3.1. DTD-GC-MS Condition Optimization
Heating temperature, heating time, desorption temperature and desorption time of the direct thermal desorption process were evaluated to achieve the best overall analytical conditions. No references related with wood and the volatile compounds that it could bring to spirits and wines determined by this method were found in bibliography and, therefore, it had to be optimized.
To optimize the direct thermal desorption conditions, we chose a sequential exploration of the response, which was carried out in two stages. In the first stage, a factorial design of 2
4 was chosen to analyze the influence of heating temperature, heating time, desorption temperature and desorption time using a mixture of all the wood types studied, as described in
Section 2.1, in order to consider all the compounds that could be present in the wood samples. In the second stage, a factorial design of 3
2 was chosen to optimize the heating temperature and heating time.
3.1.1. Screening by a 24 Factorial Design
The values corresponding to the low (−) and high (+) levels for each factor are shown in
Table 1. The design involved sixteen experiments in duplicate. Total area values and chromatographic peak number of each experiment evaluated in the 2
4 factorial design are shown in
Table 2. The data obtained for the heating temperature, heating time, desorption temperature and desorption time were evaluated by ANOVA at the 0.05 significance level (
Table 3).
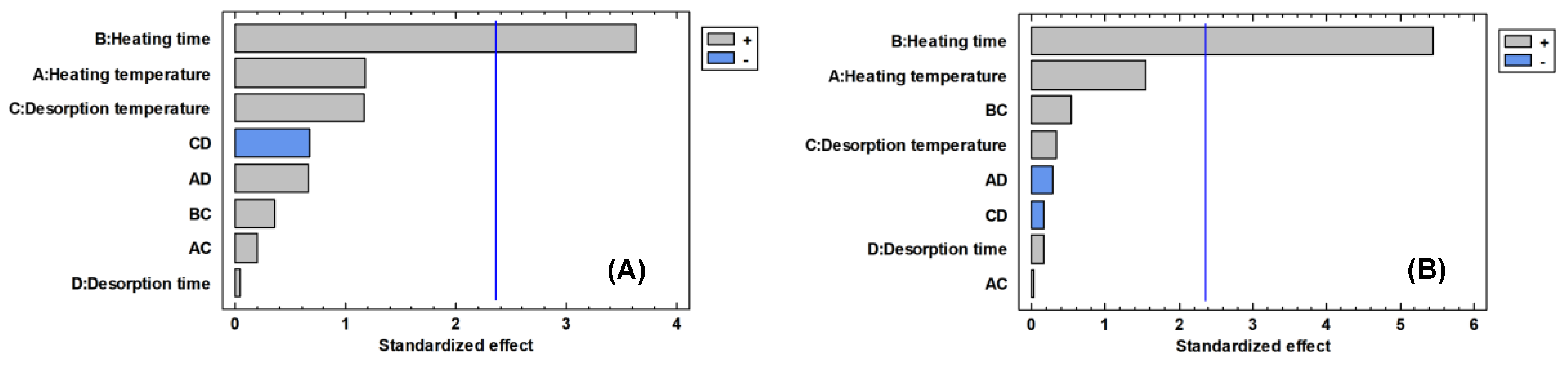
Parameters related with the heating process had a significant positive influence on the total area and the number of chromatographic peaks, appearing statistically as the most influential effect (
