteins. J. Dairy Sci. 1985, 68, 273–280. [Google Scholar] [CrossRef]
- Martin, G.J.; Williams, R.; Dunstan, D. Comparison of casein micelles in raw and reconstituted skim milk. J. Dairy Sci. 2007, 90, 4543–4551. [Google Scholar] [CrossRef]
- Meyer, P.; Mayer, A.; Kulozik, U. High concentration of skim milk proteins by ultrafiltration: Characterisation of a dynamic membrane system with a rotating membrane in comparison with a spiral wound membrane. Int. Dairy J. 2015, 51, 75–83. [Google Scholar] [CrossRef]
- Nöbel, S.; Weidendorfer, K.; Hinrichs, J. Apparent voluminosity of casein micelles determined by rheometry. J. Colloid Interface Sci. 2012, 386, 174–180. [Google Scholar] [CrossRef]
- Sood, S.M.; Herbert, P.J.; Slatter, C.W. Structural studies on casein micelles of human milk: Dissociation of β-casein of different phosphorylation levels induced by cooling and ethylenediaminetetraacetate. J. Dairy Sci. 1997, 80, 628–633. [Google Scholar] [CrossRef]
- Carr, A.; Southward, C.; Creamer, L. Protein hydration and viscosity of dairy fluids. In Advanced Dairy Chemistry—1 Proteins; Springer: Berlin/Heidelberg, Germany, 2003; pp. 1289–1323. [Google Scholar]
- Qi, X.L.; Brownlow, S.; Holt, C.; Sellers, P. Thermal denaturation of β-lactoglobulin: Effect of protein concentration at pH 6.75 and 8.05. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzymol. 1995, 1248, 43–49. [Google Scholar] [CrossRef]
- Steinhauer, T.; Hanély, S.; Bogendörfer, K.; Kulozik, U. Temperature dependent membrane fouling during filtration of whey and whey proteins. J. Membr. Sci. 2015, 492, 364–370. [Google Scholar] [CrossRef]
- Barbano, D.; Sciancalepore, V.; Rudan, M. Characterization of milk proteins in ultrafiltration permeate. J. Dairy Sci. 1988, 71, 2655–2657. [Google Scholar] [CrossRef]
- Permiakov, E.A. Alpha-Lactalbumin; Nova Science Publishers: New York, NY, USA, 2005. [Google Scholar]
- Lu, D.D.; Nielsen, S.S. Heat inactivation of native plasminogen activators in bovine milk. J. Food Sci. 1993, 58, 1010–1012. [Google Scholar] [CrossRef]
- Bastian, E.D.; Hansen, K.G.; Brown, R.J. Inhibition of plasmin by β-lactoglobulin using casein and a synthetic substrate. J. Dairy Sci. 1993, 76, 3354–3361. [Google Scholar] [CrossRef]
- Ismail, B.; Choi, L.; Were, L.M.; Nielsen, S. Activity and nature of plasminogen activators associated with the casein micelle. J. Dairy Sci. 2006, 89, 3285–3295. [Google Scholar] [CrossRef]
- Schaller, J.; Gerber, S.S. The plasmin–antiplasmin system: Structural and functional aspects. Cell. Mol. Life Sci. 2011, 68, 785–801. [Google Scholar] [CrossRef]



| Parameter | Feed | UF Retentate | UF Permeate | ||||
|---|---|---|---|---|---|---|---|
| 5 °C | 20 °C | 50 °C | 5 °C | 20 °C | 50 °C | ||
| TS (%, w/w) | 8.44 ± 0.11 | 18.6 b ± 1.96 | 15.6 a ± 1.38 | 20.4 c ± 1.34 | 5.19 b ± 0.04 | 5.15 a ± 0.01 | 5.31 c ± 0.04 |
| Protein (%, w/w) | 3.03 ± 0.13 | 12.1 a ± 1.29 | 9.83 a ± 1.12 | 13.7 b ± 1.15 | 0.10 a ± 0.01 | 0.11 ab ± 0.01 | 0.12 b ± 0.01 |
| Lactose (g/L) | 58.7 ± 3.69 | 42.0 c ± 0.78 | 38.1 b ± 2.26 | 25.3 a ± 0.96 | 44.7 a ± 5.23 | 43.9 a ± 2.43 | 50.7 a ± 4.75 |
| Ash (%, w/w) | 0.62 ± 0.06 | 1.31 a ± 0.16 | 1.18 a ± 0.20 | 1.58 b ± 0.13 | 0.35 a ± 0.04 | 0.35 a ± 0.10 | 0.40 a ± 0.06 |
