Review of Quantitative Microbial Risk Assessment in Poultry Meat: The Central Position of Consumer Behavior
Abstract
1. Introduction
2. Materials and Methods
- WoS: TITLE: (poultry OR chicken* OR broiler* OR poulet* OR volaille* OR duck* OR geese* OR turkey* OR dinde* OR oie* OR canard*) AND TITLE: (risk* OR risque* OR “risk assessment” OR aqr OR QMRA OR exposure OR “modelling”)
- Scopus: (TITLE (poultry OR chicken* OR broiler* OR poulet* OR volaille* OR duck* OR geese* OR turkey* OR dinde* OR oie* OR canard*) AND TITLE (risk* OR risque* OR “risk assessment” OR aqr OR qmra OR exposure OR modeling OR modelling).
3. Results
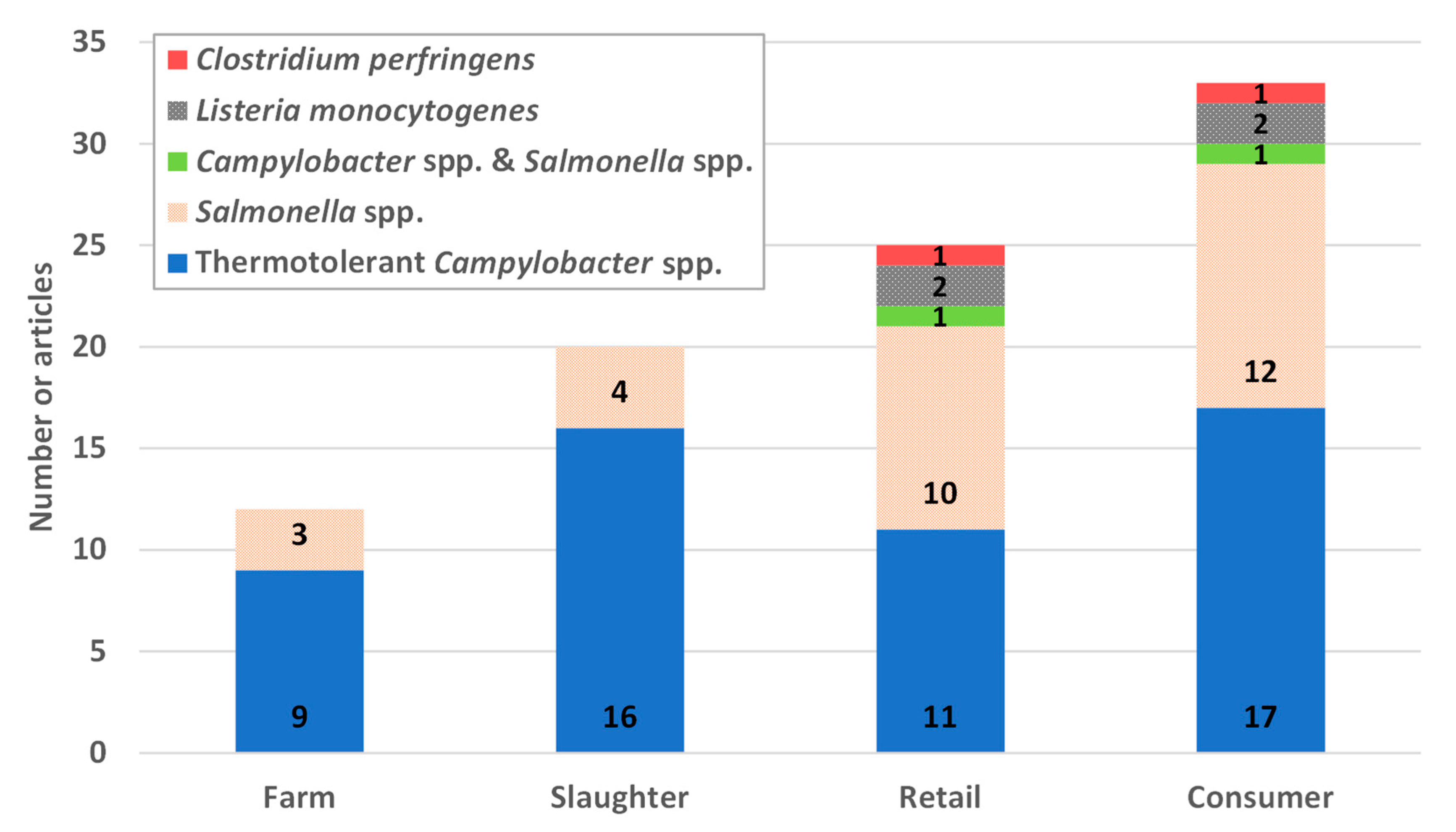
3.1. Summary of Collected Papers
- Identification of risk factors along the meat chain causing microbial contamination or growth [77,78,80,81,82,83,84,85,87,89,90,91,92,93,97,98,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147];
3.2. Poultry Farm-to-Fork Chain
3.2.1. Farm
3.2.2. Slaughtering and Processing
3.2.3. Retail
3.2.4. Consumer
3.3. Pathogens Included in the Review of Quantitative Microbial Risk Assessment (QMRA) Studies
3.4. Parts of the Farm-to-Fork Chain Considered in QMRA Studies
3.5. Focus on the Consumer Step
3.5.1. Effect of Poultry Storage
3.5.2. Impact of Food Preparation
3.5.3. Inactivation of Bacteria during Cooking
3.5.4. Influence of the Consumer Behavior during Serving
4. Discussion
4.1. Summary of Mitigation Interventions Applicable at the Consumer Step
4.2. Geographical Specificities within the Poultry Meat Chain
4.3. Consumer Education with Regard to the Whole Farm-to-Fork Chain
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kirk, M.D.; Pires, S.M.; Black, R.E.; Caipo, M.; Crump, J.A.; Devleesschauwer, B.; Döpfer, D.; Fazil, A.; Fischer-Walker, C.L.; Hald, T.; et al. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med. 2015, 12, e1001921. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, 05926. [Google Scholar] [CrossRef]
- Kelly, L.A.; Hartnett, E.; Gettinby, G.; Fazil, A.; Snary, E.; Wooldridge, M. Microbiological safety of poultry meat: Risk assessment as a way forward. Worlds Poult. Sci. J. 2003, 59, 495–508. [Google Scholar] [CrossRef]
- Humphrey, T.; O’Brien, S.; Madsen, M. Campylobacters as zoonotic pathogens: A food production perspective. Int. J. Food Microbiol. 2007, 117, 237–257. [Google Scholar] [CrossRef] [PubMed]
- Global Epidemiology of Campylobacter Infection|Clinical Microbiology Reviews. Available online: https://cmr.asm.org/content/28/3/687 (accessed on 5 May 2020).
