Green Extraction Methods for Extraction of Polyphenolic Compounds from Blueberry Pomace
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
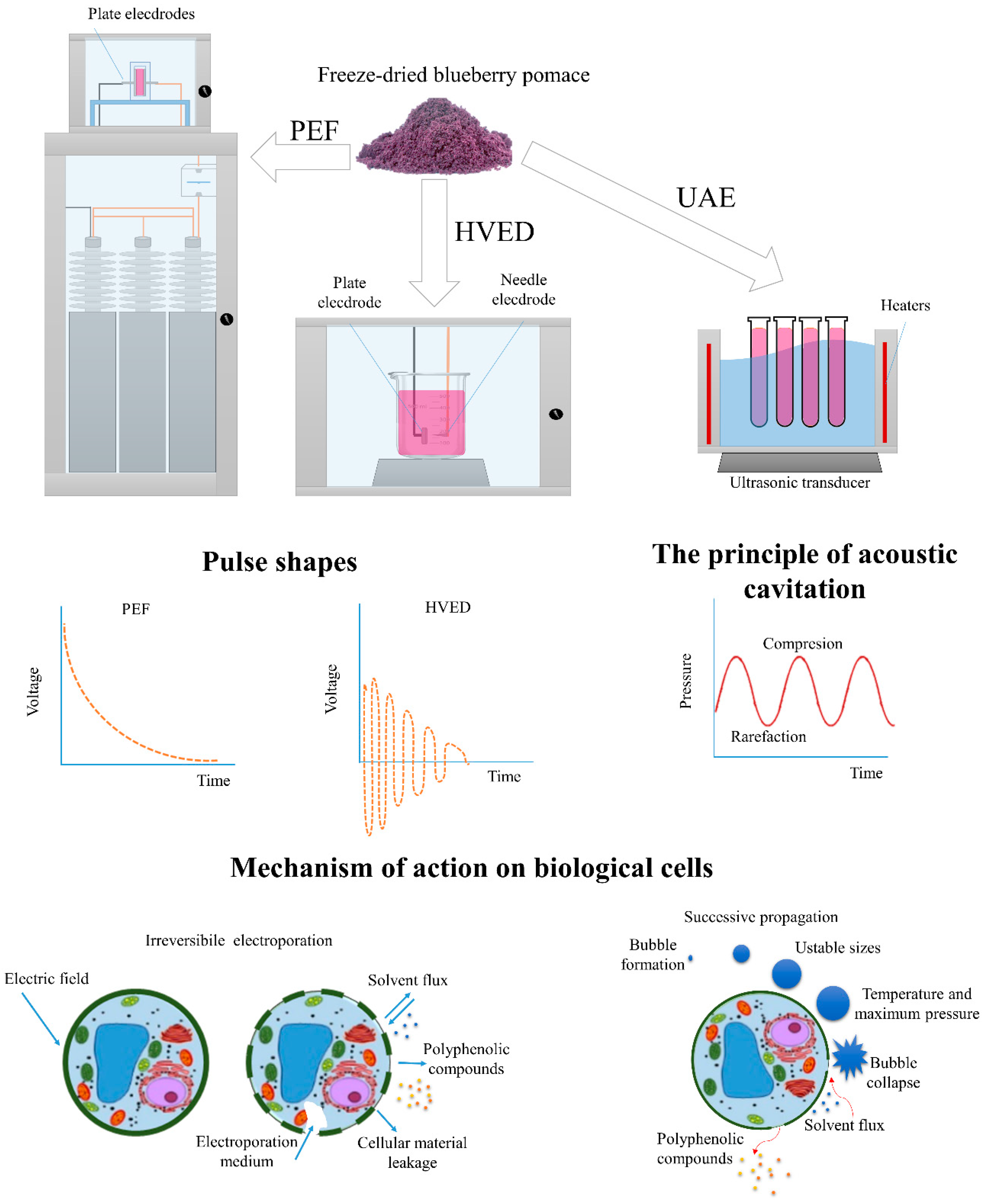
2.3. Polyphenols Extraction
2.4. High Voltage Electrical Discharge-Assisted Extraction (HVED)
2.5. Pulsed Electric Field-Assisted Extraction (PEF)
2.6. Ultrasound-Assisted Extraction (UAE)
2.7. Determination of Total Polyphenolic Content and Antiradical Activity
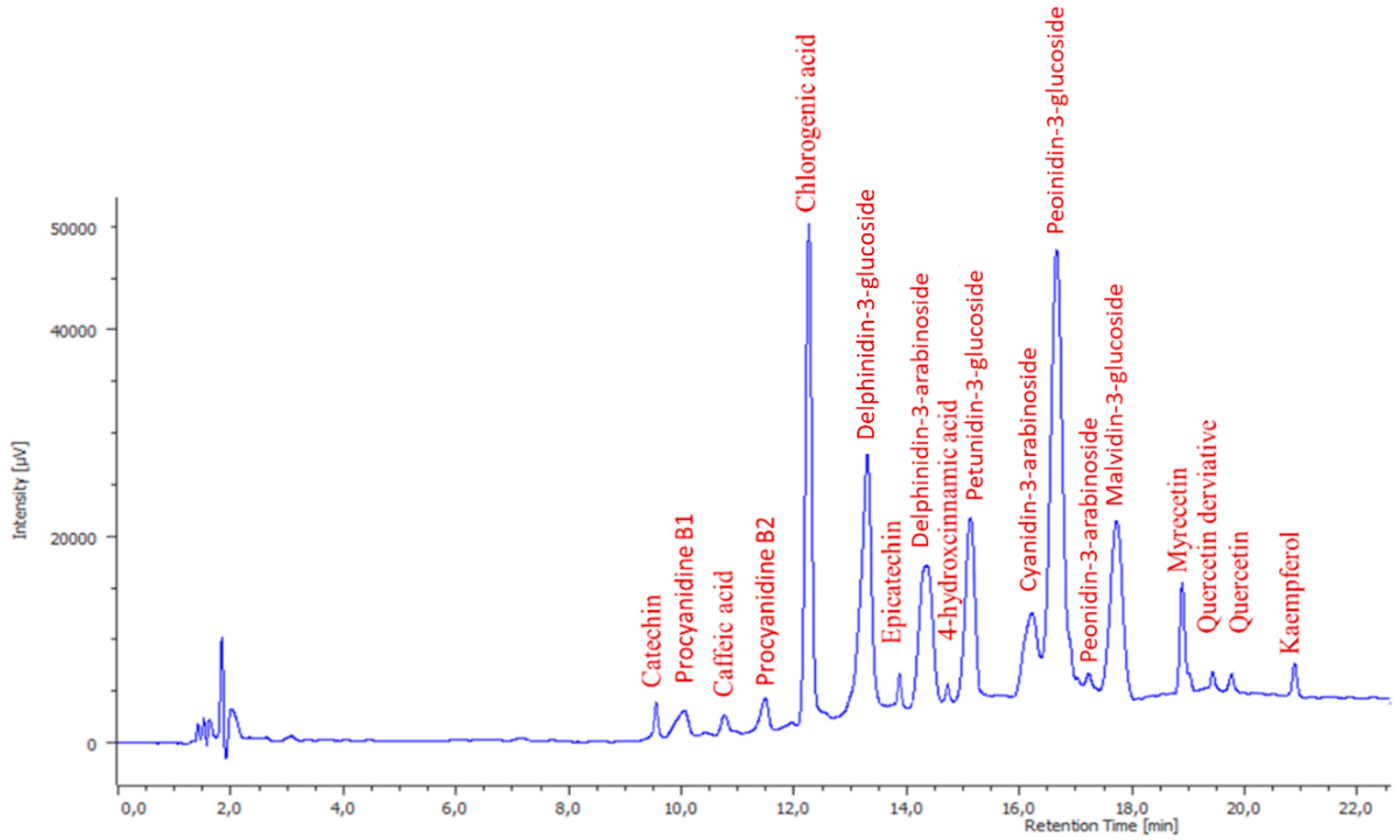
2.8. High-Performance Liquid Chromatography with the Diode-Array Detector (HPLC-DAD)
2.9. Phenolic Identification by LC-MS-MS
2.10. Statistical Analysis
3. Results
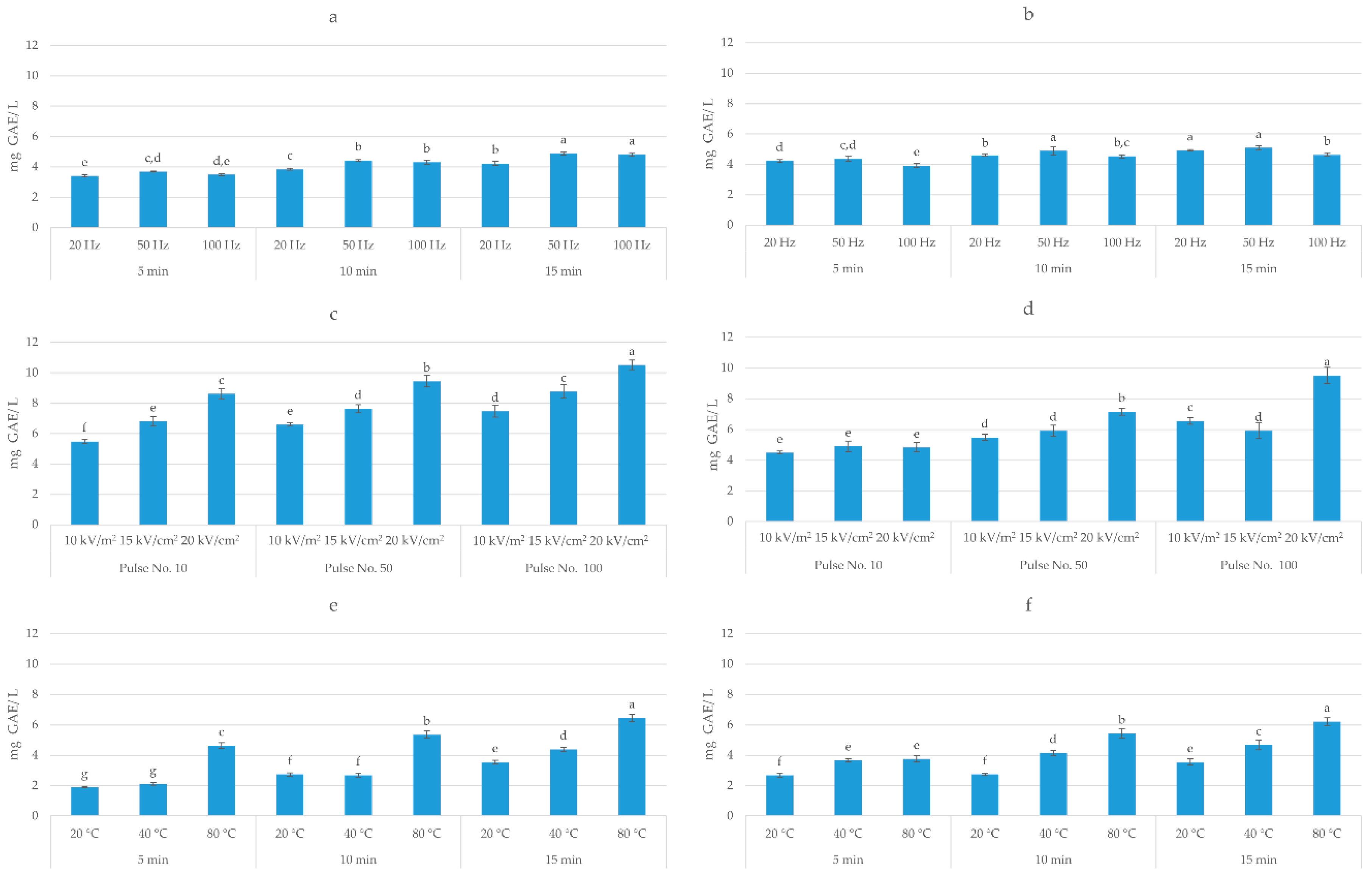
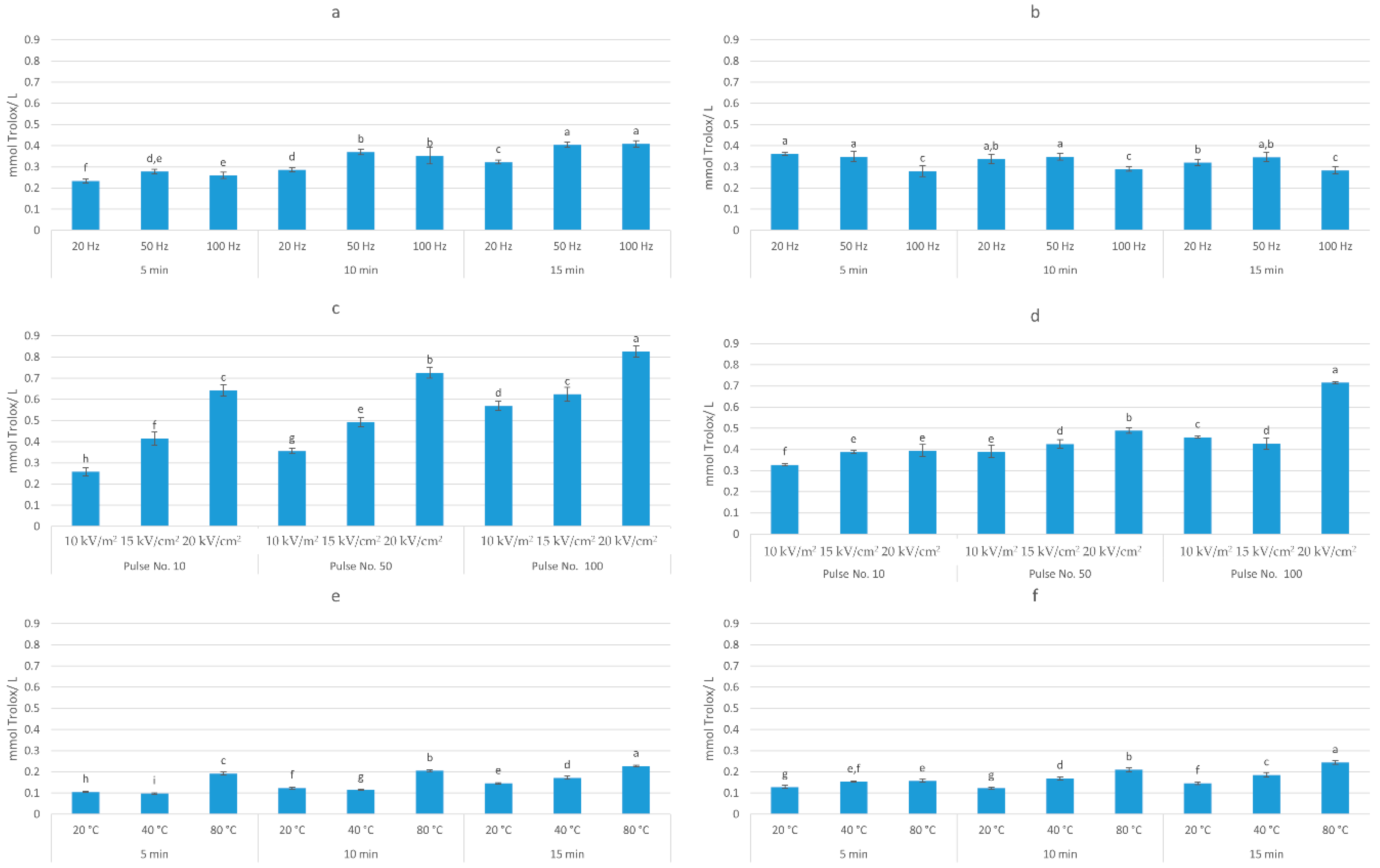
3.1. Total Polyphenolic Content and Antioxidant Activity of Extracts
3.2. Extraction of Anthocyanins
3.3. Extraction of Phenolic Acids
3.4. Extraction of Flavanols
3.5. Extraction of Flavonols
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Michalska, A.; Łysiak, G. Bioactive compounds of blueberries: Post-harvest factors influencing the nutritional value of products. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef] [PubMed]
- Tagliani, C.; Perez, C.; Curutchet, A.; Arcia, P.; Cozzano, S. Blueberry pomace, valorization of an industry by-product source of fibre with antioxidant capacity. Food Sci. Technol. 2019, 39, 644–651. [Google Scholar] [CrossRef]
- Bamba, B.S.B.; Shi, J.; Tranchant, C.C.; Xue, S.J.; Forney, C.F.; Lim, L.T. Influence of extraction conditions on ultrasound-assisted recovery of bioactive phenolics from blueberry pomace and their antioxidant activity. Molecules 2018, 23, 1685. [Google Scholar] [CrossRef] [PubMed]
- Abdulkhaleq, L.A.; Assi, M.A.; Noor, M.H.M.; Abdullah, R.; Saad, M.Z.; Taufiq-Yap, Y.H. Therapeutic uses of epicatechin in diabetes and cancer. Vet. World 2017, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Lú-Chau, T.A.; Moreira, M.T.; Lema, J.M.; Eibes, G. Rutin: A review on extraction, identification and purification methods, biological activities and approaches to enhance its bioavailability. Trends Food Sci. Technol. 2017, 67, 220–235. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Ul-Haq, I.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Šarić, B.; Mišan, A.; Mandić, A.; Nedeljković, N.; Pojić, M.; Pestorić, M.; Đilas, S. Valorisation of raspberry and blueberry pomace through the formulation of value-added gluten-free cookies. J. Food Sci. Technol. 2016, 53, 1140–1150. [Google Scholar] [CrossRef]
- Da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC–Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. The role of green extraction techniques in Green Analytical Chemistry. TrAC–Trends Anal. Chem. 2015, 71, 2–8. [Google Scholar] [CrossRef]
- Galanakis, C.M. Emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food Bioprod. Process. 2013, 91, 575–579. [Google Scholar] [CrossRef]
- Li, Z.; Fan, Y.; Xi, J. Recent advances in high voltage electric discharge extraction of bioactive ingredients from plant materials. Food Chem. 2019, 277, 246–260. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, X.; Huang, H. Effects of Pulsed Electric Fields on Anthocyanin Extraction Yield of Blueberry Processing By-Products. J. Food Process. Preserv. 2015, 39, 1898–1904. [Google Scholar] [CrossRef]
- Barba, F.J.; Galanakis, C.M.; Esteve, M.J.; Frigola, A.; Vorobiev, E. Potential use of pulsed electric technologies and ultrasounds to improve the recovery of high-added value compounds from blackberries. J. Food Eng. 2015, 167, 38–44. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef]
- Brianceau, S.; Turk, M.; Vitrac, X.; Vorobiev, E. High voltage electric discharges assisted extraction of phenolic compounds from grape stems: Effect of processing parameters on flavan-3-ols, flavonols and stilbenes recovery. Innov. Food Sci. Emerg. Technol. 2016, 35, 67–74. [Google Scholar] [CrossRef]
- Boussetta, N.; Lebovka, N.; Vorobiev, E.; Adenier, H.; Bedel-Cloutour, C.; Lanoisellé, J.L. Electrically assisted extraction of soluble matter from chardonnay grape skins for polyphenol recovery. J. Agric. Food Chem. 2009, 57, 1491–1497. [Google Scholar] [CrossRef]
- Boussetta, N.; Lanoisellé, J.L.; Bedel-Cloutour, C.; Vorobiev, E. Extraction of soluble matter from grape pomace by high voltage electrical discharges for polyphenol recovery: Effect of sulphur dioxide and thermal treatments. J. Food Eng. 2009, 95, 192–198. [Google Scholar] [CrossRef]
