Preliminary Study of Microelements, Phenolics as well as Antioxidant Activity in Local, Homemade Wines from North-East Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sample Collection
2.3. Determination of Heavy Metals and Trace Elements
2.4. Determination of Colour
2.5. Determination of Total Phenolic Content and Total Flavonoid Content
2.6. Determination of Antioxidant Activity
2.7. Statistical Analysis
3. Results
3.1. Metals in Organic Wine Samples
3.2. Colour of Wines
3.3. Total Phenolics and Flavonoids Content and Antioxidant Activity of Wines
3.4. Exploratory Data Analysis of Wines
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Lopez-Velez, M.; Martinez-Martinez, F.; Del Valle-Ribes, C. The study of phenolic compounds as natural antioxidants in wine. Crit. Rev. Food Sci. Nutr. 2003, 43, 233–244. [Google Scholar] [CrossRef] [PubMed]
- Lachman, J.; Šulc, M.; Faitová, K.; Pivec, V. Major factors influencing antioxidant contents and antioxidant activity in grapes and wines. Int. J. Wine Res. 2009, 1, 101–121. [Google Scholar] [CrossRef]
- Jackson, R.S. Chapter 12—Wine, food, and health. In Wine Science, 5th ed.; Jackson, R.S., Ed.; Academic Press: San Diego, CA, USA, 2020; pp. 947–978. [Google Scholar] [CrossRef]
- Díaz, C.; Conde, J.E.; Estévez, D.; Pérez Olivero, S.J.; Pérez Trujillo, J.P. Application of multivariate analysis and artificial neural networks for the differentiation of red wines from the Canary Islands according to the island of origin. J. Agric. Food. Chem. 2003, 51, 4303–4307. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P. What do metals tell us about wine? TracTrends Anal. Chem. 2007, 26, 941–949. [Google Scholar] [CrossRef]
- Tariba, B. Metals in Wine—Impact on Wine Quality and Health Outcomes. Biol. Trace. Elem. Res. 2011, 144, 143–156. [Google Scholar] [CrossRef]
- Galani-Nikolakaki, S.; Kallithrakas-Kontos, N.; Katsanos, A.A. Trace element analysis of Cretan wines and wine products. Sci. Total. Environ. 2002, 285, 155–163. [Google Scholar] [CrossRef]
- Kment, P.; Mihaljevič, M.; Ettler, V.; Šebek, O.; Strnad, L.; Rohlová, L. Differentiation of Czech wines using multielement composition—A comparison with vineyard soil. Food Chem. 2005, 91, 157–165. [Google Scholar] [CrossRef]
- Redan, B.W.; Jablonski, J.E.; Halverson, C.; Jaganathan, J.; Mabud, M.A.; Jackson, L.S. Factors Affecting Transfer of the Heavy Metals Arsenic, Lead, and Cadmium from Diatomaceous-Earth Filter Aids to Alcoholic Beverages during Laboratory-Scale Filtration. J. Agric. Food Chem. 2019, 67, 2670–2678. [Google Scholar] [CrossRef]
- Aguilar, M.V.; Martinez, M.C.; Masoud, T.A. Arsenic content in some Spanish wines Influence of the wine-making technique on arsenic content in musts and wines. Z. Lebensm. Unters. Forsch. 1987, 185, 185–187. [Google Scholar] [CrossRef]
- EC. Commission Regulation (EC) No.1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, L 364, 5–24. [Google Scholar]
- OIV. International code of oenological practices. International Organization of Vine and Wine 2020. Available online: http://www.oiv.int/public/medias/7213/oiv-international-code-of-oenological-practices-2020-en.pdf (accessed on 7 October 2020).
- Towle, K.M.; Garnick, L.C.; Monnot, A.D. A human health risk assessment of lead (Pb) ingestion among adult wine consumers. Int. J. Food Contam. 2017, 4, 7. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Catarino, S.; Madeira, M.; Monteiro, F.; Rocha, F.; Curvelo-Garcia, A.S.; de Sousa, R.B. Effect of Bentonite Characteristics on the Elemental Composition of Wine. J. Agric. Food. Chem. 2008, 56, 158–165. [Google Scholar] [CrossRef]
- Dordoni, R.; Galasi, R.; Colangelo, D.; De Faveri, D.M.; Lambri, M. Effects of fining with different bentonite labels and doses on colloidal stability and colour of a Valpolicella red wine. Int. J. Food Sci. Technol. 2015, 50, 2246–2254. [Google Scholar] [CrossRef]
- Hellenic Ministry of Rural Development and Food. Statistical data. Available online: http://www.minagric.gr/index.php/el/pinakas-3-dendrodeis-monimes-kalliergeies/file/ (accessed on 7 October 2020).