Figure 1). The effect of the parameters heating time and heating temperature is positive for the two variables considered, that is, high temperature levels and high heating time produce the extraction of larger amounts of volatile compounds (
Figure 2), being the heating time the only parameter that presents a significant effect (
p value < 0.05,
Table 3), both for the number of peaks and for the total area. The heating temperature is the next parameter that most affects the variables considered, although its effect is not significant (
p value > 0.05,
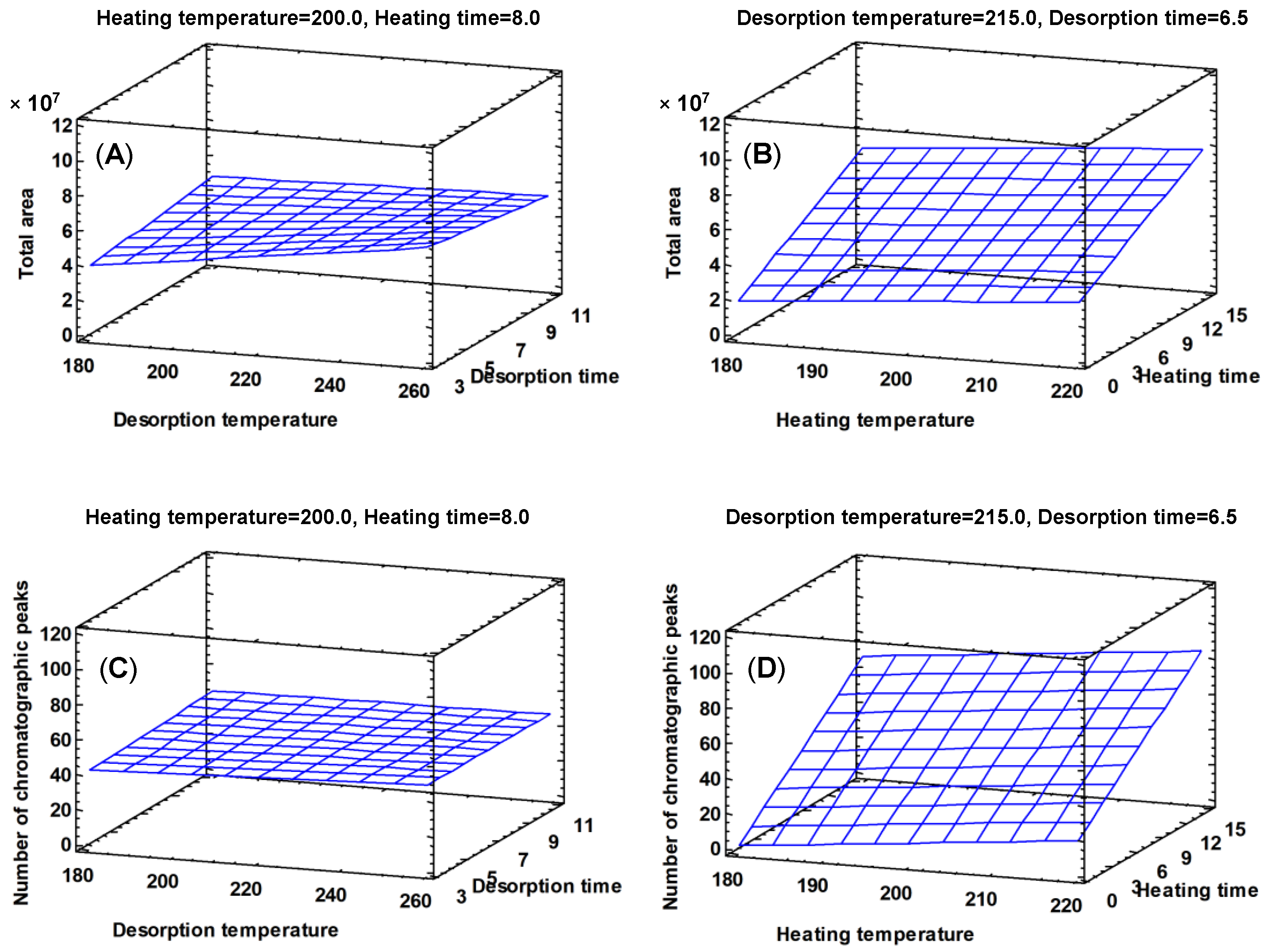
Table 3). As the heating time and the heating temperature increase (15 min and 220 °C, respectively), the response obtained in both variables is greater (
Figure 2).
The parameters related with the desorption process, temperature desorption and time desorption do not show a significant influence on the total area or the number of chromatographic peaks (
p value > 0.05,
Table 3). However, the desorption at 250 °C showed better results than the desorption at 180 °C, being selected as the optimal value for the following analysis (
Figure 2). No differences between the high and low level of the desorption time were found, so an average value (6 min) was selected.
Heating parameters turned out to be the most influential ones in the direct thermal desorption process. For these analysis, 20 mg of a mixture of the five woods was used. Some chromatographic peaks were saturated and so, for the optimized method experiments, the sample amount employed was lower than before in the following factorial design.
