Onboard Evaluation of Variable Water Flow and Recirculation Effects on Bleeding of Atlantic Cod (Gadus morhua)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Chemical Analyses
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paquette, J.C.; Mercier, S.; Marcos, B.; Morasse, S. Modeling the thermal performance of a multilayer box for the transportation of perishable food. Food Bioprod. Process. 2017, 105, 77–85. [Google Scholar] [CrossRef]
- Margeirsson, B.; Palsson, H.; Popov, V.; Gospavic, R.; Arason, S.; Sveinsdóttir, K.; Jónsson, M.T. Numerical modelling of temperature fluctuations in superchilled fish loins packaged in expanded polystyrene and stored at dynamic temperature conditions. Int. J. Refrig. 2012, 35, 1318–1326. [Google Scholar] [CrossRef]
- Lauzon, H.L.; Margeirsson, B.; Sveinsdottir, K.; Gudjonsdottir, M.; Karlsdottir, M.G.; Martinsdottir, E. Overview on Fish Quality Research-Impact of Fish Handling, Processing, Storage and Logistics on Fish Quality Deterioration; Icelandic Food and Biotech R&D: Reykjavik, Iceland, 2010. [Google Scholar]
- Olafsdottir, G.; Lauzon, H.L.; Martinsdottir, E.; Oehlenschlauger, J.; Kristbergsson, K. Evaluation of Shelf Life of Superchilled Cod (Gadus morhua) Fillets and the Influence of Temperature Fluctuations During Storage on Microbial and Chemical Quality Indicators. J. Food Sci. 2006, 71, 97–109. [Google Scholar] [CrossRef]
- Borderías, A.J.; Sanchez, I. First Processing Steps and the Quality of Wild and Farmed Fish. J. Food Sci. 2010, 76, R1–R5. [Google Scholar] [CrossRef]
- Huss, H.H. Quality and Changes in Fresh Fish. In FAO Fisheries Technical Paper; No.348.; FAO Press: Rome, Italy, 1995. [Google Scholar]
- Richards, M.P.; Hultin, H.O. Contributions of Blood and Blood Components to Lipid Oxidation in Fish Muscle. J. Agric. Food Chem. 2002, 50, 555–564. [Google Scholar] [CrossRef]
- Botta, J.R.; Squires, B.E.; Johnson, J. Effect of Bleeding/Gutting Procedures on the Sensory Quality of Fresh Raw Atlantic Cod (Gadus morhua). Can. Inst. Food Sci. Technol. J. 1986, 19, 186–190. [Google Scholar] [CrossRef]
- Olsen, S.H.; Joensen, S.; Tobiassen, T.; Heia, K.; Akse, L.; Nilsen, H. Quality consequences of bleeding fish after capture. Fish. Res. 2014, 153, 103–107. [Google Scholar] [CrossRef]
- Eliasson, S.; Arason, S.; Margeirsson, B.; Bergsson, A.B.; Palsson, O.P. Effects of on-board bleeding methods and superchilling on quality of cod and saithe. In Proceedings of the 25th International Congress of Refrigeration (ICR 2019), Montreal, QC, Canada, 24–30 August 2019. [Google Scholar]
- Misimi, E.; Erikson, U.; Digre, H.; Skavhaug, A.; Mathiassen, J. Computer Vision-Based Evaluation of Pre- and Postrigor Changes in Size and Shape of Atlantic Cod (Gadus morhua) and Atlantic Salmon (Salmo salar) Fillets during Rigor Mortis and Ice Storage: Effects of Perimortem Handling Stress. J. Food Sci. 2008, 73, E57–E68. [Google Scholar] [CrossRef]
- Poli, B.M.; Parisi, G.; Scappini, F.; Zampacavallo, G. Fish welfare and quality as affected by pre-slaughter and slaughter management. Aquac. Int. 2005, 13, 29–49. [Google Scholar] [CrossRef]
- Digre, H.; Erikson, U.; Misimi, E.; Standal, I.; Gallart-Jornet, L.; Riebroy, S.; Rustad, T. Bleeding of Farmed Atlantic Cod: Residual Blood, Color, and Quality Attributes of Pre- and Postrigor Fillets as Affected by Perimortem Stress and Different Bleeding Methods. J. Aquat. Food Prod. Technol. 2011, 20, 391–411. [Google Scholar] [CrossRef]
- Roth, B.; Obach, A.; Hunter, D.; Nortvedt, R.; Oyarzún, F. Factors affecting residual blood and subsequent effect on bloodspotting in smoked Atlantic salmon fillets. Aquaculture 2009, 297, 163–168. [Google Scholar] [CrossRef]
- Skjervold, P.O.; Fjæra, S.O.; Østby, P.B.; Einen, O. Live-chilling and crowding stress before slaughter of Atlantic salmon (Salmo salar). Aquaculture 2001, 192, 265–280. [Google Scholar] [CrossRef]
- Karlsdottir, M.G.; Minh, N.V.; Arason, S.; Olafsdottir, A.; Romotowska, P.E.; Bergsson, A.B.; Bjornsson, S. Effects of Bleeding Methods on Quality and Storage Life of Cod and Saithe Products; Icelandic Food and Biotech R&D: Reykjavik, Iceland, 2014. [Google Scholar]
- Ackman, R.G. Fish lipids. Part 1. In Advances in Fish Science and Technology, Farnham, Surrey; Connell, J., Ed.; Fishing News (Books) Ltd.: Surrey, UK, 1980. [Google Scholar]