- Roussis, I.G.; Lambropoulos, I.; Tzimas, P.; Gkoulioti, A.; Marinos, V.; Tsoupeis, D.; Boutaris, I. Antioxidant activities of some Greek wines and wine phenolic extracts. J. Food Compos. Anal. 2008, 21, 614–621. [Google Scholar] [CrossRef]
- Drava, G.; Minganti, V. Mineral composition of organic and conventional white wines from Italy. Heliyon 2019, 5, e02464. [Google Scholar] [CrossRef]
- IUPAC. International Union of Pure and Applied Chemistry. Nomenclature in Evaluation of Analytical Methods Including Detection and Quantification Capabilities. Pure Appl. Chem. 1995, 67, 1699–1723. [Google Scholar] [CrossRef]
- Sudraud, P. Interpretation des courbes d’absorption du vins rouges. Ann. De Technol. Agric. 1958, 7, 203–208. [Google Scholar]
- Glories, Y. La couleur des vins rouges. Connaiss. Vigne Vin 1984, 18, 195–217. [Google Scholar]
- Simpson, R.F. Factors affecting oxidative browning of white wine. Vitis 1982, 21, 233–239. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology; John Wileys Sons, Ltd.: New York, NY, USA, 2006; Volume 2. [Google Scholar]
- Mayén, M.; Barón, R.; Mérida, J.; Medina, M. Changes in phenolic compounds during accelerated browning in white wines from cv. Pedro Ximenez and cv. Baladi grapes. Food Chem. 1997, 58, 89–95. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods Enzymol; Academic Press: New York, NY, USA, 1998; Volume 299, pp. 152–178. [Google Scholar]
- Bao, J.; Cai, Y.; Sun, M.; Wang, G.; Corke, H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese bayberry (Myrica rubra) extracts and their color properties and stability. J. Agric. Food. Chem. 2005, 53, 2327–2332. [Google Scholar] [CrossRef] [PubMed]
- Skendi, A.; Irakli, M.N.; Chatzopoulou, P. Analysis of phenolic compounds in Greek plants of Lamiaceae family by HPLC. J. Appl. Res. Med. Aromat. Plants 2017, 6, 62–69. [Google Scholar] [CrossRef]
- Larcher, R.; Nicolini, G. Elements and inorganic anions in winemaking: Analysis and applications. In Hyphenated Techniques in Grape and Wine Chemistry; Flamini, R., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2008; p. 292. [Google Scholar]