In summary, the best conditions obtained in this first optimization study were the following: heating time, 15 min; heating temperature, 220 °C; desorption time, 6 min; and desorption temperature, 250 °C.
3.1.2. Optimization by a 32 Factorial Design
In order to optimize the parameters of the direct thermal desorption method, the most influent variables resulting from the first factorial design were studied. Three levels of heating temperature and heating time were established. The design involved nine experiments in duplicate. The values corresponding to the low (−) and high (+) levels for each factor are shown in
Table 4.
After the results obtained in the 2
4 factorial design experiments, the desorption conditions established for the analysis were the following: 6 min and 250 °C. A total of 10 mg of the mixture sample was used in the study. Total area values and chromatographic peak number of each experiment evaluated in the 3
2 factorial design were shown in
Table 5. The data obtained for the heating temperature and the heating time were evaluated by ANOVA at the 5% significance level (
Table 6).
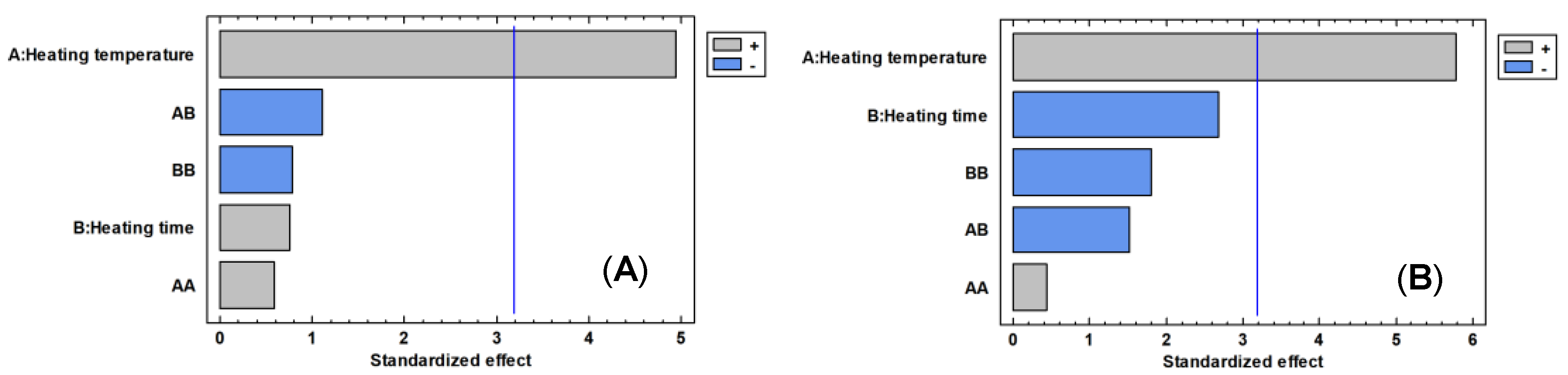
Heating temperature had a significant positive influence on the total area and the number of chromatographic peaks, appearing as the statistically main effect (
Figure 3). The effect of heating time and heating temperature is positive for the two variables considered, that is, high temperature levels produce the extraction of larger amounts of volatile compounds as the heating time value is between 5 and 10 min (
Figure 4). The heating temperature is the only parameter that presents a significant effect (
p value < 0.05,
Table 6), both for the number of peaks and for the total area. The heating temperature does not significantly affect the total area or the number of chromatographic peaks (
p value > 0.05,
Table 6). However, the heating time range from 5 to 10 min showed the best results, and so, an average value (7 min) was selected as the optimum value.
Taking into account all the results obtained, the final direct thermal desorption conditions considered to be optimal were as follows: heating 10 mg of the sample at 250 °C during 7 min and desorbing the sample at 250 °C during 6 min.
3.2. Analysis of Volatile Compounds of Wood Powder by DTD-GC-MS
Five different types of wood (American oak, Spanish oak, French oak, Chestnut and Cherry) were analyzed (in duplicate) employing the DTD-GC-MS method optimized. For this analysis, the wood chips were grounded to a 0.25 µm grain size. All the factorial design experiments were carried out in a splitless mode. High peak densities were obtained in all of them. In order to avoid detector saturation during the analysis of the real samples, they were injected in split mode. Different split ratios were tested 1:30, 1:20, 1:10 and 1:5. The split ratio 1:10 showed the best results.