- Vellinga, A. The Dioxin Crisis as Experiment to Determine Poultry-Related Campylobacter Enteritis. Emerg. Infect. Dis. 2002, 8, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization-Organization for Economic Co-operation and Development. Chapter 6: Meat. In OECD-FAO Agricultural Outlook 2018–2027; OECD Publishing: Paris, France; Food and Agriculture Organization of the United Nations: Rome, Italy, 2018; pp. 149–162. [Google Scholar]
- Anses. State of Knowledge Relating to the Contamination of Broilers with Campylobacter and Assessment of the Impact of Interventions at Different Stages of the Food Chain in France; Collective Expert Appraisal Report; Anses: Fougères, France, 2018; p. 104. [Google Scholar]
- Chapman, B.; Otten, A.; Fazil, A.; Ernst, N.; Smith, B.A. A review of quantitative microbial risk assessment and consumer process models for Campylobacter in broiler chickens. Microb. Risk Anal. 2016, 2, 3–15. [Google Scholar] [CrossRef]
- Membré, J.-M.; Boué, G. Quantitative microbiological risk assessment in food industry: Theory and practical application. Food Res. Int. 2018, 106, 1132–1139. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Bucher, O.; Fazil, A.; Rajić, A.; Farrar, A.; Wills, R.; McEWEN, S.A. Evaluating interventions against Salmonella in broiler chickens: Applying synthesis research in support of quantitative exposure assessment. Epidemiol. Infect. 2012, 140, 925–945. [Google Scholar] [CrossRef]
- Collineau, L.; Chapman, B.; Bao, X.; Sivapathasundaram, B.; Carson, C.A.; Fazil, A.; Reid-Smith, R.J.; Smith, B.A. A farm-to-fork quantitative risk assessment model for Salmonella Heidelberg resistant to third-generation cephalosporins in broiler chickens in Canada. Int. J. Food Microbiol. 2020, 330, 108559. [Google Scholar] [CrossRef]
- Oscar, T.P. The Development of a Risk Assessment Model for Use in the Poultry Industry. J. Food Saf. 1998, 18, 371–381. [Google Scholar] [CrossRef]
- Oscar, T.P. A quantitative risk assessment model for Salmonella and whole chickens. Int. J. Food Microbiol. 2004, 93, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Oscar, T.P. Risk of Salmonellosis from Chicken Parts Prepared from Whole Chickens Sold in Flow Pack Wrappers and Subjected to Temperature Abuse. J. Food Prot. 2017, 80, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Maijala, R.; Ranta, J.; Seuna, E.; Pelkonen, S.; Johansson, T. A quantitative risk assessment of the public health impact of the Finnish Salmonella control program for broilers. Int. J. Food Microbiol. 2005, 102, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Bai, Y.; Wang, Y.; Song, X.; Cui, S.; Xu, H.; Jiao, X.; Li, F. A risk assessment of salmonellosis linked to chicken meals prepared in households of China. Food Control 2017, 79, 279–287. [Google Scholar] [CrossRef]
- Smadi, H.; Sargeant, J.M. Quantitative Risk Assessment of Human Salmonellosis in Canadian Broiler Chicken Breast from Retail to Consumption. Risk Anal. 2013, 33, 232–248. [Google Scholar] [CrossRef]
- Straver, J.M.; Janssen, A.F.W.; Linnemann, A.R.; van Boekel, M.A.J.S.; Beumer, R.R.; Zwietering, M.H. Number of Salmonella on Chicken Breast Filet at Retail Level and Its Implications for Public Health Risk. J. Food Prot. 2007, 70, 2045–2055. [Google Scholar] [CrossRef]
- Bemrah, N.; Bergis, H.; Colmin, C.; Beaufort, A.; Millemann, Y.; Dufour, B.; Benet, J.J.; Cerf, O.; Sanaa, M. Quantitative risk assessment of human salmonellosis from the consumption of a turkey product in collective catering establishments. Int. J. Food Microbiol. 2003, 80, 17–30. [Google Scholar] [CrossRef]
- Jeong, J.; Chon, J.-W.; Kim, H.; Song, K.-Y.; Seo, K.-H. Risk Assessment for Salmonellosis in Chicken in South Korea: The Effect of Salmonella Concentration in Chicken at Retail. Korean J. Food Sci. Anim. Resour. 2018, 38, 1043–1054. [Google Scholar] [CrossRef]
- Sampedro, F.; Wells, S.J.; Bender, J.B.; Hedberg, C.W. Developing a risk management framework to improve public health outcomes by enumerating Salmonella in ground turkey. Epidemiol. Infect. 2019, 147, e69. [Google Scholar] [CrossRef] [PubMed]
- Oscar, T.P. Process risk model for Salmonella and ground chicken. J. Appl. Microbiol. 2019, 127, 1236–1245. [Google Scholar] [CrossRef]
- Hartnett, E.; Kelly, L.; Newell, D.; Wooldridge, M.; Gettinby, G. A quantitative risk assessment for the occurrence of Campylobacter in chickens at the point of slaughter. Epidemiol. Infect. 2001, 127, 195–206. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Crotta, M.; Georgiev, M.; Guitian, J. Quantitative risk assessment of Campylobacter in broiler chickens—Assessing interventions to reduce the level of contamination at the end of the rearing period. Food Control 2017, 75, 29–39. [Google Scholar] [CrossRef][Green Version]
- European Food Safety Authority. Scientific Opinion on Campylobacter in broiler meat production: Control options and performance objectives and/or targets at different stages of the food chain. EFSA J. 2011, 9, 2105. [Google Scholar] [CrossRef]
- Romero-Barrios, P.; Hempen, M.; Messens, W.; Stella, P.; Hugas, M. Quantitative microbiological risk assessment (QMRA) of food-borne zoonoses at the European level. Food Control 2013, 29, 343–349. [Google Scholar] [CrossRef]
- Dogan, O.B.; Clarke, J.; Mattos, F.; Wang, B. A quantitative microbial risk assessment model of Campylobacter in broiler chickens: Evaluating processing interventions. Food Control 2019, 100, 97–110. [Google Scholar] [CrossRef]
- Nauta, M.J.; Jacobs-Reitsma, W.F.; Evers, E.G.; van Pelt, W.; Havelaar, A.H. Risk Assessment of Campylobacter in the Netherlands via Broiler Meat and Other Routes; RIVM: Bilthoven, The Netherlands, 2005; p. 128. [Google Scholar]
- Havelaar, A.H.; Mangen, M.-J.J.; Koeijer, A.A.D.; Bogaardt, M.-J.; Evers, E.G.; Jacobs-Reitsma, W.F.; Pelt, W.V.; Wagenaar, J.A.; Wit, G.A.D.; Zee, H.V.D.; et al. Effectiveness and efficiency of controlling Campylobacter on broiler chicken meat. Risk Anal. 2007, 27, 831–844. [Google Scholar] [CrossRef]
- Katsma, W.E.A.; De Koeijer, A.A.; Jacobs-Reitsma, W.F.; Mangen, M.-J.J.; Wagenaar, J.A. Assessing Interventions to Reduce the Risk of Campylobacter Prevalence in Broilers. Risk Anal. 2007, 27, 863–876. [Google Scholar] [CrossRef]
- Nauta, M.J.; Jacobs-Reitsma, W.F.; Havelaar, A.H. A Risk Assessment Model for Campylobacter in Broiler Meat. Risk Anal. 2007, 27, 845–861. [Google Scholar] [CrossRef]
- Huang, J.; Zang, X.; Zhai, W.; Guan, C.; Lei, T.; Jiao, X. Campylobacter spp. in chicken-slaughtering operations: A risk assessment of human campylobacteriosis in East China. Food Control 2018, 86, 249–256. [Google Scholar] [CrossRef]
- Wang, J.; Guo, Y.C.; Li, N. Prevalence and Risk Assessment of Campylobacter jejuni in Chicken in China. Biomed. Environ. Sci. 2013, 26, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Lindqvist, R.; Lindblad, M. Quantitative risk assessment of thermophilic Campylobacter spp. and cross-contamination during handling of raw broiler chickens evaluating strategies at the producer level to reduce human campylobacteriosis in Sweden. Int. J. Food Microbiol. 2008, 121, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Osiriphun, S.; Iamtaweejaloen, P.; Kooprasertying, P.; Koetsinchai, W.; Tuitemwong, K.; Erickson, L.E.; Tuitemwong, P. Exposure assessment and process sensitivity analysis of the contamination of Campylobacter in poultry products. Poult. Sci. 2011, 90, 1562–1573. [Google Scholar] [CrossRef] [PubMed]