- Volpe, M.G.; La Cara, F.; Volpe, F.; De Mattia, A.; Serino, V.; Petitto, F.; Zavalloni, C.; Limone, F.; Pellecchia, R.; De Prisco, P.P.; et al. Heavy metal uptake in the enological food chain. Food Chem. 2009, 117, 553–560. [Google Scholar] [CrossRef]
- Ostapczuk, P.; Eschnauer, H.R.; Scollary, G.R. Determination of cadmium, lead and copper in wine by potentiometric stripping analysis. Fresenius’ J. Anal. Chem. 1997, 358, 723–727. [Google Scholar] [CrossRef]
- Papageorgiou, F.; Karampatea, K.; Mitropoulos, A.C.; Kyzas, G.Z. Determination of metals in Greek wines. Int. J. Environ. Sci. Technol. 2019, 16, 347–356. [Google Scholar] [CrossRef]
- Banović, M.; Kirin, J.; Ćurko, N.; Kovačević Ganić, K. Influence of vintage on Cu, Fe, Zn and Pb content in some Croatian red wines. Czech J. Food Sci. 2009, 27, S401–S403. [Google Scholar] [CrossRef]
- Mena, C.M.; Cabrera, C.; Lorenzo, M.L.; Lopez, M.C. Determination of Lead Contamination in Spanish Wines and Other Alcoholic Beverages by Flow Injection Atomic Absorption Spectrometry. J. Agric. Food. Chem. 1997, 45, 1812–1815. [Google Scholar] [CrossRef]
- Malavolti, M.; Fairweather-Tait, S.J.; Malagoli, C.; Vescovi, L.; Vinceti, M.; Filippini, T. Lead exposure in an Italian population: Food content, dietary intake and risk assessment. Food Res. Int. 2020, 137, 109370. [Google Scholar] [CrossRef]
- Lazos, E.S.; Alexakis, A. Metal ion content of some Greek wines. Int. J. Food Sci. Technol. 1989, 24, 39–46. [Google Scholar] [CrossRef]
- Teissedre, P.L.; Vique, C.C.; Cabanis, M.T.; Cabanis, J.C. Determination of Nickel in French Wines and Grapes. Am. J. Enol. Vitic. 1998, 49, 205. [Google Scholar]
- McRae, J.M.; Day, M.P.; Bindon, K.A.; Kassara, S.; Schmidt, S.A.; Schulkin, A.; Kolouchova, R.; Smith, P.A. Effect of early oxygen exposure on red wine colour and tannins. Tetrahedron 2015, 71, 3131–3137. [Google Scholar] [CrossRef]
- Bekker, M.Z.; Day, M.P.; Smith, P.A. Changes in Metal Ion Concentrations in a Chardonnay Wine Related to Oxygen Exposure during Vinification. Molecules 2019, 24, 1523. [Google Scholar] [CrossRef]