The amount of the volatile compounds detected in each type of wood has been obtained by means of the relative integration with respect to the internal standard, 4-methyl-2-pentanol (
Table 7). The results were evaluated by ANOVA at the 5% significance level (
Table 7).
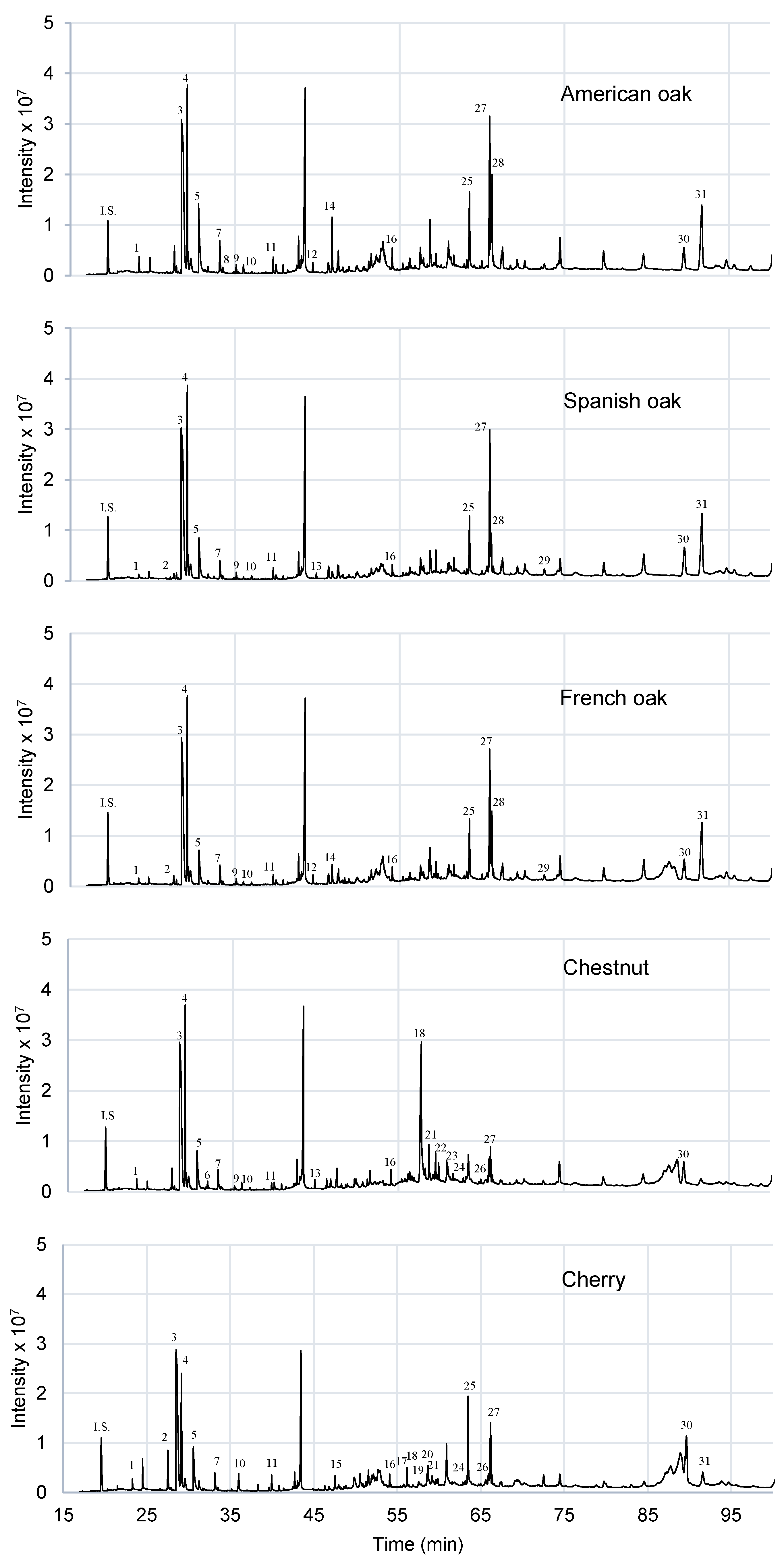
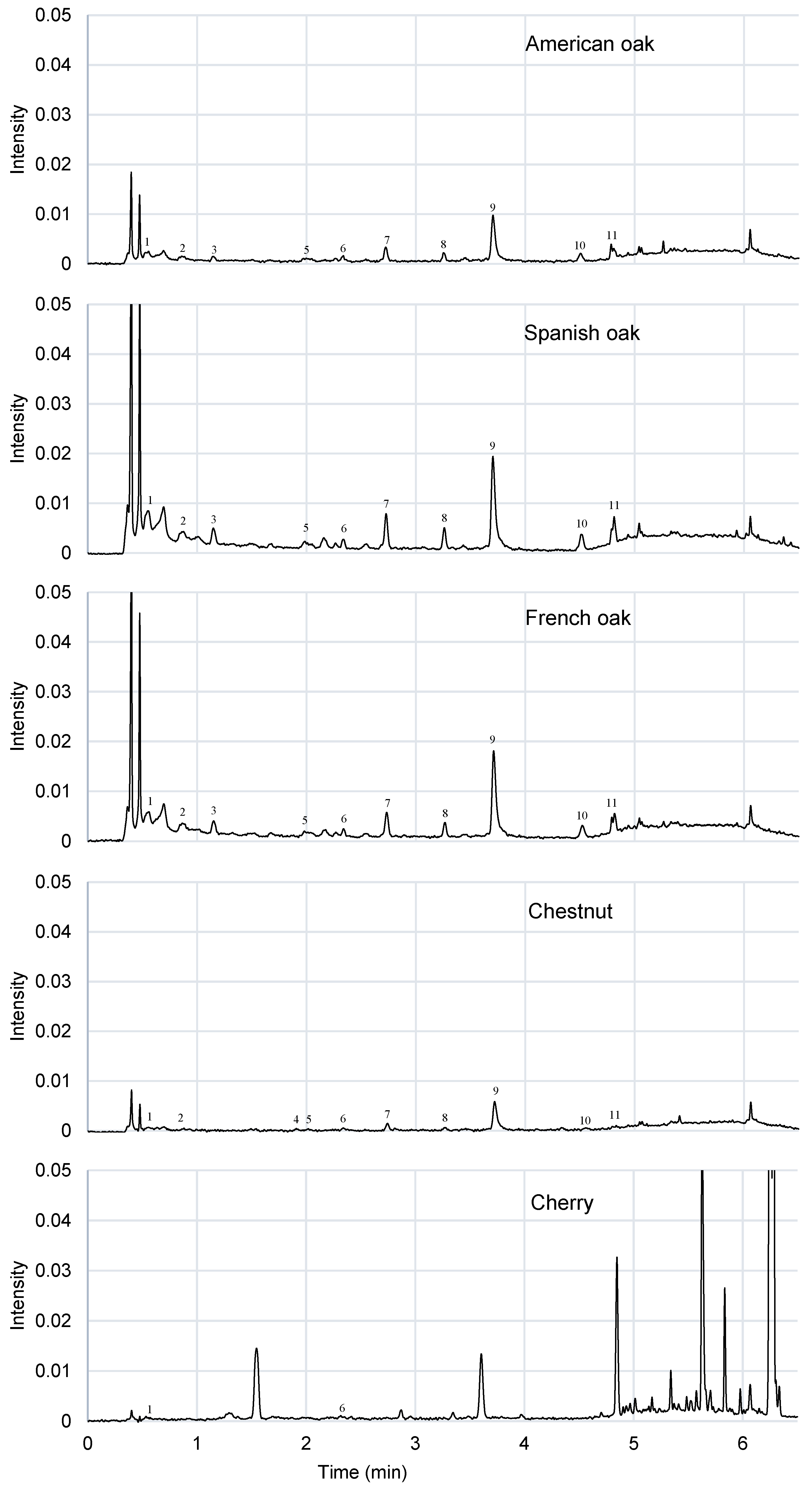
As expected, many similarities were found regarding the volatile composition of three oak woods studied. As can be seen in
Figure 5, their aroma profile is very similar. However, there are some differences, as the levels of formic acid, acrylic acid and furanone that are significantly different, at 5% of significance level, in American oak with respect to Spanish and French oaks (
Table 7). There are significant differences between the amount of vanillin and syringaldehyde between American and French oak, but there are none as compared to Spanish oak (
Table 7). These compounds could be affected by the toasting level of the chip wood. All the studied oaks have a medium toasting level. Acetic acid, furfural, formic acid, 5-methylfurfural, vanillin and syringaldehyde are the most abundant components [
20,
23,
26,
27]. Many of them are generated during the heat treatment processes during the toasting of the chips. There are compounds as whiskey lactones that are only identified in American and French oaks. According to the literature, these compounds are commonly present in oak wood, being more abundant in the
Quercus alba species [
20,
22]. 2-phenylethanol is only detected in Spanish oak and chestnut. 4-cyclopentene-1,3-dione is only detected in American oak. Myristic acid is detected in French and Spanish oak [
5] but it is not detected in American oak.