- Xi, J.; He, L.; Yan, L.G. Continuous extraction of phenolic compounds from pomegranate peel using high voltage electrical discharge. Food Chem. 2017. [Google Scholar] [CrossRef]
- Yan, L.G.; Deng, Y.; Ju, T.; Wu, K.; Xi, J. Continuous high voltage electrical discharge extraction of flavonoids from peanut shells based on “annular gap type” treatment chamber. Food Chem. 2018, 256, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Impact of pulsed electric fields and high voltage electrical discharges on extraction of high-added value compounds from papaya peels. Food Res. Int. 2014, 65, 337–343. [Google Scholar] [CrossRef]
- Puértolas, E.; Luengo, E.; Álvarez, I.; Raso, J. Improving Mass Transfer to Soften Tissues by Pulsed Electric Fields: Fundamentals and Applications. Annu. Rev. Food Sci. Technol. 2012. [Google Scholar] [CrossRef]
- Barba, F.J.; Grimi, N.; Vorobiev, E. New Approaches for the Use of Non-conventional Cell Disruption Technologies to Extract Potential Food Additives and Nutraceuticals from Microalgae. Food Eng. Rev. 2014, 7, 45–62. [Google Scholar] [CrossRef]
- Luengo, E.; Álvarez, I.; Raso, J. Improving the pressing extraction of polyphenols of orange peel by pulsed electric fields. Innov. Food Sci. Emerg. Technol. 2013, 17, 79–84. [Google Scholar] [CrossRef]
- Puértolas, E.; Cregenzán, O.; Luengo, E.; Álvarez, I.; Raso, J. Pulsed-Electric-Field-Assisted extraction of anthocyanins from purple-fleshed potato. Food Chem. 2013. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E.; Le, L.H.; Cordin-Falcimaigne, A.; Lanoisellé, J.L. Application of electrical treatments in alcoholic solvent for polyphenols extraction from grape seeds. LWT–Food Sci. Technol. 2012, 46, 127–134. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC–Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Wang, T.; Guo, N.; Wang, S.X.; Kou, P.; Zhao, C.J.; Fu, Y.J. Ultrasound-Negative pressure cavitation extraction of phenolic compounds from blueberry leaves and evaluation of its DPPH radical scavenging activity. Food Bioprod. Process. 2018, 108, 69–80. [Google Scholar] [CrossRef]
- Zhang, H.; Tchabo, W.; Ma, Y. Quality of extracts from blueberry pomace by high hydrostatic pressure, ultrasonic, microwave and heating extraction: A comparison study. Emir. J. Food Agric. 2017, 29, 815–819. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT–Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Barnes, J.S.; Nguyen, H.P.; Shen, S.; Schug, K.A. General method for extraction of blueberry anthocyanins and identification using high performance liquid chromatography-electrospray ionization-ion trap-time of flight-mass spectrometry. J. Chromatogr. A 2009, 1216, 4728–4735. [Google Scholar] [CrossRef] [PubMed]
- El Kantar, S.; Boussetta, N.; Lebovka, N.; Foucart, F.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed electric field treatment of citrus fruits: Improvement of juice and polyphenols extraction. Innov. Food Sci. Emerg. Technol. 2018, 46, 153–161. [Google Scholar] [CrossRef]
- Stein-Chisholm, R.; Beaulieu, J.; Grimm, C.; Lloyd, S. LC–MS/MS and UPLC–UV Evaluation of Anthocyanins and Anthocyanidins during Rabbiteye Blueberry Juice Processing. Beverages 2017, 3, 56. [Google Scholar] [CrossRef]
- You, Q.; Wang, B.; Chen, F.; Huang, Z.; Wang, X.; Luo, P.G. Comparison of anthocyanins and phenolics in organically and conventionally grown blueberries in selected cultivars. Food Chem. 2011, 125, 201–208. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Barba, F.J.; Parniakov, O.; Galanakis, C.M.; Lebovka, N.; Grimi, N.; Vorobiev, E. High Voltage Electrical Discharges, Pulsed Electric Field, and Ultrasound Assisted Extraction of Protein and Phenolic Compounds from Olive Kernel. Food Bioprocess Technol. 2015, 8, 885–894. [Google Scholar] [CrossRef]
- Rajha, H.N.; Abi-Khattar, A.M.; El Kantar, S.; Boussetta, N.; Lebovka, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Comparison of aqueous extraction efficiency and biological activities of polyphenols from pomegranate peels assisted by infrared, ultrasound, pulsed electric fields and high-voltage electrical discharges. Innov. Food Sci. Emerg. Technol. 2019, 58, 102212. [Google Scholar] [CrossRef]
- Taruscio, T.G.; Barney, D.L.; Exon, J. Content and Profile of Flavanoid and Phenolic Acid Compounds in Conjunction with the Antioxidant Capacity for a Variety of Northwest Vaccinium Berries. J. Agric. Food Chem. 2004, 52, 3169–3176. [Google Scholar] [CrossRef]