- Álvarez, M.; Moreno, I.M.; Jos, Á.; Cameán, A.M.; Gustavo González, A. Differentiation of ‘two Andalusian DO ‘fino’ wines according to their metal content from ICP-OES by using supervised pattern recognition methods. Microchem. J. 2007, 87, 72–76. [Google Scholar] [CrossRef]
- de Freitas, V.A.P.; Fernandes, A.; Oliveira, J.; Teixeira, N.; Mateus, N. A review of the current knowledge of red wine colour. Oeno One 2017, 51. [Google Scholar] [CrossRef]
- Iland, I.; Ewart, A.; Sitters, J.; Markides, A.; Bruer, N. Techniques for Chemical Analysis and Quality Monitoring During Winemaking; Patrick Iland Wine Promotions: Campbelltown, Australia, 2000. [Google Scholar]
- Somers, T.C.; Evans, M.E. Wine quality: Correlations with colour density and anthocyanin equilibria in a group of young red wines. J. Sci. Food Agric. 1974, 25, 1369–1379. [Google Scholar] [CrossRef]
- Somers, T.C.; Verette, E. Phenolic composition of natural wine types. In Modern Methods of Plant Analysis, Wine Analysis; Linskens, H.F., Jackson, J.F., Eds.; Springer: Berlin, German, 1988; Volume 6, pp. 219–257. [Google Scholar]
- Brouillard, R. Chapter 1. Chemical Structure of Anthocyanins. In Anthocyanins as Food Colors; Academic Press: New York, NY, USA, 1982; pp. 1–40. [Google Scholar]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Zurbano, P.; Ferreira, V.; Escudero, A.; Cacho, J. Role of Hydroxycinnamic Acids and Flavanols in the Oxidation and Browning of White Wines. J. Agric. Food. Chem. 1998, 46, 4937–4944. [Google Scholar] [CrossRef]
- Philippidis, A.; Poulakis, E.; Basalekou, M.; Strataridaki, A.; Kallithraka, S.; Velegrakis, M. Characterization of Greek Wines by Ultraviolet–Visible Absorption Spectroscopy and Statistical Multivariate Methods. Anal. Lett. 2017, 50, 1950–1963. [Google Scholar] [CrossRef]
- Burns, J.; Gardner, P.T.; O’Neil, J.; Crawford, S.; Morecroft, I.; McPhail, D.B.; Lister, C.; Matthews, D.; MacLean, M.R.; Lean, M.E.J.; et al. Relationship among Antioxidant Activity, Vasodilation Capacity, and Phenolic Content of Red Wines. J. Agric. Food. Chem. 2000, 48, 220–230. [Google Scholar] [CrossRef]