- Rosenquist, H.; Nielsen, N.L.; Sommer, H.M.; Nørrung, B.; Christensen, B.B. Quantitative risk assessment of human campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int. J. Food Microbiol. 2003, 83, 87–103. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Risk Assessment of Campylobacter spp. in Broiler Chickens: Technical Report; World Health Organization/Food and Agriculture Organization of the United Nations, FAO, Eds.; Microbiological Risk Assessment Series; World Health Organization: Geneva, Switzerland, 2009; ISBN 978-92-4-154736-9. [Google Scholar]
- Food and Agriculture Organization of the United Nations. Risk Assessment of Campylobacter spp. in Broiler Chickens: Interpretary Summary; World Health Organization/Food and Agriculture Organization of the United Nations, Ed.; Microbiological Risk Assessment Series; World Health Organization: Geneva, Switzerland, 2009; ISBN 978-92-4-154735-2. [Google Scholar]
- Nauta, M.; Lindqvist, R.; Georgsson, F.; Hogåsen, H.; Hielm, S.; Tuominen, P.; Ranta, J.; Rosenquist, H.; Andersen, J.K. Establishment of Risk Based Microbiological Criteria in the Nordic Countries: A Case Study on Campylobacter in Broiler Meat; National Food Institute, Technical University of Denmark, DTU: Copenhagen, Denmark, 2013; ISBN 978-87-92763-83-9. [Google Scholar]
- Boysen, L. Campylobacter in Denmark: Control, Human Risk and Source Attribution. Ph.D. Thesis, National Food Institute, Technical University of Denmark, Søborg, Denmark, 2012. ISBN 978-87-92763-22-8. [Google Scholar]
- Boysen, L.; Nauta, M.; Duarte, A.S.R.; Rosenquist, H. Human risk from thermotolerant Campylobacter on broiler meat in Denmark. Int. J. Food Microbiol. 2013, 162, 129–134. [Google Scholar] [CrossRef]
- Christensen, B.B.; Nauta, M.; Korsgaard, H.; Sørensen, A.I.V.; Rosenquist, H.; Boysen, L.; Perge, A.; Nørrung, B. Case-by-case risk assessment of broiler meat batches: An effective control strategy for Campylobacter. Food Control 2013, 31, 485–490. [Google Scholar] [CrossRef]
- Brynestad, S.; Braute, L.; Luber, P.; Bartelt, E. Quantitative microbiological risk assessment of campylobacteriosis cases in the German population due to consumption of chicken prepared in homes. Int. J. Risk Assess. Manag. 2008, 8, 194. [Google Scholar] [CrossRef]
- Calistri, P.; Giovannini, A. Quantitative risk assessment of human campylobacteriosis related to the consumption of chicken meat in two Italian regions. Int. J. Food Microbiol. 2008, 128, 274–287. [Google Scholar] [CrossRef]
- Signorini, M.L.; Zbrun, M.V.; Romero-Scharpen, A.; Olivero, C.; Bongiovanni, F.; Soto, L.P.; Frizzo, L.S.; Rosmini, M.R. Quantitative risk assessment of human campylobacteriosis by consumption of salad cross-contaminated with thermophilic Campylobacter spp. from broiler meat in Argentina. Prev. Vet. Med. 2013, 109, 37–46. [Google Scholar] [CrossRef]
- Nauta, M.J.; Sanaa, M.; Havelaar, A.H. Risk based microbiological criteria for Campylobacter in broiler meat in the European Union. Int. J. Food Microbiol. 2012, 158, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Uyttendaele, M.; Baert, K.; Ghafir, Y.; Daube, G.; De Zutter, L.; Herman, L.; Dierick, K.; Pierard, D.; Dubois, J.J.; Horion, B.; et al. Quantitative risk assessment of Campylobacter spp. in poultry based meat preparations as one of the factors to support the development of risk-based microbiological criteria in Belgium. Int. J. Food Microbiol. 2006, 111, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Pouillot, R.; Garin, B.; Ravaonindrina, N.; Diop, K.; Ratsitorahina, M.; Ramanantsoa, D.; Rocourt, J. A Risk Assessment of Campylobacteriosis and Salmonellosis Linked to Chicken Meals Prepared in Households in Dakar, Senegal. Risk Anal. 2012, 32, 1798–1819. [Google Scholar] [CrossRef] [PubMed]
- Foerster, C.; Figueroa, G.; Evers, E. Risk assessment of Listeria monocytogenes in poultry and beef. Br. Food J. 2015, 117, 779–792. [Google Scholar] [CrossRef]
- Aarnisalo, K.; Vihavainen, E.; Rantala, L.; Maijala, R.; Suihko, M.; Hielm, S.; Tuominen, P.; Ranta, J.; Raaska, L. Use of results of microbiological analyses for risk-based control of Listeria monocytogenes in marinated broiler legs. Int. J. Food Microbiol. 2008, 121, 275–284. [Google Scholar] [CrossRef]
- Golden, N.J.; Crouch, E.A.; Latimer, H.; Kadry, A.-R.; Kause, J. Risk Assessment for Clostridium perfringens in Ready-to-Eat and Partially Cooked Meat and Poultry Products†. J. Food Prot. 2009, 72, 1376–1384. [Google Scholar] [CrossRef]
- Chien, S.-Y.; Sheen, S.; Sommers, C.H.; Sheen, L.-Y. Modeling the Inactivation of Intestinal Pathogenic Escherichia coli O157:H7 and Uropathogenic E. coli in Ground Chicken by High Pressure Processing and Thymol. Front. Microbiol. 2016, 7, 920. [Google Scholar] [CrossRef]
- Dominguez, S.A.; Schaffner, D.W. Modeling the Growth of Salmonella in Raw Poultry Stored under Aerobic Conditions. J. Food Prot. 2008, 71, 2429–2435. [Google Scholar] [CrossRef]
- Genigeorgis, C.A.; Meng, J.; Baker, D.A. Behavior of Nonproteolytic Clostridium botulinum Type B and E Spores in Cooked Turkey and Modeling Lag Phase and Probability of Toxigenesis. J. Food Sci. 1991, 56, 373–379. [Google Scholar] [CrossRef]
- Ghollasi-Mood, F.; Mohsenzadeh, M.; Hoseindokht, M.-R.; Varidi, M. Quality changes of air-packaged chicken meat stored under different temperature conditions and mathematical modelling for predicting the microbial growth and shelf life: Ghollasi-Mood, et al. J. Food Saf. 2017, 37, e12331. [Google Scholar] [CrossRef]
- Guentert, A.M.; Mohtar, R.H.; Linton, R.H.; Tamplin, M.; Luchansky, J.B. Modeling the Behavior of Listeria monocytogenes in pH-modified Chicken Salad During Cold Storage and Temperature Abuse Conditions. J. Food Process Eng. 2006, 29, 89–117. [Google Scholar] [CrossRef]
- Hu, J.; Lin, L.; Chen, M.; Yan, W. Modeling for Predicting the Time to Detection of Staphylococcal Enterotoxin A in Cooked Chicken Product. Front. Microbiol. 2018, 9, 1536. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Sheen, S.; Sommers, C.; Sheen, L.-Y. Modeling the Survival of Escherichia coli O157:H7 under Hydrostatic Pressure, Process Temperature, Time and Allyl Isothiocyanate Stresses in Ground Chicken Meat. Front. Microbiol. 2018, 9, 1871. [Google Scholar] [CrossRef] [PubMed]
- Juneja, V.K.; Melendres, M.V.; Huang, L.; Gumudavelli, V.; Subbiah, J.; Thippareddi, H. Modeling the effect of temperature on growth of Salmonella in chicken. Food Microbiol. 2007, 24, 328–335. [Google Scholar] [CrossRef]
- Keklik, N.M.; Demirci, A.; Puri, V.M.; Heinemann, P.H. Modeling the Inactivation of Salmonella Typhimurium, Listeria monocytogenes, and Salmonella Enteritidis on Poultry Products Exposed to Pulsed UV Light. J. Food Prot. 2012, 75, 281–288. [Google Scholar] [CrossRef]
- Li, M.; Huang, L.; Zhu, Y.; Wei, Q. Growth of Clostridium Perfringens in roasted chicken and braised beef during cooling—One-step dynamic analysis and modeling. Food Control 2019, 106, 106739. [Google Scholar] [CrossRef]
- McCarthy, Z.; Smith, B.; Fazil, A.; Wu, J.; Ryan, S.D.; Munther, D. Individual based modeling and analysis of pathogen levels in poultry chilling process. Math. Biosci. 2017, 294, 172–180. [Google Scholar] [CrossRef]