- Huang, W.Y.; Zhang, H.C.; Liu, W.X.; Li, C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Redondo, D.; Venturini, M.E.; Luengo, E.; Raso, J.; Arias, E. Pulsed electric fields as a green technology for the extraction of bioactive compounds from thinned peach by-products. Innov. Food Sci. Emerg. Technol. 2018, 45, 335–343. [Google Scholar] [CrossRef]
- Martín-García, B.; Tylewicz, U.; Verardo, V.; Pasini, F.; Gómez-Caravaca, A.M.; Caboni, M.F.; Dalla Rosa, M. Pulsed electric field (PEF) as pre-treatment to improve the phenolic compounds recovery from brewers’ spent grains. Innov. Food Sci. Emerg. Technol. 2020, 64, 102402. [Google Scholar] [CrossRef]
- Wang, Y.; Fong, S.K.; Singh, A.P.; Vorsa, N.; Johnson-Cicalese, J. Variation of anthocyanins, proanthocyanidins, flavonols, and organic acids in cultivated and wild diploid blueberry species. HortScience 2019, 54, 576–585. [Google Scholar] [CrossRef]
- Boussetta, N.; Vorobiev, E.; Deloison, V.; Pochez, F.; Falcimaigne-Cordin, A.; Lanoisellé, J.L. Valorisation of grape pomace by the extraction of phenolic antioxidants: Application of high voltage electrical discharges. Food Chem. 2011, 128, 364–370. [Google Scholar] [CrossRef]
- Trouillas, P.; Sancho-García, J.C.; De Freitas, V.; Gierschner, J.; Otyepka, M.; Dangles, O. Stabilizing and modulating color by copigmentation: Insights from theory and experiment. Chem. Rev. 2016, 116, 4937–4982. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, C.Y.; Wang, S.Y.; Zheng, W. Effect of High-Oxygen Atmospheres on Blueberry Phenolics, Anthocyanins, and Antioxidant Capacity. J. Agric. Food Chem. 2003, 51, 7162–7169. [Google Scholar] [CrossRef]
- Bansal, V.; Sharma, A.; Ghanshyam, C.; Singla, M.L. Optimization and characterization of pulsed electric field parameters for extraction of quercetin and ellagic acid in emblica officinalis juice. J. Food Meas. Charact. 2014, 8, 225–233. [Google Scholar] [CrossRef]
- Gharibi Tehrani, M.; Elhamirad, A.H.; Azarpazhooh, E.; Pedramnia, A.; Sharayei, P. Natural valuable compound extraction from onion by-products using a pulsed electric field. Int. J. Biol. Chem. 2019, 12, 171–180. [Google Scholar] [CrossRef]
- Razmara, R.S.; Daneshfar, A.; Sahraei, R. Solubility of quercetin in water + methanol and water + ethanol from (292.8 to 333.8). KJ Chem. Eng. Data 2010, 55, 3934–3936. [Google Scholar] [CrossRef]
- Rababah, T.M.; Banat, F.; Rababah, A.; Ereifej, K.; Yang, W. Optimization of extraction conditions of total phenolics, antioxidant activities, and anthocyanin of oregano, thyme, terebinth, and pomegranate. J. Food Sci. 2010, 75, C626–C632. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, N.; Colombi, B.; Salvatore, S.; Brenna, O.V.; Galaverna, G.; Del Rio, D.; Bianchi, M.; Bennett, R.N.; Brighenti, F. Evaluation of antioxidant capacity of some fruit and vegetable foods: Efficiency of extraction of a sequence of solvents. J. Sci. Food Agric. 2007, 87, 103–111. [Google Scholar] [CrossRef]
- Boussetta, N.; Grimi, N.; Vorobiev, E. Pulsed Electrical Technologies Assisted Polyphenols Extraction from Agricultural Plants and Bioresources: A Review. Int. J. Food Process. Technol. 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Cholet, C.; Delsart, C.; Petrel, M.; Gontier, E.; Grimi, N.; L’Hyvernay, A.; Ghidossi, R.; Vorobiev, E.; Mietton-Peuchot, M.; Gény, L. Structural and biochemical changes induced by pulsed electric field treatments on cabernet sauvignon grape berry skins: Impact on cell wall total tannins and polysaccharides. J. Agric. Food Chem. 2014, 62, 2925–2934. [Google Scholar] [CrossRef]
| Phenolic Compound | Structure | Molecular Formula | [M − H]− (m/z) a | MS/MS (m/z) b | Collision Energy (eV) | Rt (min) HPLC c |
|---|---|---|---|---|---|---|
| Catechin | 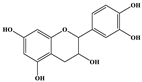 | C15H14O6 | 289.006 | 245.020 203.115 | 17 22 | 9.53 |
| Procyanidin B1 |  | C30H26O12 | 577.033 | 407.066 288.931 424.977 | 26 25 26 | 10.07 |
| Caffeic Acid | 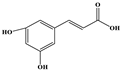 | C9H8O4 | 178.978 | 135.033 134.010 | 19 28 | 10.66 |
| Procyanidin B2 | 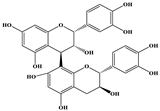 | C30H26O12 | 577.033 | 407.066 288.931 424.977 | 26 25 26 | 11.58 |
| Chlorogenic Acid | 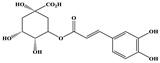 | C16H18O9 | 353.000 | 191.074 85.090 93.073 | 22 43 45 | 12.38 |
| Epicatechin |  | C15H14O6 | 289.006 | 245.020 203.115 | 17 22 | 13.79 |
| 4-Hydroxcinnamic acid | 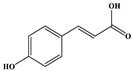 | C9H8O3 | 163.016 | 119.072 163.016 163.016 | 18 37 38 | 14.70 |
| Myricetin + |  | C15H10O8 | 319.000 | 153.027 217.062 245.062 | 31 31 27 | 19.00 |