In
Figure 5, chestnut and cherry show a slightly different aromatic profile both between them and with respect to the oak types studied. Most of the detected compounds were in significant different amounts with respect the other woods studied (
Table 7). However, they contain many compounds present in oak wood too, like acetic acid, furfural, formic acid, 5-methylfurfural and palmitic acid [
20,
22,
26], and their content is not significantly different among woods at 5% significance level (
Table 7), except for formic acid content. It should be noted that the cherry and chestnut wood studied in both cases is an untoasted wood; however, they are also rich in compounds that come from the toasting of the wood, such as furfurals. This is due to the prior heating of the sample as part of the analysis method. The peak profile during the first 40 min of the analysis is very similar for all the wood chips studied. Cherry is the wood that has the most different volatile composition profile. 2,3-butanediol, glycerin and ethyl hydrogen succinate are only present in chestnut. This wood is similar to the studied oak woods, but also shares some similarities with cherry wood. Compounds as 2-phenylethanol or acrylic acid are detected in oak and chestnut wood, but are not present in cherry wood. On the other hand, compounds as trans-isoeugenol, 2,3-dihydrobenzofuran, benzoic acid and methoxyeugenol are present in chestnut and cherry wood but not in oak wood. According to the literature, methoxyeugenol is present in significant levels in cherry and chestnut wood, it could be present in oak wood too but, in this case it was not detected [
5,
28]. Cherry wood has the most particular aroma profile. Cyclopropyl carbinol, pyranone [
20], levulinic acid and p-acetylacetophenone are only detected in this wood. There are some compounds as 5-HMF, ethriol and syringaldehyde that are detected also in oak wood, but they are not in chestnut wood. Therefore, there are compounds or profiles that are characteristics of each type of wood studied, thus being able to be targets to identify each species in DTD-GC-MS analysis.
All the mentioned studies analyzed the volatile compounds through a previous hydroalcoholic extraction of the wood; the novelty of this work is the direct analysis of wood aromas by DTD-GC-MS. Although there are studies in oak wood that work with DTD-GC-MS (but using a solid support to trap the volatile compounds) [
29], in the rest of cited references, the woods have not been studied in this way. The previous direct thermal desorption stage could alter the sample, since heating could increase the toasting level of wood sample studied and increase the concentration of those compounds that are related to this process. However, there is not a loss of information, as could happen in extraction processes, since the aromatic profile is measured directly.
3.3. Analysis of Volatile Compounds of Wood Extracts by SBSE-GC-MS and GC-MS
An analysis on hydroalcoholic wood extracts was carried out to compare the compounds that are present in the wood detected by the analysis by DTD-GC-MS with those volatile compounds that could be transferred to the spirit or the wine during their ageing through the wood chips. In order to determine if they could be detected in aged alcoholic beverages, an hydroalcoholic ultrasound assisted extraction was carried out.
As regards the volatile composition of wood extracts, a low amount of the compounds was found in the samples. Relative area values of volatile compounds determined by SBSE-GC-MS of wood extracts are shown in
Table 8. The aromatic profile is different in all the studied wood but the amount of each detected compound is very low. In order to complement this information, the samples were analyzed by GC-MS. However, any compounds were not detected with this technique.