- Meng, J.; Genigeorgis, C.A. Modeling lag phase of nonproteolytic Clostridium botulinum toxigenesis in cooked turkey and chicken breast as affected by temperature, sodium lactate, sodium chloride and spore inoculum. Int. J. Food Microbiol. 1993, 19, 109–122. [Google Scholar] [CrossRef]
- Osaili, T.M.; Griffis, C.L.; Martin, E.M.; Gbur, E.E.; Marcy, J.A. Modeling Cooking Time to Inactivate Salmonella in Chicken Leg Quarters Cooked in an Air Steam Impingement Oven. J. Food Sci. 2006, 71, M146–M149. [Google Scholar] [CrossRef]
- Oscar, T.P. Simulation Model for Enumeration of Salmonella on Chicken as a Function of PCR Detection Time Score and Sample Size: Implications for Risk Assessment. J. Food Prot. 2004, 67, 1201–1208. [Google Scholar] [CrossRef]
- Oscar, T.P. General Regression Neural Network and Monte Carlo Simulation Model for Survival and Growth of Salmonella on Raw Chicken Skin as a Function of Serotype, Temperature, and Time for Use in Risk Assessment. J. Food Prot. 2009, 72, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Oscar, T.P. Neural network models for growth of Salmonella serotypes in ground chicken subjected to temperature abuse during cold storage for application in HACCP and risk assessment. Int. J. Food Sci. Technol. 2017, 52, 214–221. [Google Scholar] [CrossRef]
- Ritz, M.; Nauta, M.J.; Teunis, P.F.M.; van Leusden, F.; Federighi, M.; Havelaar, A.H. Modelling of Campylobacter survival in frozen chicken meat. J. Appl. Microbiol. 2007, 103, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Sommers, C.; Huang, C.-Y.; Sheen, L.-Y.; Sheen, S.; Huang, L. Growth modeling of Uropathogenic Escherichia coli in ground chicken meat. Food Control 2018, 86, 397–402. [Google Scholar] [CrossRef]
- Takhar, P.S.; Head, K.L.; Hendrix, K.M.; Smith, D.M. Predictive Modeling of Salmonella Species Inactivation in Ground Pork and Turkey during Cooking. Int. J. Food Eng. 2009, 5. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, W.; Zhang, J.; Liao, M.; Yang, H.; Fang, W.; Li, Y. Modeling the Reduction and Cross-Contamination of Salmonella in Poultry Chilling Process in China. Microorganisms 2019, 7, 448. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, W.; Zhang, X.; Zhang, J.; Liao, M.; Yang, H.; Zhang, Q.; Rainwater, C.; Li, Y. Modeling the Reduction of Salmonella spp. on Chicken Breasts and Wingettes during Scalding for QMRA of the Poultry Supply Chain in China. Microorganisms 2019, 7, 165. [Google Scholar] [CrossRef]
- Marcotte, M.; Chen, C.R.; Grabowski, S.; Ramaswamy, H.S.; Piette, J.-P.G. Modelling of cooking-cooling processes for meat and poultry products. Int. J. Food Sci. Technol. 2008, 43, 673–684. [Google Scholar] [CrossRef]
- Ahmed, M.F.E.M.; El-Adawy, H.; Hotzel, H.; Tomaso, H.; Neubauer, H.; Kemper, N.; Hartung, J.; Hafez, H.M. Prevalence, genotyping and risk factors of thermophilic Campylobacter spreading in organic turkey farms in Germany. Gut Pathog. 2016, 8, 28. [Google Scholar] [CrossRef][Green Version]
- Allain, V.; Chemaly, M.; Laisney, M.-J.; Rouxel, S.; Quesne, S.; Le Bouquin, S. Prevalence of and risk factors for Campylobacter colonisation in broiler flocks at the end of the rearing period in France. Br. Poult. Sci. 2014, 55, 452–459. [Google Scholar] [CrossRef]
- Ansari-Lari, M.; Hosseinzadeh, S.; Shekarforoush, S.S.; Abdollahi, M.; Berizi, E. Prevalence and risk factors associated with campylobacter infections in broiler flocks in Shiraz, southern Iran. Int. J. Food Microbiol. 2011, 144, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, J.; Letellier, A.; Quessy, S.; Morin, J.-P.; Boulianne, M. Prevalence and Risk Factors for Salmonella and Campylobacter spp. Carcass Contamination in Turkeys Slaughtered in Quebec, Canada. J. Food Prot. 2007, 70, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, J.; Letellier, A.; Quessy, S.; Boulianne, M. Prevalence and Risk Factors for Salmonella and Campylobacter spp. Carcass Contamination in Broiler Chickens Slaughtered in Quebec, Canada. J. Food Prot. 2007, 70, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, J.; Letellier, A.; Quessy, S.; Normand, V.; Boulianne, M. Prevalence and risk factors for Salmonella spp. and Campylobacter spp. caecal colonization in broiler chicken and turkey flocks slaughtered in Quebec, Canada. Prev. Vet. Med. 2007, 81, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Aury, K.; Chemaly, M.; Petetin, I.; Rouxel, S.; Picherot, M.; Michel, V.; Le Bouquin, S. Prevalence and risk factors for Salmonella enterica subsp. enterica contamination in French breeding and fattening turkey flocks at the end of the rearing period. Prev. Vet. Med. 2010, 94, 84–93. [Google Scholar] [CrossRef]
- Carron, M.; Chang, Y.-M.; Momanyi, K.; Akoko, J.; Kiiru, J.; Bettridge, J.; Chaloner, G.; Rushton, J.; O’Brien, S.; Williams, N.; et al. Campylobacter, a zoonotic pathogen of global importance: Prevalence and risk factors in the fast-evolving chicken meat system of Nairobi, Kenya. PLoS Negl. Trop. Dis. 2018, 12, e0006658. [Google Scholar] [CrossRef]
- Djeffal, S.; Mamache, B.; Elgroud, R.; Hireche, S.; Bouaziz, O. Prevalence and risk factors for Salmonella spp. contamination in broiler chicken farms and slaughterhouses in the northeast of Algeria. Vet. World 2018, 11, 1102–1108. [Google Scholar] [CrossRef]
- Dubey, J.P.; Hill, D.E.; Jones, J.L.; Hightower, A.W.; Kirkland, E.; Roberts, J.M.; Marcet, P.L.; Lehmann, T.; Vianna, M.C.B.; Miska, K.; et al. Prevalence of viable toxoplasma gondii in beef, chicken, and pork from retail meat stores in the united states: Risk assessment to consumers. J. Parasitol. 2005, 91, 1082–1093. [Google Scholar] [CrossRef]
- El Allaoui, A.; Rhazi Filali, F.; Ameur, N.; Bouchrif, B. Contamination of broiler turkey farms by Salmonella spp. in Morocco: Prevalence, antimicrobial resistance and associated risk factors. Rev. Sci. Tech. OIE 2017, 36, 935–946. [Google Scholar] [CrossRef]
- Habib, I.; Sampers, I.; Uyttendaele, M.; De Zutter, L.; Berkvens, D. A Bayesian modelling framework to estimate Campylobacter prevalence and culture methods sensitivity: Application to a chicken meat survey in Belgium. J. Appl. Microbiol. 2008, 105, 2002–2008. [Google Scholar] [CrossRef]
- Henry, I.; Reichardt, J.; Denis, M.; Cardinale, E. Prevalence and risk factors for Campylobacter spp. in chicken broiler flocks in Reunion Island (Indian Ocean). Prev. Vet. Med. 2011, 100, 64–70. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Shekarforoush, S.S.; Ansari-Lari, M.; EsalatPanah-Fard Jahromi, M.; Berizi, E.; Abdollahi, M. Prevalence and Risk Factors for Listeria monocytogenes in Broiler Flocks in Shiraz, Southern Iran. Foodborne Pathog. Dis. 2012, 9, 568–572. [Google Scholar] [CrossRef]
- Hue, O.; Le Bouquin, S.; Laisney, M.-J.; Allain, V.; Lalande, F.; Petetin, I.; Rouxel, S.; Quesne, S.; Gloaguen, P.-Y.; Picherot, M.; et al. Prevalence of and risk factors for Campylobacter spp. contamination of broiler chicken carcasses at the slaughterhouse. Food Microbiol. 2010, 27, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Hue, O.; Le Bouquin, S.; Lalande, F.; Allain, V.; Rouxel, S.; Petetin, I.; Quesne, S.; Laisney, M.-J.; Gloaguen, P.-Y.; Picherot, M.; et al. Prevalence of Salmonella spp. on broiler chicken carcasses and risk factors at the slaughterhouse in France in 2008. Food Control 2011, 22, 1158–1164. [Google Scholar] [CrossRef]