| Quercetin + | 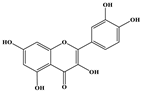 | C15H10O7 | 303.098 | 229.106 153.046 | 28 33 | 19.86 |
| Kaempferol | 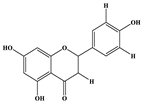 | C15H10O6 | 285.078 | 184.919 239.126 | 30 35 | 20.97 |
| Anthocyanins * | ||||||
| Del-3-glu + | 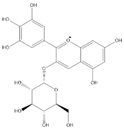 | C21H21O12 | 465.1027 | 13.39 | ||
| Del-3-ara + | 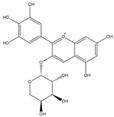 | C20H19O11 | 435.0922 | 14.47 | ||
| Pet-3-glu + |  | C22H23O12 | 479.1184 | 15.24 | ||
| Cya-3-ara + | 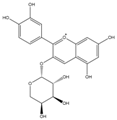 | C20H19O10 | 419.0928 | 16.37 | ||
| Peo-3-glu + | 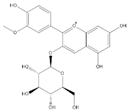 | C22H23O11 | 463.1235 | 16.61 | ||
| Peo-3-ara + |  | C21H21O11 | 433.1129 | 17.18 | ||
| Mal-3-glu + | 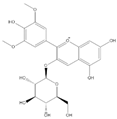 | C23H25O12 | 493.1340 | 17.68 | ||
| Ethanol | Anthocyanins | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HVED (Hz) | Minutes | Del-3-Glu | Del-3-ara | Pet-3-glu | Cya-3-ara | Peo-3-glu | Peo-3-ara | Mal-3-glu | Total |
| 20 | 5 | 122.23 ± 3.08 | 87.77 ± 2.33 | 171.12 ± 4.91 | 44.73 ± 1.71 | 192.38 ± 5.3 | 7.14 ± 0.28 | 78.11 ± 2.69 | 703.48 |
| 20 | 10 | 157.04 ± 2.05 | 110.96 ± 1.34 | 188.35 ± 3.54 | 39.05 ± 1.14 | 228.56 ± 2.92 | 8.42 ± 0.16 | 100.74 ± 1.33 | 833.12 |
| 20 | 15 | 174.12 ± 4.22 | 124.02 ± 3.16 | 212.94 ± 4.97 | 44.99 ± 1.19 | 255.62 ± 5.96 | 9.36 ± 0.29 | 112.22 ± 2.84 | 933.27 |
| 50 | 5 | 146.66 ± 3.36 | 105.27 ± 2.44 | 178.60 ± 4.81 | 35.80 ± 0.89 | 213.30 ± 5.89 | 7.96 ± 0.3 | 93.88 ± 3.51 | 781.47 |
| 50 | 10 | 185.50 ± 2.90 | 133.14 ± 2.46 | 226.25 ± 4.94 | 43.09 ± 1.13 | 269.41 ± 3.89 | 10.49 ± 0.29 | 120.33 ± 2.3 | 988.21 |
| 50 | 15 | 102.24 ± 2.32 | 74.03 ± 1.81 | 128.17 ± 3.22 | 26.16 ± 0.81 | 152.06 ± 4.06 | 1.00 ± 0.02 | 67.03 ± 1.34 | 550.69 |
| 100 | 5 | 133.76 ± 3.86 | 95.75 ± 2.55 | 171.07 ± 11.34 | 37.44 ± 7.16 | 196.93 ± 10.19 | 7.65 ± 0.26 | 83.75 ± 2.54 | 726.35 |
| 100 | 10 | 178.95 ± 5.66 | 126.32 ± 3.61 | 222.76 ± 5.90 | 46.02 ± 1.57 | 259.50 ± 8.23 | 10.35 ± 0.26 | 113.66 ± 3.67 | 957.56 |
| 100 | 15 | 203.96 ± 5.67 | 144.19 ± 3.30 | 253.84 ± 5.37 | 52.92 ± 1.94 | 299.82 ± 8.56 | 11.93 ± 0.43 | 120.52 ± 3.93 | 1087.18 |
| PEF (kV/cm) | Pulse No. | ||||||||
| 10 | 10 | 185.94 ± 13.92 | 132.99 ± 3.71 | 225 ± 8.04 | 48.20 ± 1.14 | 298.26 ± 10.54 | 5.47 ± 0.07 | 127.01 ± 4.18 | 1022.87 |
| 10 | 50 | 187.96 ± 5.08 | 135.52 ± 3.1 | 241.02 ± 4.53 | 50.98 ± 2.60 | 316.21 ± 7.63 | 5.23 ± 0.14 | 131.25 ± 3.72 | 1068.17 |
| 10 | 100 | 190.89 ± 5.23 | 143.61 ± 3.97 | 256.63 ± 5.52 | 55.03 ± 2.34 | 348.97 ± 7.09 | 6.70 ± 0.24 | 147.45 ± 3.67 | 1149.28 |
| 15 | 10 | 175.75 ± 8.54 | 127.31 ± 6.84 | 217.25 ± 8.19 | 47.09 ± 1.66 | 279.67 ± 11.45 | 4.95 ± 0.23 | 117.26 ± 7.54 | 969.28 |
| 15 | 50 | 169.08 ± 2.75 | 126.12 ± 1.82 | 222.3 ± 4.18 | 47.60 ± 1.00 | 293.73 ± 4.69 | 4.69 ± 0.24 | 126.44 ± 2.05 | 989.96 |
| 15 | 100 | 193.49 ± 9.05 | 153.4 ± 9.19 | 289.65 ± 14.8 | 60.66 ± 2.96 | 400.71 ± 17.97 | 7.07 ± 0.29 | 169.94 ± 7.39 | 1274.92 |
| 20 | 10 | 209.32 ± 8.70 | 152.16 ± 6.09 | 262.42 ± 8.91 | 55.95 ± 2.77 | 340.43 ± 13.96 | 5.92 ± 0.27 | 145.56 ± 6.77 | 1171.76 |
| 20 | 50 | 220.56 ± 7.80 | 162.98 ± 4.34 | 301.2 ± 23.05 | 67.17 ± 15.76 | 386.54 ± 19.83 | 7.36 ± 0.16 | 162.10 ± 5.93 | 1307.91 |
| 20 | 100 | 279.39 ± 10.73 | 205.24 ± 7.90 | 370.58 ± 14.00 | 79.13 ± 2.9 | 477.61 ± 17.45 | 11.33 ± 0.51 | 201.26 ± 8.81 | 1624.54 |
| US (T °C) | Minutes | ||||||||
| 20 | 5 | 52.95 ± 0.87 | 40.80 ± 1.05 | 64.36 ± 1.22 | 12.40 ± 0.26 | 80.07 ± 2.45 | 1.40 ± 0.02 | 33.75 ± 0.95 | 285.73 |
| 20 | 10 | 76.36 ± 2.23 | 56.41 ± 1.76 | 94.01 ± 3.01 | 18.59 ± 0.61 | 114.16 ± 3.89 | 2.28 ± 0.06 | 49.33 ± 2.05 | 411.14 |
| 20 | 15 | 100.01 ± 5.53 | 72.44 ± 3.93 | 122.48 ± 6.23 | 23.44 ± 0.85 | 146.78 ± 7.75 | 4.12 ± 0.28 | 63.90 ± 3.98 | 533.17 |
| 40 | 5 | 62.29 ± 2.27 | 46.44 ± 1.87 | 73.13 ± 3.27 | 13.45 ± 0.36 | 82.88 ± 4.75 | 1.41 ± 0.04 | 37.10 ± 2.45 | 316.70 |
| 40 | 10 | 79.30 ± 3.16 | 58.12 ± 2.57 | 93.87 ± 4.98 | 17.38 ± 0.58 | 105.80 ± 5.8 | 1.90 ± 0.06 | 47.52 ± 2.61 | 403.89 |
| 40 | 15 | 120.50 ± 2.84 | 88.06 ± 2.19 | 153.57 ± 12.24 | 32.90 ± 8.39 | 181.80 ± 10.59 | 5.08 ± 0.13 | 77.01 ± 2.09 | 658.92 |
| 80 | 5 | 124.94 ± 2.59 | 92.61 ± 2.16 | 155.35 ± 3.96 | 29.97 ± 0.88 | 201.58 ± 4.94 | 4.13 ± 0.11 | 86.36 ± 1.95 | 694.94 |
| 80 | 10 | 146.36 ± 3.28 | 107.94 ± 2.15 | 183.81 ± 4.09 | 36.57 ± 0.65 | 226.42 ± 4.54 | 4.80 ± 0.10 | 98.60 ± 2.56 | 804.50 |