Attending to the results, shown in
Table 8, wood related compounds were not detected. As regards the relative area values of the compounds present in the extractant (1:1 hydroalcoholic mixture of rectified wine distillate at 96% vol. and water solution), similarities with the relative area values of wood extracts were found. The relative area values obtained were evaluated by ANOVA at the 5% significance level (
Table 8). As it can be seen, most of them are not significantly different, which means that the compounds detected are not influenced by wood. It seems that it only contributes to trace levels of them. According to the literature, there are fatty acids as caprylic acid, myristic acid or palmitic acid present in wood composition [
30,
31,
32]. The contribution of these compounds to the extracts and their respective esters could be due to the extraction procedure, in which the wood powder was extracted with an hydroalcoholic solution under 40 °C. The esterification of the fatty acids in the presence of ethanol at this temperature resulting in the corresponding esters as ethyl caprylate, ethyl myristate, ethyl palmitate or ethyl laureate could take place during the extraction process. This fact could explain the increase of the ethyl laureate and myristic acid in all the wood extracts studied, ethyl caprylate in chestnut and cherry wood extracts and ethyl palmitate in the American oak extract. The only compound detected in both analysis (DTD-GC-MS and SBSE-GC-MS) was myristic acid.
In the direct analysis of the wood powder by DTD-GC-MS, numerous volatile compounds were identified, which make it a very interesting technique. These compounds are transferred from the wood to the spirit or the wine during their ageing, modifying its sensorial profile. During ultrasound-assisted extractions, these compounds were extracted by the hydroalcoholic mixture used. However, once the hydroalcoholic extracts were analyzed, no volatile compounds were detected by GC-MS and very few compounds and at very low levels were detected by SBSE-GC-MS. Therefore, there is a loss of information regarding the analysis of volatile compounds once the ultrasound-assisted extraction is performed. However, the hydroalcoholic extracts were useful to characterize the phenolic compounds that wood could contribute to spirits and wines and to complete the aromatic profile of the woods studied. The majority of the compounds identified in SBSE-GC-MS analysis (
Table 8) are characteristic of wines, wine spirits or brandies, so their presence in the wood extracts studied could be also due to the origin of the extractant used, that has a part of a grape derived alcoholic beverage.
3.4. Phenolic Composition of the Wood Extracts and Total Polyphenol Index
The TPI data of the studied samples, expressed in mg of equivalent gallic acid (GAE) per litre, are shown in
Table 9. Of all the woods studied, Spanish oak released the highest amount of phenolic compounds into the alcoholic beverage. The lowest TPI values of oak were found in American oak wood. The TPI values for cherry wood (without toasting) are between medium toasted French and American wood. Chestnut (without toasting) has the lowest composition in phenolic compounds of all those studied. The results of the one-way analysis of the variance (ANOVA) proved that all the wood extracts are statistically different, with a probability of 95%.
The content in low molecular weight phenolic compounds determined by means of UHPLC in the wood extracts, expressed in mg L
−1, is also shown in
Table 9. As regards the phenolic acids studied, gallic acid, vanillic acid, caffeic acid, syringic acid and ellagic acid were found. Gallic and ellagic acids come from the hydrolysis of gallotannins and ellagitannins under an acidic environment [
28]. The oxidation and hydrolysis of the compounds derived from the degradation of lignin is the origin of vanillic and syringic acids [
16,
33]. A significant amount of phenolic aldehydes (p-hydroxybenzaldehyde, vanillin, syringaldehyde, coniferylaldehyde and sinapaldehyde) was found in some of the samples studied (
Table 9). Their origin is in the thermal degradation of lignin [
14,
16,
33], a process that takes place during the manufacturing of the barrel due to the toasting of the wood and its thermal treatments [
10]. 5-hydroxymethylfurfural and furfural have been detected in significant amounts in some of the samples studied (
Table 9). The presence of furfural is due to the heating of the pentoses, while 5-hydroxymethylfurfural has its origin in the thermal degradation of the glucose and cellulose. Their presence depends on the toasting of the wood [
10].