- Ibrahim, M.J.; Abdul-Aziz, S.; Bitrus, A.A.; Mohammed, D.G.; Abu, J.; Bejo, S.K.; Mohamed, M.A.; Mohamed, M.Y.I. Occurrence of Multidrug resistant (MDR) Campylobacter species isolated from retail Chicken meats in Selangor, Malaysia and their associated risk factors. Malays. J. Microbiol. 2018. [Google Scholar] [CrossRef]
- Kapperud, G.; Skjerve, E.; Vik, L.; Hauge, K.; Lysaker, A.; Aalmen, I.; Ostroff, S.M.; Potter, M. Epidemiological investigation of risk factors for Campylobacter colonization in Norwegian broiler flocks. Epidemiol. Infect. 1993, 111, 245–256. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Keeratipibul, S.; Lekroengsin, S. Risk Assessment of Listeria spp. Contamination in the Production Line of Ready-to-Eat Chicken Meat Products. J. Food Prot. 2008, 71, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Keeratipibul, S.; Meethong, S.; Techaruwichit, P.; Thephuttee, N. Prevalence of Escherichia coli and enterococci in a Thai frozen cooked chicken plant, and modeling of the cleaning and sanitizing procedure. Food Control 2010, 21, 1104–1112. [Google Scholar] [CrossRef]
- Lawes, J.R.; Vidal, A.; Clifton-Hadley, F.A.; Sayers, R.; Rodgers, J.; Snow, L.; Evans, S.J.; Powell, L.F. Investigation of prevalence and risk factors for Campylobacter in broiler flocks at slaughter: Results from a UK survey. Epidemiol. Infect. 2012, 140, 1725–1737. [Google Scholar] [CrossRef]
- Le Bouquin, S.; Allain, V.; Rouxel, S.; Petetin, I.; Picherot, M.; Michel, V.; Chemaly, M. Prevalence and risk factors for Salmonella spp. contamination in French broiler-chicken flocks at the end of the rearing period. Prev. Vet. Med. 2010, 97, 245–251. [Google Scholar] [CrossRef]
- Mamber, S.W.; Mohr, T.; Leathers, C.; Mbandi, E.; Bronstein, P.; Barlow, K. Occurrence of Salmonella in Ready-to-Eat Meat and Poultry Product Samples from U.S. Department of Agriculture–Regulated Producing Establishments. I. Results from the ALLRTE and RTE001 Random and Risk-Based Sampling Projects, from 2005 to 2012. J. Food Prot. 2018, 81, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.S.; Urdahl, A.M.; Nesse, L.L.; Slettemeås, J.S.; Ramstad, S.N.; Torp, M.; Norström, M. Occurrence of and risk factors for extended-spectrum cephalosporin-resistant Enterobacteriaceae determined by sampling of all Norwegian broiler flocks during a six month period. PLoS ONE 2019, 14, e0223074. [Google Scholar] [CrossRef] [PubMed]
- Mulders, M.N.; Haenen, A.P.J.; Geenen, P.L.; Vesseur, P.C.; Poldervaart, E.S.; Bosch, T.; Huijsdens, X.W.; Hengeveld, P.D.; Dam-Deisz, W.D.C.; Graat, E.A.M.; et al. Prevalence of livestock-associated MRSA in broiler flocks and risk factors for slaughterhouse personnel in The Netherlands. Epidemiol. Infect. 2010, 138, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Osimani, A.; Aquilanti, L.; Pasquini, M.; Clementi, F. Prevalence and risk factors for thermotolerant species of Campylobacter in poultry meat at retail in Europe. Poult. Sci. 2017, 96, 3382–3391. [Google Scholar] [CrossRef]
- Powell, L.F.; Lawes, J.R.; Clifton-Hadley, F.A.; Rodgers, J.; Harris, K.; Evans, S.J.; Vidal, A. The prevalence of Campylobacter spp. in broiler flocks and on broiler carcases, and the risks associated with highly contaminated carcases. Epidemiol. Infect. 2012, 140, 2233–2246. [Google Scholar] [CrossRef]
- Rajan, K.; Shi, Z.; Ricke, S.C. Current aspects of Salmonella contamination in the US poultry production chain and the potential application of risk strategies in understanding emerging hazards. Crit. Rev. Microbiol. 2017, 43, 370–392. [Google Scholar] [CrossRef]
- Rong, G.; Zhou, H.-L.; Hou, G.-Y.; Zhao, J.-M.; Xu, T.-S.; Guan, S. Seroprevalence, risk factors and genotyping of Toxoplasma gondii in domestic geese (Anser domestica) in tropical China. Parasit. Vectors 2014, 7, 459. [Google Scholar] [CrossRef]
- Sevilla-Navarro, S.; Marin, C.; Cortés, V.; García, C.; Catalá-Gregori, P. Campylobacter prevalence and risk factors associated with exceeding allowable limits in poultry slaughterhouses in Spain. Vet. Rec. 2020, 186, vetrec–2019–105558. [Google Scholar] [CrossRef]
- Torralbo, A.; Borge, C.; Allepuz, A.; García-Bocanegra, I.; Sheppard, S.K.; Perea, A.; Carbonero, A. Prevalence and risk factors of Campylobacter infection in broiler flocks from southern Spain. Prev. Vet. Med. 2014, 114, 106–113. [Google Scholar] [CrossRef]
- Aliyu, A.B.; Saleha, A.A.; Jalila, A.; Zunita, Z. Risk factors and spatial distribution of extended spectrum β-lactamase-producing- Escherichia coli at retail poultry meat markets in Malaysia: A cross-sectional study. BMC Public Health 2016, 16, 699. [Google Scholar] [CrossRef]
- Aury, K.; Le Bouquin, S.; Toquin, M.-T.; Huneau-Salaün, A.; Le Nôtre, Y.; Allain, V.; Petetin, I.; Fravalo, P.; Chemaly, M. Risk factors for Listeria monocytogenes contamination in French laying hens and broiler flocks. Prev. Vet. Med. 2011, 98, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Avrain, L.; Humbert, F.; L’Hospitalier, R.; Sanders, P.; Kempf, I. Antimicrobial resistance in Campylobacter from broilers. Br. Poult. Sci. 2001, 42, 32–43. [Google Scholar] [CrossRef]
- Barrios, P.R.; Reiersen, J.; Lowman, R.; Bisaillon, J.-R.; Michel, P.; Fridriksdóttir, V.; Gunnarsson, E.; Stern, N.; Berke, O.; McEwen, S.; et al. Risk factors for Campylobacter spp. colonization in broiler flocks in Iceland. Prev. Vet. Med. 2006, 74, 264–278. [Google Scholar] [CrossRef] [PubMed]
- Borck Høg, B.; Sommer, H.M.; Larsen, L.S.; Sørensen, A.I.V.; David, B.; Hofshagen, M.; Rosenquist, H. Farm specific risk factors for Campylobacter colonisation in Danish and Norwegian broilers. Prev. Vet. Med. 2016, 130, 137–145. [Google Scholar] [CrossRef]
- Bouwknegt, M.; van de Giessen, A.W.; Dam-Deisz, W.D.C.; Havelaar, A.H.; Nagelkerke, N.J.D.; Henken, A.M. Risk factors for the presence of Campylobacter spp. in Dutch broiler flocks. Prev. Vet. Med. 2004, 62, 35–49. [Google Scholar] [CrossRef]
- Bryan, F.L.; Doyle, M.P. Health Risks and Consequences of Salmonella and Campylobacter jejuni in Raw Poultry. J. Food Prot. 1995, 58, 326–344. [Google Scholar] [CrossRef]
- Cardinale, E.; Tall, F.; Guèye, E.F.; Cisse, M.; Salvat, G. Risk factors for Campylobacter spp. infection in Senegalese broiler-chicken flocks. Prev. Vet. Med. 2004, 64, 15–25. [Google Scholar] [CrossRef]
- Cardinale, E.; Tall, F.; Cissé, M.; Guèye, E.F.; Salvat, G.; Mead, G. Risk factors associated with Salmonella enterica subsp. enterica contamination of chicken carcases in Senegal. Br. Poult. Sci. 2005, 46, 293–299. [Google Scholar] [CrossRef]
- Cardinale, E.; Perrier Gros-Claude, J.D.; Tall, F.; Guèye, E.F.; Salvat, G. Risk factors for contamination of ready-to-eat street-vended poultry dishes in Dakar, Senegal. Int. J. Food Microbiol. 2005, 103, 157–165. [Google Scholar] [CrossRef]
- Carson, C.; Li, X.-Z.; Agunos, A.; Loest, D.; Chapman, B.; Finley, R.; Mehrotra, M.; Sherk, L.M.; Gaumond, R.; Irwin, R. Ceftiofur-resistant Salmonella enterica serovar Heidelberg of poultry origin—A risk profile using the Codex framework. Epidemiol. Infect. 2019, 147, e296. [Google Scholar] [CrossRef]