| 80 | 15 | 182.33 ± 6.15 | 131.75 ± 4.68 | 204.11 ± 7.91 | 43.37 ± 1.36 | 264.54 ± 9.86 | 6.29 ± 0.31 | 116.89 ± 4.68 | 949.28 |
| Methanol | |||||||||
| HVED (Hz) | Minutes | ||||||||
| 20 | 5 | 183.81 ± 4.19 | 128.97 ± 3.40 | 228.02 ± 6.46 | 46.92 ± 1.45 | 265.21 ± 7.32 | 10.79 ± 0.17 | 114.86 ± 2.85 | 978.58 |
| 20 | 10 | 205.46 ± 10.31 | 144.44 ± 7.08 | 254.27 ± 13.45 | 53.32 ± 2.80 | 299.05 ± 15.64 | 10.54 ± 0.53 | 131.10 ± 7.07 | 1098.18 |
| 20 | 15 | 222.23 ± 6.57 | 155.98 ± 4.94 | 275.22 ± 7.94 | 56.88 ± 2.42 | 325.47 ± 9.84 | 10.30 ± 0.61 | 143.05 ± 4.92 | 1189.13 |
| 50 | 5 | 187.32 ± 2.61 | 130.42 ± 2.15 | 231.34 ± 3.76 | 47.70 ± 0.90 | 271.26 ± 3.86 | 11.38 ± 0.79 | 116.95 ± 1.18 | 996.37 |
| 50 | 10 | 217.38 ± 14.17 | 152.38 ± 10.08 | 268.88 ± 18.97 | 55.38 ± 4.23 | 319.06 ± 21.7 | 11.43 ± 0.36 | 139.19 ± 9.52 | 1163.70 |
| 50 | 15 | 228.11 ± 5.61 | 158.83 ± 3.48 | 283.99 ± 6.87 | 58.86 ± 1.62 | 334.39 ± 8.52 | 11.31 ± 0.25 | 145.79 ± 3.91 | 1221.28 |
| 100 | 5 | 167.92 ± 3.18 | 117.15 ± 1.96 | 205.56 ± 3.39 | 40.96 ± 1.22 | 241.71 ± 4.65 | 8.86 ± 0.35 | 105.92 ± 2.10 | 888.08 |
| 100 | 10 | 201.16 ± 7.87 | 139.64 ± 5.13 | 247.67 ± 9.74 | 52.06 ± 1.80 | 291.32 ± 10.85 | 9.87 ± 0.52 | 127.33 ± 4.68 | 1069.05 |
| 100 | 15 | 204.98 ± 5.70 | 142.35 ± 4.02 | 253.36 ± 6.90 | 52.96 ± 1.90 | 298.10 ± 9.05 | 9.83 ± 0.22 | 130.07 ± 3.38 | 1091.65 |
| PEF (kV/cm) | Pulse No. | ||||||||
| 10 | 10 | 177.70 ± 10.78 | 125.50 ± 5.33 | 222.39 ± 12.60 | 48.79 ± 3.37 | 270.18 ± 14.44 | 1.41 ± 0.20 | 116.78 ± 8.72 | 962.75 |
| 10 | 50 | 202.38 ± 10.85 | 143.46 ± 11.05 | 255.74 ± 20.02 | 52.43 ± 4.92 | 320.43 ± 23.07 | 1.50 ± 0.17 | 136.94 ± 11.36 | 1112.88 |
| 10 | 100 | 206.96 ± 3.71 | 146.98 ± 1.52 | 263.92 ± 12.29 | 58.49 ± 3.05 | 341.00 ± 10.18 | 1.73 ± 0.19 | 148.07 ± 3.24 | 1167.15 |
| 15 | 10 | 210.21 ± 11.62 | 149.62 ± 9.02 | 265.86 ± 13.47 | 59.77 ± 3.31 | 332.22 ± 16.36 | 1.56 ± 0.39 | 141.98 ± 6.19 | 1161.22 |
| 15 | 50 | 239.46 ± 6.99 | 174.41 ± 8.29 | 295.52 ± 20.43 | 62.89 ± 5.79 | 347.15 ± 24.64 | 1.83 ± 0.19 | 155.87 ± 11.05 | 1277.13 |
| 15 | 100 | 248.33 ± 12.46 | 178.99 ± 10.51 | 326.64 ± 17.45 | 68.57 ± 4.26 | 419.18 ± 27.02 | 1.83 ± 0.18 | 180.08 ± 9.31 | 1423.62 |
| 20 | 10 | 228.41 ± 7.58 | 161.90 ± 7.95 | 294.41 ± 11.87 | 60.80 ± 2.34 | 367.03 ± 13.34 | 1.72 ± 0.06 | 156.65 ± 6.17 | 1270.92 |
| 20 | 50 | 263.36 ± 5.38 | 183.62 ± 3.98 | 343.53 ± 2.73 | 77.21 ± 7.35 | 472.62 ± 40.17 | 2.01 ± 0.18 | 189.68 ± 10.29 | 1532.03 |
| 20 | 100 | 325.13 ± 10.14 | 230.93 ± 7.35 | 408.30 ± 12.17 | 87.65 ± 2.80 | 485.47 ± 18.70 | 2.63 ± 0.09 | 217.21 ± 6.38 | 1757.32 |
| US (T °C) | Minutes | ||||||||
| 20 | 5 | 73.60 ± 3.27 | 53.96 ± 2.26 | 90.33 ± 4.12 | 18.59 ± 0.86 | 114.96 ± 5.35 | 0.66 ± 0.04 | 49.84 ± 2.37 | 401.94 |
| 20 | 10 | 76.36 ± 2.23 | 56.41 ± 1.76 | 94.01 ± 3.01 | 18.59 ± 0.61 | 114.16 ± 3.89 | 2.28 ± 0.06 | 49.33 ± 2.05 | 411.14 |
| 20 | 15 | 100.01 ± 5.53 | 72.44 ± 3.93 | 122.48 ± 6.23 | 23.44 ± 0.85 | 146.78 ± 7.75 | 4.12 ± 0.28 | 63.90 ± 3.98 | 533.17 |
| 40 | 5 | 102.24 ± 2.32 | 74.03 ± 1.81 | 128.17 ± 3.22 | 26.16 ± 0.81 | 152.06 ± 4.06 | 1.00 ± 0.02 | 67.03 ± 1.34 | 550.69 |
| 40 | 10 | 116.83 ± 5.12 | 84.49 ± 3.55 | 145.41 ± 6.57 | 29.93 ± 1.29 | 169.60 ± 7.37 | 1.12 ± 0.06 | 75.61 ± 3.64 | 622.99 |
| 40 | 15 | 132.09 ± 8.63 | 94.68 ± 6.02 | 164.35 ± 11.45 | 33.62 ± 1.85 | 191.19 ± 12.97 | 1.28 ± 0.12 | 85.19 ± 5.80 | 702.40 |
| 80 | 5 | 108.87 ± 4.58 | 79.96 ± 3.46 | 132.15 ± 6.63 | 28.00 ± 2.11 | 148.29 ± 8.72 | 0.89 ± 0.09 | 67.40 ± 3.76 | 565.56 |
| 80 | 10 | 156.31 ± 8.85 | 114.30 ± 6.40 | 190.59 ± 10.71 | 41.67 ± 2.64 | 214.49 ± 13.5 | 1.40 ± 0.12 | 97.52 ± 5.94 | 816.28 |
| 80 | 15 | 175.59 ± 7.32 | 128.90 ± 5.07 | 236.13 ± 7.99 | 45.28 ± 1.44 | 252.73 ± 10.43 | 2.16 ± 0.07 | 113.12 ± 4.67 | 953.91 |
| Ethanol | Phenolic Acids | ||||
|---|---|---|---|---|---|
| HVED (Hz) | Minutes | Chlorogenic Acid | Caffeic Acid | 4-HCA | Total |
| 20 | 5 | 224.30 ± 7.73 | 2.52 ± 0.06 | 16.82 ± 0.76 | 243.64 |
| 20 | 10 | 282.73 ± 5.57 | 2.91 ± 0.11 | 21.20 ± 0.21 | 306.84 |
| 20 | 15 | 322.65 ± 7.94 | 3.18 ± 0.12 | 23.29 ± 0.69 | 349.12 |
| 50 | 5 | 302.03 ± 5.67 | 2.98 ± 0.08 | 20.27 ± 0.37 | 325.28 |
| 50 | 10 | 373.83 ± 7.18 | 3.57 ± 0.10 | 24.59 ± 0.42 | 401.99 |
| 50 | 15 | 425.42 ± 14.87 | 1.63 ± 0.05 | 15.85 ± 0.31 | 442.90 |
| 100 | 5 | 256.38 ± 8.84 | 2.78 ± 0.07 | 18.53 ± 0.57 | 277.69 |
| 100 | 10 | 334.03 ± 11.74 | 3.32 ± 0.11 | 23.93 ± 0.48 | 361.28 |
| 100 | 15 | 378.60 ± 12.13 | 3.63 ± 0.10 | 26.67 ± 0.90 | 408.90 |
| PEF (kV/cm) | Pulse No. | ||||