- Di Majo, D.; La Guardia, M.; Giammanco, S.; La Neve, L.; Giammanco, M. The antioxidant capacity of red wine in relationship with its polyphenolic constituents. Food Chem. 2008, 111, 45–49. [Google Scholar] [CrossRef]
- Hosu, A.; Cristea, V.-M.; Cimpoiu, C. Analysis of total phenolic, flavonoids, anthocyanins and tannins content in Romanian red wines: Prediction of antioxidant activities and classification of wines using artificial neural networks. Food Chem. 2014, 150, 113–118. [Google Scholar] [CrossRef] [PubMed]
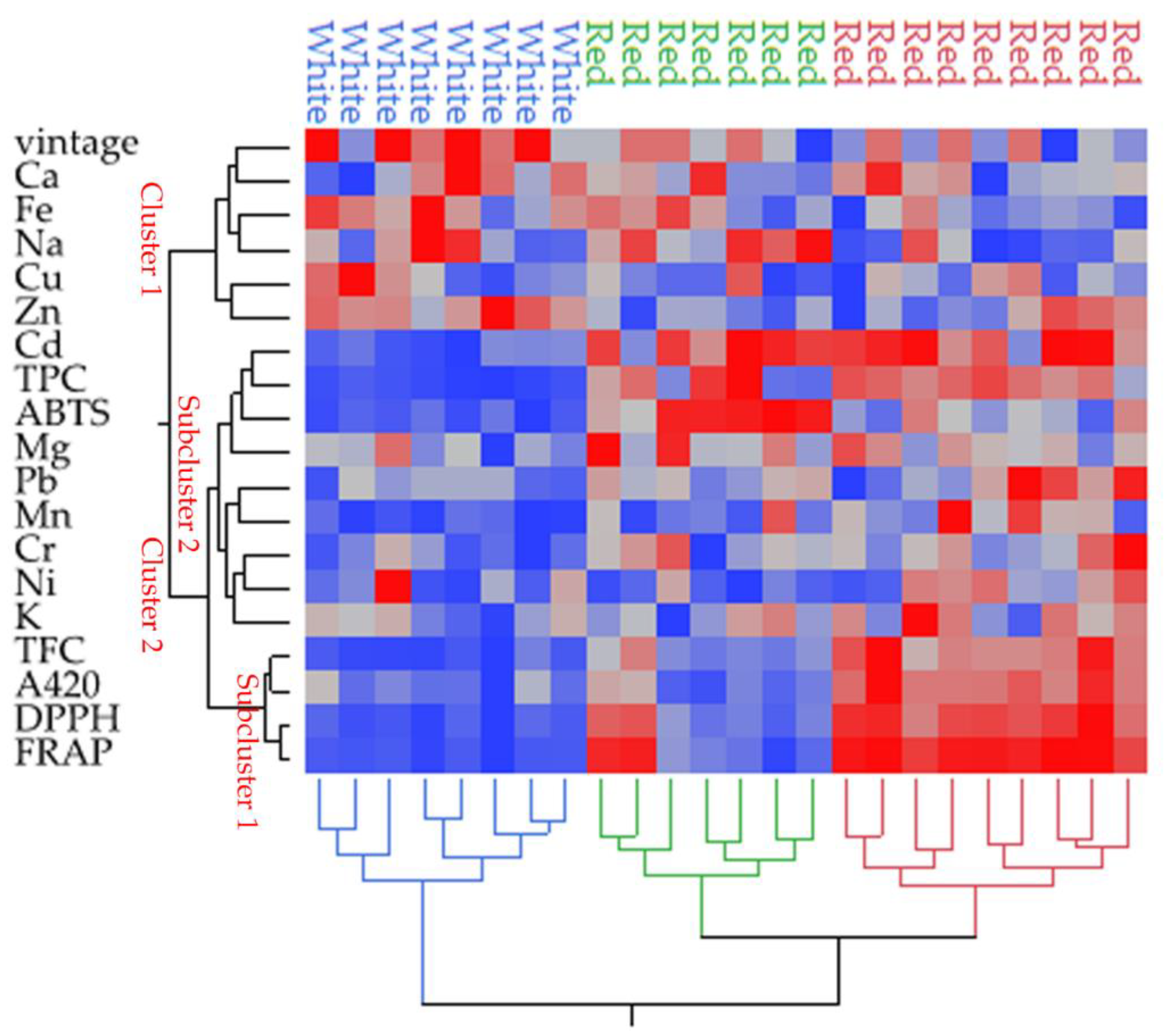
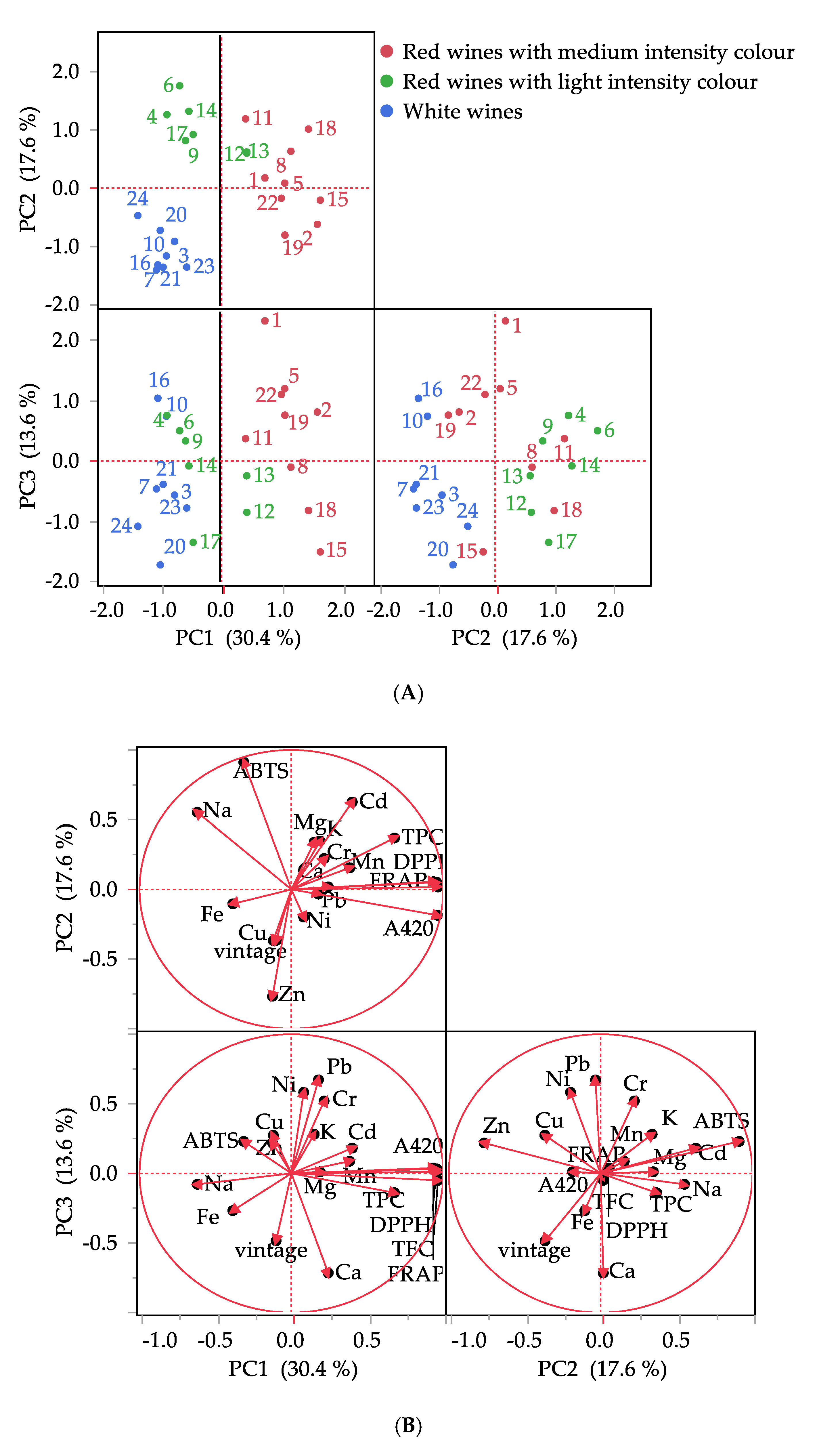
| Sample | Type of Wine | Grape Variety | Vintage |
|---|---|---|---|
| S1 | Red | Xinomavro/Merlot | 2013 |
| S2 | Red | Merlot | 2014 |
| S3 | White | Chardonnay/Muscat of Alexandria | 2014 |
| S4 | Red | Merlot | 2011 |
| S5 | Red | NR | 2011 |
| S6 | Red | Merlot/Syrah | 2014 |
| S7 | White | NR | 2015 |
| S8 | Red | NR | 2015 |
| S9 | Red | NR | 2015 |
| S10 | White | Malagousia | 2016 |
| S11 | Red | NR | 2013 |
| S12 | Red | NR | 2015 |
| S13 | Red | NR | 2014 |
| S14 | Red | NR | 2015 |
| S15 | Red | NR | 2015 |
| S16 | White | Assyrtiko | 2013 |
| S17 | Red | NR | 2014 |
| S18 | Red | NR | 2013 |
| S19 | Red | NR | 2015 |
| S20 | White | Malagousia/Roditis/Assyrtiko | 2016 |
| S21 | White | NR | 2016 |
| S22 | Red | Limnio/Cabernet Sauvignon | 2013 |
| S23 | White | NR | 2016 |
| S24 | White | NR | 2015 |
| Element | OIV (International Organization of Vine and Wine) Maximum Levels (mg/L) | Mean (mg/L) ± Std. Deviation | Median (mg/L) | Minimum (mg/L) | Maximum (mg/L) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Red | White | Red | White | Red | White | Red | White | ||
| As (Arsenic) | 0.2 | <LOD ± | <LOD ± | <LOD | <LOD | <LOD | <LOD | <LOD | |
| Cd (Cadmium) | 0.01 | 0.004 ± 0.001 | 0.002 ± 0.000 | 0.005 a | 0.002 b | 0.003 | 0.002 | 0.005 | 0.003 |