- Chaves, R.D.; Silva, A.R.; Alvarenga, V.O.; Pereira, J.L.; Mousavi Khaneghah, A. The modeling of time to enterotoxin detection of Staphylococcus aureus in chicken meat. J. Food Saf. 2017, 37, e12342. [Google Scholar] [CrossRef]
- Chowdhury, S.; Sandberg, M.; Themudo, G.E.; Ersbøll, A.K. Risk factors for Campylobacter infection in Danish broiler chickens. Poult. Sci. 2012, 91, 2701–2709. [Google Scholar] [CrossRef] [PubMed]
- Chutia, R. Fuzzy risk analysis using similarity measure of interval-valued fuzzy numbers and its application in poultry farming. Appl. Intell. 2018, 48, 3928–3949. [Google Scholar] [CrossRef]
- Cufaoglu, G.; Ayaz, N.D. Listeria monocytogenes risk associated with chicken at slaughter and biocontrol with three new bacteriophages. J. Food Saf. 2019, 39, e12621. [Google Scholar] [CrossRef]
- Ellis-Iversen, J.; Jorgensen, F.; Bull, S.; Powell, L.; Cook, A.J.; Humphrey, T.J. Risk factors for Campylobacter colonisation during rearing of broiler flocks in Great Britain. Prev. Vet. Med. 2009, 89, 178–184. [Google Scholar] [CrossRef]
- Featherstone, C.A.; Reichel, R.; Snow, L.C.; Davies, R.H.; Christiansen, K.H.; Carrique-Mas, J.J.; Evans, S.J. Investigation of risk factors for Salmonella on fattening-turkey farms. Epidemiol. Infect. 2010, 138, 1427–1438. [Google Scholar] [CrossRef]
- Guerin, M.T.; Martin, S.W.; Reiersen, J.; Berke, O.; McEwen, S.A.; Friðriksdóttir, V.; Bisaillon, J.-R.; Lowman, R. Temperature-related risk factors associated with the colonization of broiler-chicken flocks with Campylobacter spp. in Iceland, 2001–2004. Prev. Vet. Med. 2008, 86, 14–29. [Google Scholar] [CrossRef]
- Guerin, M.T.; Martin, W.; Reiersen, J.; Berke, O.; McEwen, S.A.; Bisaillon, J.-R.; Lowman, R. House-level risk factors associated with the colonization of broiler flocks with Campylobacter spp. in Iceland, 2001–2004. BMC Vet. Res. 2007, 3, 30. [Google Scholar] [CrossRef]
- Hansson, I.; Engvall, E.O.; Vågsholm, I.; Nyman, A. Risk factors associated with the presence of Campylobacter-positive broiler flocks in Sweden. Prev. Vet. Med. 2010, 96, 114–121. [Google Scholar] [CrossRef]
- Huneau-Salaün, A.; Denis, M.; Balaine, L.; Salvat, G. Risk factors for Campylobacter spp. colonization in French free-range broiler-chicken flocks at the end of the indoor rearing period. Prev. Vet. Med. 2007, 80, 34–48. [Google Scholar] [CrossRef]
- Kuana, S.; Santos, L.; Rodrigues, L.; Borsoi, A.; Moraes, H.; Salle, C.; Nascimento, V. Risk factors and likelihood of Campylobacter colonization in broiler flocks. Rev. Bras. Ciênc. Avícola 2007, 9, 201–204. [Google Scholar] [CrossRef]
- Lyngstad, T.M.; Jonsson, M.E.; Hofshagen, M.; Heier, B.T. Risk Factors Associated with the Presence of Campylobacter Species in Norwegian Broiler Flocks. Poult. Sci. 2008, 87, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Marin, C.; Hernandiz, A.; Lainez, M. Biofilm development capacity of Salmonella strains isolated in poultry risk factors and their resistance against disinfectants. Poult. Sci. 2009, 88, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Mataragas, M.; Skandamis, P.; Drosinos, E. Risk profiles of pork and poultry meat and risk ratings of various pathogen/product combinations. Int. J. Food Microbiol. 2008, 126, 1–12. [Google Scholar] [CrossRef] [PubMed]
- McDowell, S.W.J.; Menzies, F.D.; McBride, S.H.; Oza, A.N.; McKenna, J.P.; Gordon, A.W.; Neill, S.D. Campylobacter spp. in conventional broiler flocks in Northern Ireland: Epidemiology and risk factors. Prev. Vet. Med. 2008, 84, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Mezali, L.; Mebkhout, F.; Nouichi, S.; Boudjellaba, S.; Hamdi, T.-M. Serotype diversity and slaughterhouse-level risk factors related to Salmonella contamination on poultry carcasses in Algiers. J. Infect. Dev. Ctries. 2019, 13, 384–393. [Google Scholar] [CrossRef]
- Milanov, D.; Ljubojević, D.; Čabarkapa, I.; Karabasil, N.; Velhner, M. Biofilm as risk factor for Salmonella contamination in various stages of poultry production. Poult. Sci. 2017, 81. [Google Scholar] [CrossRef]
- Mo, S.S.; Kristoffersen, A.B.; Sunde, M.; Nødtvedt, A.; Norström, M. Risk factors for occurrence of cephalosporin-resistant Escherichia coli in Norwegian broiler flocks. Prev. Vet. Med. 2016, 130, 112–118. [Google Scholar] [CrossRef]
- Näther, G.; Alter, T.; Martin, A.; Ellerbroek, L. Analysis of risk factors for Campylobacter species infection in broiler flocks. Poult. Sci. 2009, 88, 1299–1305. [Google Scholar] [CrossRef]
- Pieskus, J.; Butrimaite-Ambrazeviciene, C.; Kazeniauskas, E.; Stanevicius, Z.; Mauricas, M. Risk factors for the presence of Campylobacter sp. in Lithuanian broiler flocks. Int. J. Poult. Sci. 2008, 7, 1242–1246. [Google Scholar] [CrossRef]
- Refrégier-Petton, J.; Rose, N.; Denis, M.; Salvat, G. Risk factors for Campylobacter spp. contamination in French broiler-chicken flocks at the end of the rearing period. Prev. Vet. Med. 2001, 50, 89–100. [Google Scholar] [CrossRef]
- Rivera-Pérez, W.; Barquero-Calvo, E.; Zamora-Sanabria, R. Salmonella Contamination Risk Points in Broiler Carcasses during Slaughter Line Processing. J. Food Prot. 2014, 77, 2031–2034. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.; Beaudeau, F.; Drouin, P.; Toux, J.Y.; Rose, V.; Colin, P. Risk factors for Salmonella enterica subsp. enterica contamination in French broiler-chicken flocks at the end of the rearing period. Prev. Vet. Med. 1999, 39, 265–277. [Google Scholar] [CrossRef]
- Rushton, S.P.; Humphrey, T.J.; Shirley, M.D.F.; Bull, S.; Jørgensen, F. Campylobacter in housed broiler chickens: A longitudinal study of risk factors. Epidemiol. Infect. 2009, 137, 1099–1110. [Google Scholar] [CrossRef] [PubMed]
- Sarr, A.; Galal, L.; Boumediene, F.; Hamidović, A.; Dardé, M.-L.; Diallo, M.; Sow, A.; Niang, Y.; Cuny, T.; Mercier, A. Seroprevalence and Risk Factors of Toxoplasma gondii Infection in Free-Range Chickens in Senegal, West Africa. Vector-Borne Zoonotic Dis. 2020, 20, 15–21. [Google Scholar] [CrossRef]
- Sasaki, Y.; Tsujiyama, Y.; Tanaka, H.; Yoshida, S.; Goshima, T.; Oshima, K.; Katayama, S.; Yamada, Y. Risk Factors for Campylobacter Colonization in Broiler Flocks in Japan: Campylobacter in Broiler Farms in Japan. Zoonoses Public Health 2011, 58, 350–356. [Google Scholar] [CrossRef]
- Schaumburg, F.; Alabi, A.S.; Frielinghaus, L.; Grobusch, M.P.; Köck, R.; Becker, K.; Issifou, S.; Kremsner, P.G.; Peters, G.; Mellmann, A. The risk to import ESBL-producing Enterobacteriaceae and Staphylococcus aureus through chicken meat trade in Gabon. BMC Microbiol. 2014, 14, 286. [Google Scholar] [CrossRef]
- Sommer, H.M.; Nauta, M.J.; Rosenquist, H. Translation of risk factor estimates into on-farm interventions and their effect on Campylobacter broiler flock prevalence. Microb. Risk Anal. 2016, 2, 27–37. [Google Scholar] [CrossRef]
- Sommer, H.M.; Høg, B.B.; Larsen, L.S.; Sørensen, A.I.V.; Williams, N.; Merga, J.Y.; Cerdà-Cuéllar, M.; Urdaneta, S.; Dolz, R.; Wieczorek, K.; et al. Analysis of farm specific risk factors for Campylobacter colonization of broilers in six European countries. Microb. Risk Anal. 2016, 2, 16–26. [Google Scholar] [CrossRef]
- Munther, D.; Sun, X.; Xiao, Y.; Tang, S.; Shimozako, H.; Wu, J.; Smith, B.A.; Fazil, A. Modeling cross-contamination during poultry processing: Dynamics in the chiller tank. Food Control 2016, 59, 271–281. [Google Scholar] [CrossRef]