| pH | 6.75 ± 0.06 | 6.73 a ± 0.04 | 6.78 a ± 0.02 | 6.75 a ± 0.05 | 6.86 b ± 0.08 | 6.82 b ± 0.03 | 6.59 a ± 0.06 |
| Ionic Ca2+ (mM) | 2.22 ± 0.33 | 2.35 ab ± 0.40 | 3.00 b ± 0.44 | 2.20 a ± 0.15 | 2.53 b ± 0.51 | 3.43 b ± 0.59 | 1.57 a ± 0.01 |
| Conductivity (mS/cm) | 4.80 ± 0.05 | 3.57 a ± 0.37 | 4.01 a ± 0.18 | 3.71 a ± 0.18 | 5.09 a ± 0.11 | 5.14 a ± 0.05 | 5.15 a ± 0.06 |
| Feed | UF Retentate | ||||||
| 5 °C | 20 °C | 50 °C | 5 °C | 20 °C | 50 °C | ||
| Zeta potential (mV) | −26.4 b ± 3.25 | −27.8 ab ± 0.88 | −28.9 a ± 1.34 | −26.6 a ± 1.19 | −26.8 a ± 1.11 | −26.5 a ± 1.01 | |
| Particle size (nm) | 187 b ± 4.17 | 189 b ± 3.01 | 179 a ± 4.46 | 190 b ± 11.8 | 189 b ± 4.06 | 179 a ± 4.11 | |
| Minerals (mg/100 g) | Feed | UF Retentate | UF Permeate | ||||
|---|---|---|---|---|---|---|---|
| 5 °C | 20 °C | 50 °C | 5 °C | 20 °C | 50 °C | ||
| Calcium | 94.9 ± 4.9 | 319 a ± 33.5 | 271 a ± 39.9 | 395 b ± 22.7 | 27.9 b ± 1.0 | 26.0 b ± 1.8 | 20.9 a ± 1.3 |
| Phosphorus | 93.7 ± 4.5 | 257 ab ± 42.9 | 214 a ± 17.3 | 281 b ± 13.8 | 40.2 b ± 1.6 | 37.8 ab ± 1.4 | 35.3 a ± 1.2 |
| Sodium | 29.1 ± 0.1 | 32.0 b ± 0.7 | 30.1 a ± 0.3 | 32.3 b ± 0.9 | 28.0 a ± 1.0 | 28.0 a ± 0.4 | 28.3 a ± 2.1 |
| Magnesium | 9.3 ± 0.2 | 20.1 b ± 1.7 | 16.9 a ± 1.7 | 21.2 b ± 1.1 | 5.3 a ± 0.2 | 5.3 a ± 0.1 | 5.5 a ± 0.4 |
| Potassium | 137 ± 0.6 | 155 b ± 3.3 | 147 a ± 1.8 | 160 b ± 3.5 | 133 a ± 4.1 | 135 a ± 1.5 | 138 a ± 9.9 |
| Sulphur | 34.8 ± 1.8 | 106 ab ± 10.6 | 87.6 a ± 11 | 119 b ± 6.8 | 13.0 a ± 0.8 | 13.3 a ± 0.3 | 13.5 a ± 0.6 |
| Iron | 0.5 ± 0.7 | 0.1 a ± 0.1 | 0.1 a ± 0.1 | 0.1 a ± 0.0 | 0.1 a ± 0.0 | 0.1 b ± 0.1 | 0.0a ± 0.0 |
| Copper | 0.0 ± 0.0 | 0.0 ab ± 0.0 | 0.0 a ± 0.0 | 0.0 b ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Zinc | 0.3 ± 0.0 | 1.3 ab ± 0.2 | 1.1 a ± 0.1 | 1.5 b ± 0.1 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Ca: Phosphorus | 1.0 ± 0.1 | 1.3 a ± 0.1 | 1.3 a ± 0.2 | 1.4 a ± 0.0 | 0.7 b ± 0.0 | 0.7 b ± 0.0 | 0.6 a ± 0.0 |
| Ca: Protein (mg/g) | 31.2 ± 1.6 | 26.4 ± 0.9 | 27.6 ± 1.5 | 28.9 ± 2.6 | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puri, R.; Singh, U.; A. O’Mahony, J. Influence of Processing Temperature on Membrane Performance and Characteristics of Process Streams Generated during Ultrafiltration of Skim Milk. Foods 2020, 9, 1721. https://doi.org/10.3390/foods9111721
Puri R, Singh U, A. O’Mahony J. Influence of Processing Temperature on Membrane Performance and Characteristics of Process Streams Generated during Ultrafiltration of Skim Milk. Foods. 2020; 9(11):1721. https://doi.org/10.3390/foods9111721
Chicago/Turabian StylePuri, Ritika, Upendra Singh, and James A. O’Mahony. 2020. "Influence of Processing Temperature on Membrane Performance and Characteristics of Process Streams Generated during Ultrafiltration of Skim Milk" Foods 9, no. 11: 1721. https://doi.org/10.3390/foods9111721
APA StylePuri, R., Singh, U., & A. O’Mahony, J. (2020). Influence of Processing Temperature on Membrane Performance and Characteristics of Process Streams Generated during Ultrafiltration of Skim Milk. Foods, 9(11), 1721. https://doi.org/10.3390/foods9111721