| 10 | 10 | 297.91 ± 10.07 | 3.39 ± 0.08 | 30.43 ± 1.23 | 331.73 |
| 10 | 50 | 216.55 ± 5.45 | 3.70 ± 0.14 | 32.55 ± 1.06 | 252.80 |
| 10 | 100 | 279.64 ± 8.22 | 3.47 ± 0.12 | 33.43 ± 1.37 | 316.54 |
| 15 | 10 | 197.80 ± 10.44 | 3.46 ± 0.16 | 28.74 ± 0.81 | 230.00 |
| 15 | 50 | 188.52 ± 4.82 | 3.66 ± 0.11 | 29.89 ± 0.67 | 222.07 |
| 15 | 100 | 253.91 ± 11.81 | 4.99 ± 0.17 | 35.49 ± 1.73 | 294.39 |
| 20 | 10 | 270.83 ± 3.46 | 3.80 ± 0.24 | 33.90 ± 1.13 | 308.53 |
| 20 | 50 | 372.75 ± 15.78 | 4.36 ± 0.11 | 37.52 ± 1.29 | 414.63 |
| 20 | Minutes | 568.86 ± 4.83 | 6.86 ± 0.19 | 49.75 ± 1.51 | 625.47 |
| UAE (T °C) | Minutes | ||||
| 20 | 5 | 170.20 ± 5.84 | 1.37 ± 0.04 | 10.13 ± 0.10 | 181.70 |
| 20 | 10 | 197.97 ± 21.29 | 1.61 ± 0.05 | 13.22 ± 0.29 | 212.80 |
| 20 | 15 | 250.41 ± 4.87 | 1.77 ± 0.15 | 16.69 ± 0.21 | 268.87 |
| 40 | 5 | 143.46 ± 2.92 | 1.44 ± 0.05 | 10.66 ± 0.54 | 155.56 |
| 40 | 10 | 184.11 ± 9.56 | 1.66 ± 0.06 | 12.61 ± 0.29 | 198.38 |
| 40 | 15 | 442.98 ± 13.05 | 2.13 ± 0.06 | 18.40 ± 0.47 | 463.51 |
| 80 | 5 | 350.38 ± 33.72 | 2.25 ± 0.04 | 18.85 ± 0.38 | 371.48 |
| 80 | 10 | 451.21 ± 11.93 | 2.57 ± 0.34 | 19.35 ± 0.28 | 473.13 |
| 80 | 15 | 471.95 ± 2.17 | 2.69 ± 0.1 | 20.24 ± 0.85 | 494.88 |
| Methanol | |||||
| HVED (Hz) | |||||
| 20 | 5 | 168.27 ± 5.04 | 2.81 ± 0.06 | 23.79 ± 0.59 | 194.87 |
| 20 | 10 | 190.52 ± 11.7 | 2.68 ± 0.14 | 23.91 ± 0.23 | 217.11 |
| 20 | 15 | 210.23 ± 9.57 | 2.63 ± 0.16 | 26.02 ± 0.97 | 238.88 |
| 50 | 5 | 166.81 ± 2.19 | 2.68 ± 0.10 | 26.93 ± 1.70 | 196.42 |
| 50 | 10 | 197.98 ± 14.93 | 2.67 ± 0.16 | 27.74 ± 0.92 | 228.39 |
| 50 | 15 | 209.00 ± 7.29 | 2.67 ± 0.17 | 27.89 ± 0.61 | 239.56 |
| 100 | 5 | 142.83 ± 2.73 | 2.46 ± 0.09 | 21.62 ± 0.32 | 166.91 |
| 100 | 10 | 173.46 ± 8.48 | 2.37 ± 0.14 | 25.11 ± 0.80 | 200.94 |
| 100 | 15 | 179.62 ± 6.80 | 2.27 ± 0.17 | 25.59 ± 0.64 | 207.48 |
| PEF (kV/cm) | Pulse No. | ||||
| 10 | 10 | 130.87 ± 6.51 | 2.78 ± 0.20 | 26.88 ± 0.57 | 160.53 |
| 10 | 50 | 116.84 ± 10.36 | 3.37 ± 0.21 | 29.47 ± 2.23 | 149.68 |
| 10 | 100 | 85.53 ± 2.56 | 2.90 ± 0.11 | 30.78 ± 1.05 | 119.21 |
| 15 | 10 | 97.50 ± 4.74 | 3.55 ± 0.29 | 28.42 ± 1.44 | 129.47 |
| 15 | 50 | 126.95 ± 3.15 | 3.51 ± 0.24 | 32.55 ± 2.12 | 163.01 |
| 15 | 100 | 133.41 ± 5.44 | 3.95 ± 0.27 | 36.27 ± 2.71 | 173.63 |
| 20 | 10 | 97.88 ± 6.61 | 4.06 ± 0.14 | 32.43 ± 1.43 | 134.37 |
| 20 | 50 | 157.15 ± 14.03 | 4.30 ± 0.42 | 40.30 ± 2.97 | 201.75 |
| 20 | 100 | 551.29 ± 5.08 | 4.93 ± 0.21 | 44.57 ± 1.43 | 600.79 |
| UAE (T °C) | Minutes | ||||
| 20 | 5 | 220.06 ± 15.75 | 1.31 ± 0.11 | 12.10 ± 0.42 | 233.47 |
| 20 | 10 | 342.73 ± 6.23 | 1.61 ± 0.05 | 13.22 ± 0.29 | 357.56 |
| 20 | 15 | 439.57 ± 29.82 | 1.77 ± 0.15 | 16.69 ± 0.21 | 458.03 |
| 40 | 5 | 425.42 ± 14.87 | 1.63 ± 0.05 | 15.85 ± 0.31 | 442.90 |
| 40 | 10 | 461.50 ± 14.64 | 1.77 ± 0.07 | 17.76 ± 0.74 | 481.03 |
| 40 | 15 | 539.92 ± 20.67 | 1.93 ± 0.08 | 19.41 ± 1.10 | 561.26 |
| 80 | 5 | 227.08 ± 11.94 | 1.79 ± 0.06 | 16.86 ± 0.69 | 245.73 |
| 80 | 10 | 332.86 ± 20.71 | 2.19 ± 0.10 | 22.58 ± 1.35 | 357.63 |
| 80 | 15 | 372.99 ± 14.32 | 2.32 ± 0.07 | 26.24 ± 0.86 | 401.55 |
| Ethanol | Flavanols | |||||
|---|---|---|---|---|---|---|
| HVED (Hz) | Minutes | Catechin | PCB1 | PCB2 | Epicatechin | Total |
| 20 | 5 | 18.77 ± 1.09 | 5.21 ± 0.16 | 44.13 ± 0.67 | 31.13 ± 0.70 | 99.24 |
| 20 | 10 | 21.63 ± 0.90 | 5.40 ± 0.26 | 54.50 ± 0.75 | 35.22 ± 2.10 | 116.75 |
| 20 | 15 | 26.19 ± 0.85 | 5.56 ± 0.20 | 61.00 ± 2.03 | 42.37 ± 1.64 | 135.12 |
| 50 | 5 | 23.38 ± 0.76 | 5.37 ± 0.07 | 56.75 ± 0.77 | 35.61 ± 1.33 | 121.11 |
| 50 | 10 | 27.38 ± 0.51 | 5.69 ± 0.15 | 68.75 ± 1.30 | 44.52 ± 0.87 | 146.34 |
| 50 | 15 | 20.00 ± 0.70 | 7.20 ± 0.18 | 37.71 ± 1.08 | 30.91 ± 0.77 | 95.82 |
| 100 | 5 | 21.70 ± 0.42 | 5.27 ± 0.05 | 49.59 ± 1.97 | 35.65 ± 0.91 | 112.21 |
| 100 | 10 | 28.16 ± 0.24 | 5.67 ± 0.10 | 64.23 ± 2.17 | 48.65 ± 0.79 | 146.71 |
| 100 | 15 | 30.39 ± 0.74 | 5.91 ± 0.13 | 72.98 ± 2.49 | 53.95 ± 1.18 | 163.23 |
| PEF (kV/cm) | Pulse No. | |||||
| 10 | 10 | 39.56 ± 0.81 | 5.98 ± 0.11 | 80.32 ± 3.30 | 54.43 ± 12.67 | 180.29 |
| 10 | 50 | 46.09 ± 0.38 | 5.83 ± 0.21 | 85.74 ± 1.13 | 45.54 ± 1.62 | 183.20 |
| 10 | 100 | 46.02 ± 0.88 | 5.99 ± 0.32 | 90.81 ± 3.21 | 50.74 ± 2.92 | 193.56 |
| 15 | 10 | 37.74 ± 0.90 | 5.69 ± 0.27 | 72.01 ± 3.69 | 44.35 ± 0.88 | 159.79 |
| 15 | 50 | 43.14 ± 1.86 | 5.90 ± 0.08 | 80.86 ± 3.37 | 40.47 ± 4.31 | 170.37 |
| 15 | 100 | 57.46 ± 1.76 | 6.28 ± 0.13 | 111.91 ± 5.26 | 47.91 ± 1.99 | 223.56 |
| 20 | 10 | 45.03 ± 1.86 | 6.14 ± 0.19 | 86.20 ± 3.91 | 47.86 ± 0.43 | 185.23 |