- Possas, A.M.M.; Posada-Izquierdo, G.D.; Pérez-Rodríguez, F.; García-Gimeno, R.M. Modeling the Transfer of Salmonella Enteritidis during Slicing of Ready-to-Eat Turkey Products Treated with Thyme Essential Oil. J. Food Sci. 2016, 81, M2770–M2775. [Google Scholar] [CrossRef] [PubMed]
- Cumhur, Ö.; Şeker, M.; Sadıkoğlu, H. Freeze drying of turkey breast meat: Mathematical modeling and estimation of transport parameters. Dry. Technol. 2016, 34, 584–594. [Google Scholar] [CrossRef]
- Rabeler, F.; Feyissa, A.H. Modelling of food processes under uncertainty: Mechanistic 3D model of chicken meat roasting. J. Food Eng. 2019, 262, 49–59. [Google Scholar] [CrossRef]
- Horne, P.L.M.; van Bondt, N. Competitiveness of the EU Poultry Meat Sector; The Hague: Wageningen, The Netherlands, 2013; ISBN 978-90-8615-664-1. [Google Scholar]
- de Swarte, C.; Donker, R.A. Towards an FSO/ALOP based food safety policy. Food Control 2005, 16, 825–830. [Google Scholar] [CrossRef]
- Worsfold, D.; Griffith, C.J. Assessment of the Standard of Consumer Food Safety Behavior. J. Food Prot. 1997, 60, 399–406. [Google Scholar] [CrossRef]
- Williamson, D.M.; Gravani, R.B.; Lawless, H.T. Correlating food safety knowledge with home food-preparation practices. Food Technol. USA 1992, 46, 94–100. [Google Scholar]
- Yoon, K.S.; Burnette, C.N.; Oscar, T.P. Development of Predictive Models for the Survival of Campylobacter jejuni (ATCC 43051) on Cooked Chicken Breast Patties and in Broth as a Function of Temperature. J. Food Prot. 2004, 67, 64–70. [Google Scholar] [CrossRef]
- Solow, B.T.; Cloak, O.M.; Fratamico, P.M. Effect of Temperature on Viability of Campylobacter jejuni and Campylobacter coli on Raw Chicken or Pork Skin. J. Food Prot. 2003, 66, 2023–2031. [Google Scholar] [CrossRef]
- Chan, K.F.; Tran, H.L.; Kanenaka, R.Y.; Kathariou, S. Survival of Clinical and Poultry-Derived Isolates of Campylobacter jejuni at a Low Temperature (4 °C). Appl. Environ. Microbiol. 2001, 67, 4186–4191. [Google Scholar] [CrossRef]
- Bhaduri, S.; Cottrell, B. Survival of Cold-Stressed Campylobacter jejuni on Ground Chicken and Chicken Skin during Frozen Storage. Appl. Environ. Microbiol. 2004, 70, 7103–7109. [Google Scholar] [CrossRef]
- Hänninen, M.L. Survival of Campylobacter jejuni/coli in ground refrigerated and in ground frozen beef liver and in frozen broiler carcasses. Acta Vet. Scand. 1981, 22, 566–577. [Google Scholar] [PubMed]
- Birk, T.; Rosenquist, H.; Brøndsted, L.; Ingmer, H.; Bysted, A.; Christensen, B.B. A Comparative Study of Two Food Model Systems to Test the Survival of Campylobacter jejuni at −18 °C. J. Food Prot. 2006, 69, 2635–2639. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, M.; Hofshagen, M.; Østensvik, Ø.; Skjerve, E.; Innocent, G. Survival of Campylobacter on Frozen Broiler Carcasses as a Function of Time. J. Food Prot. 2005, 68, 1600–1605. [Google Scholar] [CrossRef]
- Georgsson, F.; Þorkelsson, Á.E.; Geirsdóttir, M.; Reiersen, J.; Stern, N.J. The influence of freezing and duration of storage on Campylobacter and indicator bacteria in broiler carcasses. Food Microbiol. 2006, 23, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.; Duncan, L.; Berrang, M.; Marcy, J.; Wolfe, R. Thermal inactivation D- and Z-values of Salmonella and Listeria innocua in fully cooked and vacuum packaged chicken breast meat during postcook heat treatment. Poult. Sci. 2002, 81, 1578–1583. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations/World Health Organization. Risk Assessments of “Salmonella” in Eggs and Broiler Chickens; World Health Organization Food and Agriculture Organization of the United Nations: Geneva, Switzerland, 2002; ISBN 978-92-9156-229-9. [Google Scholar]
- The International Commission on Microbiological Specifications for Foods. Microorganisms in Foods 5: Characteristics of Microbial Pathogens; Springer Science & Business Media: Berlin, Germany, 1996; ISBN 978-0-412-47350-0. [Google Scholar]
- Joint FAO/WHO. Expert Consultation on Risk Assessment of Microbiological Hazards in Foods W. H. O. Food Safety Programme Food, Agriculture Organization of the United, Nations. Joint FAO/WHO Expert Consultation on Risk Assessment of Microbiological Hazards in Foods: Hazard Identification, Exposure Assessment and Hazard Characterization of Campylobacter spp. in Broiler Chickens and Vibrio spp. in Seafood, WHO Headquarters, Geneva, Switzerland, 23–27 July 2001. World Health Organization. Available online: https://apps.who.int/iris/handle/10665/67090 (accessed on 2 November 2020).
- Joint FAO/WHO Expert Meetings on Microbiological Risk Assessment (JEMRA) on Methodologies of Microbiological Risk Assessment FAO/WHO Public Consultation for Draft Guidance of Microbiological Risk Assessment for Food. Available online: https://www.who.int/news-room/articles-detail/public-consultation-for-draft-guidance-of-microbiological-risk-assessment-for-food (accessed on 2 November 2020).
- Henley, S.C. “Don’t Wash Your Chicken!” Results of an Interdisciplinary Approach to Reduce Incidence of Infectious Foodborne Diseases; Drexel University: Philadelphia, PA, USA, 2013. [Google Scholar]
- Bamgboje-Ayodele, A.; Ellis, L.; Turner, P. Developing a Framework for Understanding and Enhancing Consumers’ Safe Food Management Behaviors—A Literature Review. J. Agric. Food Inf. 2019, 20, 315–343. [Google Scholar] [CrossRef]
- Young, I.; Waddell, L.; Harding, S.; Greig, J.; Mascarenhas, M.; Sivaramalingam, B.; Pham, M.T.; Papadopoulos, A. A systematic review and meta-analysis of the effectiveness of food safety education interventions for consumers in developed countries. BMC Public Health 2015, 15. [Google Scholar] [CrossRef]
- Lee-Kwan, S.H.; DeLuca, N.; Bunnell, R.; Clayton, H.B.; Turay, A.S.; Mansaray, Y. Facilitators and Barriers to Community Acceptance of Safe, Dignified Medical Burials in the Context of an Ebola Epidemic, Sierra Leone, 2014. J. Health Commun. 2017, 22, 24–30. [Google Scholar] [CrossRef]
- Szaszi, B.; Palinkas, A.; Palfi, B.; Szollosi, A.; Aczel, B. A Systematic Scoping Review of the Choice Architecture Movement: Toward Understanding When and Why Nudges Work. J. Behav. Decis. Mak. 2018, 31, 355–366. [Google Scholar] [CrossRef]


| Pathogen | Product | Location Date | Population | Objective | Ref |
|---|---|---|---|---|---|
| Salmonella spp. | Broiler | Canada 2012 | All | Meta-analysis for evaluation of farm-to-processing interventions | [13] |
| Canada 2020 | All | Risk assessment, evaluation of mitigation strategies | [14] | ||
| Finland 2005 | All | Effect of two interventions of Finnish control program | [18] | ||
| China 2017 | All | Risk assessment of home-prepared chicken meals | [19] | ||
| Chicken | USA 1998 | All | Determination of Salmonella spp. contamination levels on chickens at processing plant exit and risks for consumers | [15] | |
| USA 2004 | All | Risk assessment based on updated model from [15] | [16] | ||
| Chicken meat | USA 2017 | All | Risk assessment following flow pack wrapping of whole chicken and temperature abuse | [17] | |
| Canada 2013 | All | Risk assessment of home-prepared chicken meals | [20] | ||
| The Netherlands 2007 | All | Risk assessment, impact of contamination level at retail | [21] | ||