| Pb (Lead) | 0.1 | 0.036 ± 0.015 | 0.025 ± 0.006 | 0.035 a | 0.028 b | 0.013 | 0.016 | 0.067 | 0.032 |
| Cr (Chromium) | 0.1 | 0.007 ± 0.005 | 0.003 ± 0.002 | 0.006 a | 0.002 b | < LOD | < LOD | 0.020 | 0.007 |
| Cu (Copper) | 1 | 0.050 ± 0.039 | 0.076 ± 0.065 | 0.038 | 0.051 | 0.010 | 0.016 | 0.144 | 0.206 |
| Fe (Iron) | 20 | 0.804 ± 0.325 | 1.103 ± 0.366 | 0.766 | 1.096 | 0.309 | 0.510 | 1.439 | 1.672 |
| Mn (Manganese) | - | 1.474 ± 1.262 | 0.503 ± 0.130 | 0.986 a | 0.454 b | 0.434 | 0.372 | 4.667 | 0.673 |
| Ni (Nickel) | - | 0.157 ± 0.098 | 0.156 ± 0.141 | 0.118 | 0.104 | 0.055 | 0.061 | 0.357 | 0.486 |
| Zn (Zinc) | 5 | 0.612 ± 0.212 | 0.900 ± 0.162 | 0.629 b | 0.863 a | 0.304 | 0.654 | 1.031 | 1.210 |
| K (Potassium) | - | 79.6 ± 18.4 | 70.1 ± 11.5 | 77.6 | 72.7 | 50.3 | 51.4 | 123.2 | 81.5 |
| Na (Sodium) | 80 | 2.7 ± 1.5 | 2.9 ± 1.5 | 2.7 | 2.7 | 0.8 | 1.4 | 5.2 | 5.3 |
| Ca (Calcium) | - | 6.1 ± 1.2 | 6.3 ± 1.7 | 6.2 | 6.4 | 3.7 | 3.7 | 8.4 | 8.8 |
| Mg (Magnesium) | - | 9.9 ± 1.1 | 8.9 ± 1.1 | 9.8 | 9.2 | 8.2 | 6.8 | 12.1 | 10.7 |
| Mean ± Std. Deviation | Median | Minimum | Maximum | |
|---|---|---|---|---|
| Red wine | ||||
| Color Intensity | 5.270 ± 2.237 | 5.897 | 1.411 | 9.159 |
| Color Hue | 0.922 ± 0.164 | 0.896 | 0.655 | 1.176 |
| Brilliance (%) | 41.886 ± 9.088 | 43.266 | 26.047 | 56.196 |
| Proportion of yellow% | 42.32 ± 4.20 | 42.28 | 34.54 | 47.95 |
| Proportion of red% | 46.56 ± 3.92 | 46.86 | 40.34 | 53.30 |
| Proportion of blue% | 11.13 ± 1.39 | 11.07 | 8.63 | 13.72 |
| White wine | ||||
| A280 nm | 3.935 ± 0.097 | 3.925 | 3.767 | 4.066 |
| A420 nm | 0.284 ± 0.135 | 0.236 | 0.081 | 0.502 |
| Mean (mg/mL) ± Std. Deviation | Median (mg/mL) | Minimum (mg/mL) | Maximum (mg/mL) | |||||
|---|---|---|---|---|---|---|---|---|
| Red | White | Red | White | Red | White | Red | White | |
| TPC | 2.029 ± 0.776 | 0.363 ± 0.099 | 2.272 a | 0.365 a | 0.666 | 0.226 | 3.291 | 0.527 |
| TFC | 0.838 ± 0.430 | 0.156 ± 0.086 | 0.920 a | 0.141 a | 0.304 | 0.064 | 1.667 | 0.340 |
| DPPH | 3.500 ± 1.559 | 0.705 ± 0.279 | 4.184 a | 0.737 a | 0.843 | 0.270 | 5.438 | 1.023 |