| 20 | 50 | 49.84 ± 1.45 | 6.43 ± 0.15 | 94.64 ± 2.93 | 51.20 ± 1.55 | 202.11 |
| 20 | 100 | 59.34 ± 2.54 | 7.65 ± 0.17 | 123.16 ± 4.58 | 74.72 ± 1.55 | 264.87 |
| UAE (T °C) | Minutes | |||||
| 20 | 5 | 14.30 ± 0.30 | 4.75 ± 0.16 | 26.02 ± 1.60 | 14.08 ± 0.42 | 59.15 |
| 20 | 10 | 15.98 ± 0.38 | 5.21 ± 0.10 | 30.32 ± 1.07 | 18.87 ± 0.39 | 70.38 |
| 20 | 15 | 18.19 ± 1.84 | 5.36 ± 0.39 | 36.73 ± 2.99 | 23.48 ± 1.25 | 83.76 |
| 40 | 5 | 12.41 ± 0.32 | 4.97 ± 0.13 | 22.21 ± 0.83 | 13.75 ± 1.02 | 53.34 |
| 40 | 10 | 15.09 ± 0.35 | 5.26 ± 0.11 | 26.99 ± 0.83 | 17.99 ± 0.88 | 65.33 |
| 40 | 15 | 22.79 ± 0.73 | 5.90 ± 0.14 | 45.30 ± 1.50 | 26.55 ± 1.57 | 100.54 |
| 80 | 5 | 25.67 ± 0.87 | 6.08 ± 0.13 | 54.39 ± 1.10 | 26.30 ± 1.66 | 112.44 |
| 80 | 10 | 28.04 ± 1.70 | 5.79 ± 0.10 | 57.73 ± 2.48 | 29.48 ± 2.53 | 121.04 |
| 80 | 15 | 30.34 ± 1.66 | 5.70 ± 0.25 | 63.08 ± 1.68 | 34.84 ± 2.37 | 133.96 |
| Methanol | ||||||
| HVED (Hz) | Minutes | |||||
| 20 | 5 | 26.31 ± 0.25 | 8.45 ± 0.19 | 64.38 ± 1.86 | 53.91 ± 0.83 | 153.05 |
| 20 | 10 | 28.43 ± 0.43 | 7.12 ± 0.65 | 62.25 ± 1.47 | 55.09 ± 0.70 | 152.89 |
| 20 | 15 | 30.96 ± 1.28 | 6.13 ± 0.55 | 68.87 ± 4.43 | 59.55 ± 2.82 | 165.51 |
| 50 | 5 | 32.48 ± 1.91 | 6.29 ± 0.46 | 73.07 ± 4.55 | 64.02 ± 3.79 | 175.86 |
| 50 | 10 | 33.12 ± 0.94 | 6.24 ± 0.40 | 76.06 ± 2.75 | 64.94 ± 1.45 | 180.36 |
| 50 | 15 | 34.02 ± 0.97 | 6.23 ± 0.56 | 76.34 ± 2.31 | 65.88 ± 1.58 | 182.47 |
| 100 | 5 | 25.48 ± 0.19 | 6.42 ± 0.38 | 53.89 ± 1.08 | 49.55 ± 1.22 | 135.34 |
| 100 | 10 | 29.62 ± 1.10 | 5.49 ± 0.31 | 64.01 ± 3.05 | 58.00 ± 2.02 | 157.12 |
| 100 | 15 | 30.57 ± 0.83 | 5.53 ± 0.35 | 66.73 ± 2.08 | 59.11 ± 1.59 | 161.94 |
| PEF (kV/cm) | Pulse No. | |||||
| 10 | 10 | 33.63 ± 1.64 | 7.68 ± 0.36 | 68.18 ± 3.76 | 56.48 ± 3.01 | 165.97 |
| 10 | 50 | 44.86 ± 2.38 | 8.74 ± 0.67 | 86.01 ± 5.61 | 60.54 ± 4.57 | 200.15 |
| 10 | 100 | 45.21 ± 0.34 | 8.04 ± 0.32 | 89.39 ± 2.71 | 63.01 ± 1.46 | 205.65 |
| 15 | 10 | 44.53 ± 1.98 | 8.63 ± 0.84 | 86.5 ± 3.69 | 65.15 ± 2.35 | 204.81 |
| 15 | 50 | 50.42 ± 2.96 | 9.47 ± 0.64 | 94.44 ± 6.92 | 70.83 ± 5.47 | 225.16 |
| 15 | 100 | 57.35 ± 2.54 | 10.00 ± 0.26 | 110.84 ± 7.62 | 75.88 ± 5.03 | 254.07 |
| 20 | 10 | 49.49 ± 2.08 | 8.52 ± 0.33 | 92.4 ± 5.37 | 70.75 ± 5.65 | 221.16 |
| 20 | 50 | 60.57 ± 3.81 | 10.74 ± 0.09 | 119.25 ± 8.51 | 85.50 ± 7.39 | 276.06 |
| 20 | 100 | 59.27 ± 1.40 | 12.11 ± 0.44 | 130.3 ± 4.81 | 96.18 ± 3.39 | 297.86 |
| UAE (T °C) | Minutes | |||||
| 20 | 5 | 18.11 ± 0.89 | 6.11 ± 0.71 | 32.79 ± 2.84 | 22.46 ± 1.42 | 79.47 |
| 20 | 10 | 15.98 ± 0.38 | 5.21 ± 0.10 | 30.32 ± 1.07 | 18.87 ± 0.39 | 70.38 |
| 20 | 15 | 18.19 ± 1.84 | 5.36 ± 0.39 | 36.73 ± 2.99 | 23.48 ± 1.25 | 83.76 |
| 40 | 5 | 20.00 ± 0.70 | 7.20 ± 0.18 | 37.71 ± 1.08 | 30.91 ± 0.77 | 95.82 |
| 40 | 10 | 21.77 ± 0.84 | 7.94 ± 0.23 | 41.79 ± 1.83 | 35.62 ± 1.44 | 107.12 |
| 40 | 15 | 23.30 ± 1.30 | 8.42 ± 0.29 | 45.58 ± 2.83 | 39.64 ± 2.51 | 116.94 |
| 80 | 5 | 19.58 ± 0.82 | 7.62 ± 0.11 | 37.86 ± 1.93 | 33.08 ± 1.36 | 98.14 |
| 80 | 10 | 25.65 ± 1.30 | 9.13 ± 0.33 | 52.00 ± 2.74 | 46.51 ± 3.05 | 133.29 |
| 80 | 15 | 30.98 ± 1.06 | 9.66 ± 0.31 | 63.05 ± 2.38 | 52.35 ± 2.08 | 156.04 |
| Ethanol | Flavonols | |||||
|---|---|---|---|---|---|---|
| HVED (Hz) | Minutes | Myricetin | Que-der | Quercetin | Kaempferol | Total |
| 20 | 5 | 39.08 ± 0.88 | 9.66 ± 0.20 | 8.57 ± 0.14 | 13.30 ± 0.33 | 70.61 |
| 20 | 10 | 49.10 ± 0.61 | 10.54 ± 0.40 | 9.54 ± 0.24 | 15.95 ± 0.20 | 85.13 |
| 20 | 15 | 55.08 ± 1.52 | 10.78 ± 0.27 | 9.75 ± 0.26 | 17.12 ± 0.38 | 92.73 |
| 50 | 5 | 48.18 ± 1.16 | 10.66 ± 0.26 | 9.50 ± 0.20 | 15.44 ± 0.27 | 83.78 |
| 50 | 10 | 59.87 ± 1.34 | 12.31 ± 0.23 | 10.72 ± 0.17 | 17.88 ± 0.30 | 100.78 |
| 50 | 15 | 76.89 ± 0.77 | 13.22 ± 0.16 | 9.41 ± 0.17 | 12.38 ± 0.28 | 121.90 |
| 100 | 5 | 42.90 ± 1.30 | 10.29 ± 0.20 | 9.05 ± 0.15 | 14.18 ± 0.27 | 76.42 |
| 100 | 10 | 56.06 ± 1.96 | 12.10 ± 0.27 | 10.39 ± 0.24 | 17.30 ± 0.51 | 95.85 |
| 100 | 15 | 63.97 ± 2.25 | 13.05 ± 0.28 | 11.18 ± 0.23 | 19.15 ± 0.50 | 107.35 |
| PEF (kV/cm) | Pulse No. | |||||
| 10 | 10 | 54.30 ± 1.89 | 14.00 ± 0.26 | 14.75 ± 0.36 | 25.61 ± 0.70 | 108.66 |
| 10 | 50 | 59.51 ± 1.86 | 13.87 ± 0.54 | 14.93 ± 0.33 | 26.18 ± 1.02 | 114.49 |
| 10 | 100 | 62.48 ± 2.06 | 15.14 ± 0.41 | 15.59 ± 0.40 | 28.37 ± 0.95 | 121.58 |
| 15 | 10 | 51.94 ± 3.06 | 12.46 ± 0.47 | 14.24 ± 0.63 | 22.64 ± 1.20 | 101.28 |
| 15 | 50 | 58.82 ± 1.76 | 13.52 ± 0.56 | 14.76 ± 0.50 | 25.50 ± 0.76 | 112.60 |