Phenolic composition is very similar in American, Spanish and French oak (
Figure 6). The same compounds were found in the hydroalcoholic extracts of all of them, however their proportion was not the same. Gallic acid, ellagic acid, vanillic acid, vanillin, syringaldehyde, coniferylaldehyde and sinapaldehyde are the most abundant compounds detected in the oak wood extracts. Spanish oak has the highest amount of phenolic compounds, while American oak has the lowest quantity. The amount of phenolic compounds present in each wood is influenced by the origin and the heat treatment of the wood during the manufacturing of the barrel or chips [
10]. During the ageing period, wood characteristics as porosity affect the extraction of phenolic and volatile compounds. Spanish and French oak are more porous than American oak, and this has a positive influence during the extraction process.
All the compounds detected in oak wood, except furfural, were also found in Chestnut extracts. This wood was untreated, without toasting treatment, so it explains the absence of this compound. However, due to its porosity, a great amount of phenolic compounds was found in the hydroalcoholic extracts studied. Chestnut wood has high levels of gallic acid [
34], in this case the wood studied was not toasted, so the level of this compound is lower than expected.
As regards the cherry wood chromatogram, a high level of phenolic compounds was detected. There are many signals at the end of the chromatogram indicating that, according to their retention time, these are low-polar compounds. According to the literature, they could be flavonoid-type compounds [
23] as (+)-catechin [
35], taxifolin [
36], naringenin [
27], aromadendrin [
37] or kaempferol [
37], that are very common in cherry wood. Flavonoids were only detected in cherry wood (
Figure 6), what makes its aromatic profile very interesting. It would be interesting to be able to identify these compounds in the future, because they make cherry wood an alternative material for the ageing of spirits and wines and to obtain different sensorial profiles from oak or chestnut. There are other compounds, as vanillin, vanillic acid, syringaldehyde, sinapaldehyde or coniferylaldehyde that come from lignin degradation and are also present in cherry wood [
23], but their presence is higher when the wood is toasted. In this sample, these compounds are at trace level, below the limit of quantification, and thus, they could not be quantified.
In summary, a similar profile has been observed in the three types of oak studied. Oak wood is rich in ellagitannins and low molecular weight acids and aldehydes. No flavonoids have been detected in any of them [
26]. Besides being from the same family, the three oak wood chips analyzed were toasted. During the toasting process, wood increases its concentration of compounds derived from lignin, and a different reduction between phenolic profiles was observed for the different woods [
26]. On the other hand, untoasted chestnut chip wood was studied. This wood is slightly similar to oak wood; it is also rich in ellagitannins and low molecular weight acids and aldehydes. In this wood, there is also an absence of flavonoids [
26]. However, as this wood was untoasted, it has a low concentration in compounds derived from lignin. As regards cherry wood, a phenolic profile totally different from the rest of the woods was observed. This wood is rich in flavonoid-type compounds and has a certain deficiency in ellagitannins and derivatives. It was also untoasted, so a low concentration of compounds derived from lignin was found. Cherry wood has a specific profile of low molecular weight compounds [
18,
23,
26,
38].
Furfural, hydroxymethylfurfural, vanillin and syringaldehyde were also detected in DTD-GC-MS analysis of wood powder. Furfural and hydroxymethylfurfural levels are higher in DTD-GC-MS experiments than in UHPLC analysis. In DTD-GC-MS, during the prior direct thermal desorption process there is a heating of the sample, reaching high temperatures that affect it, and these compounds are related with the toasting level of the wood. Vanillin and syringaldehyde are also found in a higher amount in DTD-GC-MS, except for chestnut wood. They are compounds that come from lignin thermal degradation, being also affected by high temperatures. During direct thermal desorption, wood is toasted, which produces an alteration of the initial sample that generates differences between the level of the compounds determined by both analyses.
The phenolic compound content determined by UHPLC was evaluated by ANOVA at the 5% significance level (
Table 9). As can be seen, most of them are significantly different, which means that the phenolic profile is characteristic of each wood. Although there are similarities in the presence of some compounds in the three oak woods studied or with chestnut wood, the proportion of them in each wood is different. Only a few similarities were found regarding syringic acid (no significant differences between American and French oak at the 5% significance level were found), vanillic acid (no significant differences between Spanish oak and chestnut at the 5% significance level were found) and hydroxymethylfurfural (no significant differences between Spanish and French oak at the 5% significance level were found).