| ABTS | 6.037 ± 3.330 | 1.142 ± 0.626 | 4.882 a | 1.096 a | 1.329 | 0.206 | 10.906 | 2.036 |
| FRAP | 5.238 ± 2.314 | 1.234 ± 0.268 | 6.623 a | 1.367 a | 0.901 | 0.722 | 7.068 | 1.419 |
| Component 1 | Component 2 | Component 3 | |
|---|---|---|---|
| vintage | −0.100600 | −0.365712 | −0.484852 |
| Cd | 0.401070 | 0.625847 | 0.181428 |
| Pb | 0.178149 | −0.033854 | 0.671428 |
| Cr | 0.215748 | 0.222721 | 0.520405 |
| Cu | −0.119180 | −0.367322 | 0.274617 |
| Fe | −0.386043 | −0.103756 | −0.267366 |
| Mn | 0.383935 | 0.155633 | 0.087432 |
| Ni | 0.080965 | −0.198611 | 0.582837 |
| Zn | −0.124327 | −0.767032 | 0.218482 |
| K | 0.150334 | 0.339505 | 0.281856 |
| Na | −0.619868 | 0.552884 | −0.078169 |
| Ca | 0.242568 | 0.018137 | −0.715440 |
| Mg | 0.189191 | 0.347689 | 0.011345 |
| TPC | 0.678959 | 0.369308 | −0.139029 |
| TFC | 0.965318 | 0.017392 | −0.049698 |
| DPPH | 0.956747 | 0.051815 | 0.032724 |
| ABTS | −0.313627 | 0.912607 | 0.229632 |
| FRAP | 0.935341 | 0.056037 | 0.038930 |
| A420 | 0.962183 | −0.185351 | 0.011776 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skendi, A.; Papageorgiou, M.; Stefanou, S. Preliminary Study of Microelements, Phenolics as well as Antioxidant Activity in Local, Homemade Wines from North-East Greece. Foods 2020, 9, 1607. https://doi.org/10.3390/foods9111607
Skendi A, Papageorgiou M, Stefanou S. Preliminary Study of Microelements, Phenolics as well as Antioxidant Activity in Local, Homemade Wines from North-East Greece. Foods. 2020; 9(11):1607. https://doi.org/10.3390/foods9111607
Chicago/Turabian StyleSkendi, Adriana, Maria Papageorgiou, and Stefanos Stefanou. 2020. "Preliminary Study of Microelements, Phenolics as well as Antioxidant Activity in Local, Homemade Wines from North-East Greece" Foods 9, no. 11: 1607. https://doi.org/10.3390/foods9111607
APA StyleSkendi, A., Papageorgiou, M., & Stefanou, S. (2020). Preliminary Study of Microelements, Phenolics as well as Antioxidant Activity in Local, Homemade Wines from North-East Greece. Foods, 9(11), 1607. https://doi.org/10.3390/foods9111607