| 15 | 100 | 72.40 ± 3.52 | 16.47 ± 0.52 | 16.95 ± 0.57 | 32.21 ± 1.55 | 138.03 |
| 20 | 10 | 61.91 ± 3.42 | 13.91 ± 0.37 | 15.96 ± 0.58 | 26.32 ± 1.18 | 118.10 |
| 20 | 50 | 67.86 ± 2.32 | 15.25 ± 0.30 | 16.91 ± 0.39 | 30.32 ± 0.85 | 130.34 |
| 20 | 100 | 84.90 ± 3.56 | 17.39 ± 0.55 | 20.17 ± 0.70 | 35.08 ± 1.24 | 157.54 |
| UAE (T °C) | Minutes | |||||
| 20 | 5 | 20.00 ± 0.84 | 7.74 ± 0.16 | 7.98 ± 0.14 | 10.08 ± 0.28 | 45.80 |
| 20 | 10 | 24.31 ± 0.89 | 8.50 ± 0.16 | 8.84 ± 0.17 | 11.39 ± 0.32 | 53.04 |
| 20 | 15 | 29.22 ± 1.74 | 9.52 ± 0.31 | 10.09 ± 0.39 | 13.23 ± 0.60 | 62.06 |
| 40 | 5 | 19.38 ± 1.00 | 7.54 ± 0.24 | 8.04 ± 0.25 | 9.57 ± 0.31 | 44.53 |
| 40 | 10 | 22.76 ± 1.09 | 8.14 ± 0.22 | 8.72 ± 0.19 | 10.67 ± 0.40 | 50.29 |
| 40 | 15 | 34.76 ± 0.98 | 10.34 ± 0.17 | 11.19 ± 0.22 | 14.74 ± 0.31 | 71.03 |
| 80 | 5 | 40.36 ± 0.90 | 11.15 ± 0.15 | 11.87 ± 0.18 | 16.11 ± 0.28 | 79.49 |
| 80 | 10 | 43.50 ± 1.07 | 11.16 ± 0.19 | 12.59 ± 0.24 | 16.74 ± 0.37 | 83.99 |
| 80 | 15 | 49.87 ± 1.94 | 11.38 ± 0.39 | 13.94 ± 0.45 | 18.11 ± 0.60 | 93.30 |
| Methanol | ||||||
| HVED (Hz) | Minutes | |||||
| 20 | 5 | 38.36 ± 1.12 | 11.63 ± 0.17 | 8.71 ± 0.13 | 16.17 ± 0.35 | 74.87 |
| 20 | 10 | 38.24 ± 0.31 | 11.28 ± 0.43 | 8.82 ± 0.06 | 16.10 ± 0.33 | 74.44 |
| 20 | 15 | 42.76 ± 2.67 | 9.90 ± 0.46 | 8.68 ± 0.29 | 16.52 ± 0.79 | 77.86 |
| 50 | 5 | 45.80 ± 3.40 | 10.80 ± 0.57 | 9.18 ± 0.43 | 17.51 ± 1.08 | 83.29 |
| 50 | 10 | 47.69 ± 2.46 | 11.31 ± 0.42 | 9.44 ± 0.23 | 18.21 ± 0.65 | 86.65 |
| 50 | 15 | 48.53 ± 1.85 | 11.07 ± 0.35 | 9.47 ± 0.29 | 18.56 ± 0.61 | 87.63 |
| 100 | 5 | 34.71 ± 0.61 | 9.86 ± 0.30 | 8.34 ± 0.12 | 14.46 ± 0.24 | 67.37 |
| 100 | 10 | 40.67 ± 1.52 | 9.09 ± 0.52 | 8.32 ± 0.30 | 15.53 ± 0.67 | 73.61 |
| 100 | 15 | 43.01 ± 1.59 | 9.39 ± 0.51 | 8.47 ± 0.28 | 16.23 ± 0.48 | 77.10 |
| PEF (kV/cm) | Pulse No. | |||||
| 10 | 10 | 43.27 ± 2.33 | 11.72 ± 0.42 | 12.33 ± 0.42 | 20.74 ± 1.08 | 88.06 |
| 10 | 50 | 52.92 ± 4.42 | 13.45 ± 0.67 | 13.48 ± 1.05 | 25.11 ± 1.67 | 104.96 |
| 10 | 100 | 54.94 ± 1.32 | 14.12 ± 0.42 | 13.23 ± 0.22 | 25.93 ± 0.61 | 108.22 |
| 15 | 10 | 52.30 ± 2.56 | 13.13 ± 0.34 | 14.15 ± 0.27 | 24.54 ± 1.35 | 104.12 |
| 15 | 50 | 61.18 ± 4.42 | 14.73 ± 0.83 | 14.93 ± 0.61 | 27.93 ± 1.21 | 118.77 |
| 15 | 100 | 66.01 ± 3.94 | 14.55 ± 0.79 | 15.13 ± 0.99 | 29.05 ± 1.81 | 124.74 |
| 20 | 10 | 57.90 ± 3.30 | 12.64 ± 0.50 | 15.01 ± 0.87 | 25.09 ± 1.19 | 110.64 |
| 20 | 50 | 72.78 ± 6.29 | 15.83 ± 1.09 | 16.29 ± 0.75 | 32.13 ± 2.45 | 137.03 |
| 20 | 100 | 83.21 ± 3.17 | 15.48 ± 0.65 | 18.45 ± 0.58 | 33.26 ± 1.23 | 150.40 |
| UAE (T °C) | Minutes | |||||
| 20 | 5 | 21.42 ± 1.00 | 8.20 ± 0.19 | 8.35 ± 0.22 | 10.89 ± 0.33 | 48.86 |
| 20 | 10 | 24.31 ± 0.89 | 8.50 ± 0.16 | 8.84 ± 0.17 | 11.39 ± 0.32 | 53.04 |
| 20 | 15 | 29.22 ± 1.74 | 9.52 ± 0.31 | 10.09 ± 0.39 | 13.23 ± 0.60 | 62.06 |
| 40 | 5 | 26.89 ± 0.77 | 9.22 ± 0.16 | 9.41 ± 0.17 | 12.38 ± 0.28 | 57.90 |
| 40 | 10 | 29.81 ± 1.44 | 9.73 ± 0.28 | 10.05 ± 0.27 | 13.29 ± 0.48 | 62.88 |
| 40 | 15 | 33.20 ± 2.15 | 10.42 ± 0.39 | 10.75 ± 0.44 | 14.53 ± 0.70 | 68.90 |
| 80 | 5 | 27.98 ± 1.38 | 9.15 ± 0.26 | 9.53 ± 0.30 | 12.11 ± 0.45 | 58.77 |
| 80 | 10 | 38.78 ± 2.40 | 10.89 ± 0.38 | 11.76 ± 0.50 | 15.04 ± 0.73 | 76.47 |
| 80 | 15 | 56.11 ± 1.82 | 12.11 ± 0.29 | 13.18 ± 0.39 | 17.23 ± 0.51 | 98.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lončarić, A.; Celeiro, M.; Jozinović, A.; Jelinić, J.; Kovač, T.; Jokić, S.; Babić, J.; Moslavac, T.; Zavadlav, S.; Lores, M. Green Extraction Methods for Extraction of Polyphenolic Compounds from Blueberry Pomace. Foods 2020, 9, 1521. https://doi.org/10.3390/foods9111521
Lončarić A, Celeiro M, Jozinović A, Jelinić J, Kovač T, Jokić S, Babić J, Moslavac T, Zavadlav S, Lores M. Green Extraction Methods for Extraction of Polyphenolic Compounds from Blueberry Pomace. Foods. 2020; 9(11):1521. https://doi.org/10.3390/foods9111521
Chicago/Turabian StyleLončarić, Ante, Maria Celeiro, Antun Jozinović, Josip Jelinić, Tihomir Kovač, Stela Jokić, Jurislav Babić, Tihomir Moslavac, Sandra Zavadlav, and Marta Lores. 2020. "Green Extraction Methods for Extraction of Polyphenolic Compounds from Blueberry Pomace" Foods 9, no. 11: 1521. https://doi.org/10.3390/foods9111521
APA StyleLončarić, A., Celeiro, M., Jozinović, A., Jelinić, J., Kovač, T., Jokić, S., Babić, J., Moslavac, T., Zavadlav, S., & Lores, M. (2020). Green Extraction Methods for Extraction of Polyphenolic Compounds from Blueberry Pomace. Foods, 9(11), 1521. https://doi.org/10.3390/foods9111521