| Meat preparation 1 | France 2003 | All | Risk assessment at catering establishments level | [22] | |
| South Korea 2018 | All | Risk assessment, impact of contamination concentration at retail | [23] | ||
| USA 2019 | All | Risk assessment for developing a risk management framework | [24] | ||
| USA 2019 | All | Process risk model for ground chicken, partly based on [17] | [25] | ||
| Thermotolerant Campylobacter spp. | Broiler | UK 2001 | All | Risk assessment for broilers at point of slaughter | [26] |
| UK 2017 | All | Assessment of mitigation interventions at farm level | [27] | ||
| EU 2011 | All | Impact of mitigation interventions at primary production and slaughter | [28] | ||
| EU 2013 | All | Impact of farm to fork interventions on human campylobacteriosis incidence | [29] | ||
| USA 2019 | All | Assessment of processing interventions | [30] | ||
| The Netherlands 2005 | All | Risk assessment, poultry processing model basis | [31] | ||
| Sweden 2008 | All | Evaluation of mitigation strategies and frequency of cross contamination due to consumer mishandling | [37] | ||
| United Nations 2009 | All | Risk assessment based on extensive review of knowledge | [40,41] | ||
| Nordic countries 2013 | Young, adult, males | Establishment of risk-based microbiological criteria | [42] | ||
| France 2018 | All | Risk - benefit assessment of mitigation interventions | [8] | ||
| Chicken | Denmark 2003 | All | Risk assessment, impact of mitigation strategies | [39] | |
| China 2013 | All | Prevalence estimation and risk assessment | [36] | ||
| Poultry | Thailand 2011 | All | Exposure assessment and processing risk factors identification | [38] | |
| China 2018 | All | Risk assessment based on poultry-processing model | [35] | ||
| Chicken meat | Denmark 2012 | All | Evaluation of control strategy for imported, meat | [43] | |
| Denmark 2013 | All | Risks associated with thermotolerant Campylobacter, based on [49] | [44] | ||
| Denmark 2013 | All | Risk assessment for individual batches of fresh poultry meat | [45] | ||
| Netherland 2007 | All | Tool for mitigation measures assessment | [32,33,34] | ||
| Germany 2008 | All | Risk assessment of frozen/fresh chicken legs and breasts, for household consumption | [46] | ||
| Italy 2008 | All | Risk assessment of human campylobacteriosis due to cross contamination | [47] | ||
| Argentina 2013 | All | Risk assessment for cross-contaminated salad | [48] | ||
| EU 2012 | All | Impact of microbial criteria at the end of industrial processing | [49] | ||
| Meat preparation 1 | Belgium 2006 | All | Support for definition of risk-based microbial criteria | [50] | |
| Campylobacter spp. and Salmonella spp. | Chicken meat | Senegal 2012 | All | Risk assessment considering from market to consumption | [51] |
| Listeria monocytogenes | Poultry | Chile 2015 | All | Risk assessment for both poultry and beef meat | [52] |
| Broiler legs | Finland 2008 | All | Plant-level risk assessment | [53] | |
| Clostridium perfringens | Meat preparation 1 | USA 2009 | All | Effect of maximum allowed growth during stabilization of ready-to-eat foods | [54] |
| Pathogen | Reference | Farm (12) | Slaughter (20) | Retail (25) | Consumer (33) |
|---|---|---|---|---|---|
| Salmonella spp. | Bucher et al. 2012 [13] | ✓ | ✓ | ||
| Collineau et al. 2020 [14] | ✓ | ✓ | ✓ | ✓ | |
| Oscar 1998 [15] | ✓ | ✓ | ✓ | ||
| Oscar 2004 [16] | ✓ | ✓ | |||
| Oscar 2017 [17] | ✓ | ✓ | |||
| Maijala et al. 2005 [18] | ✓ | ✓ | ✓ | ✓ | |
| Zhu et al. 2017 [19] | ✓ | ✓ | |||
| Smadi & Sargeant 2013 [20] | ✓ | ✓ | |||
| Straver et al. 2007 [21] | ✓ | ✓ | |||
| Bemrah et al. 2003 [22] | ✓ | ✓ | |||
| Jeong et al. 2018 [23] | ✓ | ✓ | |||
| Sampedro et al. 2019 [24] | ✓ | ||||
| Oscar et al. 2019 [25] | ✓ | ||||
| Thermotolerant Campylobacter spp. | Hartnett et al. 2001 [26] | ✓ | ✓ | ||
| Crotta et al. 2017 [27] | ✓ | ✓ | |||
| EFSA 2011 [28] | ✓ | ✓ | ✓ | ✓ | |
| Romero-Barrios et al. 2013 [29] | ✓ | ✓ | ✓ | ||
| Dogan et al. 2019 [30] | ✓ | ✓ | ✓ | ✓ | |
| Nauta et al. 2005 [31] | ✓ | ||||
| Katsma et al.; Havelaar et al.; Nauta et al. 2007 [32,33,34] | ✓ | ✓ | ✓ | ✓ | |
| Huang et al. 2018 [35] | ✓ | ||||
| Lindqvist and Lindblad 2008 [37] | ✓ | ✓ | ✓ | ||
| Osiriphun et al. 2011 [38] | ✓ | ||||
| Rosenquist et al. 2003 [39] | ✓ | ✓ | ✓ | ||
| Wang, Guo & Li, 2013 [36] | ✓ | ✓ | ✓ | ||
| FAO, WHO 2009 [40,41] | ✓ | ✓ | ✓ | ||
| Nauta et al. 2013 [42] | ✓ | ||||
| Anses 2018 [8] | ✓ | ✓ | ✓ | ✓ | |
| Boysen 2012 [43] | ✓ | ✓ | |||
| Boysen et al. 2013 [44] | ✓ | ||||
| Christensen et al. 2013 [45] | ✓ | ✓ | |||
| Brynestad et al. 2008 [46] | ✓ | ✓ | |||
| Calistri & Giovanini 2008 [47] | ✓ | ||||
| Signorini et al. 2013 [48] | ✓ | ✓ | ✓ | ||
| Nauta et al. 2012 [49] | ✓ | ||||
| Uyttendaele et al. 2006 [50] | ✓ | ✓ | |||
| Salmonella spp. and Campylobacter spp. | Pouillot et al. 2012 [51] | ✓ | ✓ | ||
| Listeria monocytogenes | Foerster et al. (2015) [52] | ✓ | ✓ | ||
| Aarnisalo et al. (2008) [53] | ✓ | ✓ | |||
| Clostridium perfringens | Golden et al. (2009) [54] | ✓ | ✓ |
| Step | Risk Factors | Risk Mitigation Measure | References |
|---|---|---|---|
| Storage | Temperature abuses | Respect of temperatures <4 °C | [14,16,17,19,21,48,50,51] |
| Monitoring | [48] | ||
| Survival of thermotolerant Campylobacter spp. in fridge | Freeze (−20 °C, ≥24 h) | [32,37,43,44,46,48] | |
| Cross-contamination | Change/wash utensils and wash hands between preparations | [8,19,20,46,48,50,51,166] | |
| Consumer education | [14,32] | ||
| Prepare raw meat after other ingredients | [48] | ||
| Cooking | Undercooking | Thaw frozen meat before cooking | [24] |
| Adapt cooking methods to product’s shape and size to facilitate heat transfer | [16,22,25] | ||
| Core temperature >70 °C | [14,16,18,20,22,23,24,25,40,41,46,51] | ||
| Consumer behavior | Storage after cooking | Store at heat (stove for example) | [22] |
| Cross-contamination | Change or wash utensils and hands between preparations | [8,16,17,20,30,32,39,40,41,45,46,47,48,50,51,166] | |
| Consumer’s education | [14] | ||
| Consumption of raw meat | Communication campaigns to limit | [23,32,50] | |
| Data gaps | National surveys on consuming behaviors | [39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalid, T.; Hdaifeh, A.; Federighi, M.; Cummins, E.; Boué, G.; Guillou, S.; Tesson, V. Review of Quantitative Microbial Risk Assessment in Poultry Meat: The Central Position of Consumer Behavior. Foods 2020, 9, 1661. https://doi.org/10.3390/foods9111661
Khalid T, Hdaifeh A, Federighi M, Cummins E, Boué G, Guillou S, Tesson V. Review of Quantitative Microbial Risk Assessment in Poultry Meat: The Central Position of Consumer Behavior. Foods. 2020; 9(11):1661. https://doi.org/10.3390/foods9111661
Chicago/Turabian StyleKhalid, Tahreem, Ammar Hdaifeh, Michel Federighi, Enda Cummins, Géraldine Boué, Sandrine Guillou, and Vincent Tesson. 2020. "Review of Quantitative Microbial Risk Assessment in Poultry Meat: The Central Position of Consumer Behavior" Foods 9, no. 11: 1661. https://doi.org/10.3390/foods9111661
APA StyleKhalid, T., Hdaifeh, A., Federighi, M., Cummins, E., Boué, G., Guillou, S., & Tesson, V. (2020). Review of Quantitative Microbial Risk Assessment in Poultry Meat: The Central Position of Consumer Behavior. Foods, 9(11), 1661. https://doi.org/10.3390/foods9111661