- Erickson, M. Lipid Oxidation of Muscle Foods. In Food Lipids: Chemistry, Nutrition and Biotechnology; Akoh, C.C., Min, D.B., Eds.; Marcel Dekker: New York, NY, USA, 2002. [Google Scholar]
- Olley, J.; Lovern, J.A. Phospholipid hydrolysis in cod flesh stored at various temperatures. J. Sci. Food Agric. 1960, 11, 644–652. [Google Scholar] [CrossRef]
- Burgaard, M.G.; Jørgensen, B.M. Effect of Temperature on Quality-Related Changes in Cod (Gadus morhua) During Short- and Long-Term Frozen Storage. J. Aquat. Food Prod. Technol. 2010, 19, 249–263. [Google Scholar] [CrossRef]
- Dang, H.T.T.; Gudjonsdottir, M.; Tomasson, T.; Nguyen, M.V.; Karlsdottir, M.G.; Arason, S. Influence of processing additives, packaging and storage conditions on the physicochemical stability of frozen Tra catfish (Pangasius hypophthalmus) fillets. J. Food Eng. 2018, 238, 148–155. [Google Scholar] [CrossRef]
- Hyldig, G.; Nielsen, J.; Jacobsen, C.; Nielsen, H. Sensory and quality properties of packaged seafood. In Advances in Meat, Poultry and Seafood Packaging; Woodhead Publishing: Cambridge, UK, 2012; pp. 154–170. ISBN 9781845697518. [Google Scholar]
- Richards, M.P.; Kelleher, S.D.; Hultin, H.O. Effect of Washing with or without Antioxidants on Quality Retention of Mackerel Fillets during Refrigerated and Frozen Storage. J. Agric. Food Chem. 1998, 46, 4363–4371. [Google Scholar] [CrossRef]
- Lizenko, M.V.; Regerand, T.I.; Bakhirev, A.M.; Lizenko, E.I. Comparative-biochemical analysis of lipids of blood serum lipoproteins of human and some animal species. J. Evol. Biochem. Physiol. 2008, 44, 581–590. [Google Scholar] [CrossRef]
- Pamplona, R. Membrane phospholipids, lipoxidative damage and molecular integrity: A causal role in aging and longevity. Biochim. Biophys. Acta 2008, 1777, 1249–1262. [Google Scholar] [CrossRef]
- Skaginn 3X. SUB-CHILLINGTM—Onboard. Technical Equipment Description from Skaginn. 2018. Available online: http://skaginn3x.com/products/sub-chilling-onboard (accessed on 26 July 2018).
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Phys. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Lowry, R.R.; Tinsley, I.J. Rapid colorimetric determination of free fatty acids. J. Am. Oil Chem. Soc. 1976, 53, 470–472. [Google Scholar] [CrossRef]
- Bernardez, M.; Pastoriza, L.; Sampedro, G.; Rodríguez, J.J.H.; Cabo, M. Modified Method for the Analysis of Free Fatty Acids in Fish. J. Agric. Food Chem. 2005, 53, 1903–1906. [Google Scholar] [CrossRef] [PubMed]
- Stewart, J.C.M. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal. Biochem. 1980, 104, 10–14. [Google Scholar] [CrossRef]
- Gomez-Basauri, J.V.; Regenstein, J.M. Processing and Frozen Storage Effects on the Iron Content of Cod and Mackerel. J. Food Sci. 1992, 57, 1332–1336. [Google Scholar] [CrossRef]
- Montgomery, D.C.; Runger, G.C. Applied Statistics and Probability for Engineers, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2003; p. 483. [Google Scholar]
- Olsen, S.H.; Tobiassen, T.; Akse, L.; Evensen, T.H.; Midling, K.Ø. Capture induced stress and live storage of Atlantic cod (Gadus morhua) caught by trawl: Consequences for the flesh quality. Fish. Res. 2013, 147, 446–453. [Google Scholar] [CrossRef]
- Ando, M.; Nishiyabu, A.; Tsukamasa, Y.; Makinodan, Y. Post-Mortem Softening of Fish Muscle During Chilled Storage as Affected by Bleeding. J. Food Sci. 1999, 64, 423–428. [Google Scholar] [CrossRef]
- Erikson, U.; Tveit, G.M.; Bondø, M.; Digre, H. On-board Live Storage of Atlantic Cod (Gadus morhua): Effects of Capture Stress, Recovery, Delayed Processing, and Frozen Storage on Fillet Color Characteristics. J. Aquat. Food Prod. Technol. 2019, 28, 1076–1091. [Google Scholar] [CrossRef]
- Karlsdóttir, M.G.; Sveinsdottir, K.; Kristinsson, H.G.; Villot, D.; Craft, B.D.; Arason, S. Effects of temperature during frozen storage on lipid deterioration of saithe (Pollachius virens) and hoki (Macruronus novaezelandiae) muscles. Food Chem. 2014, 156, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Minh, N.V.; Karlsdottir, M.G.; Olafsdottir, A.; Bergsson, A.B.; Arason, S. Sensory, Microbiological and Chemical Assessment of Cod (Gadus morhua) Fillets during Chilled Storage as Influenced by Bleeding Methods. Int. J. Nutr. Food Eng. 2013, 7, 544–551. [Google Scholar]
- Minh, V.N.; Phan, L.M.T. Influences of bleeding conditions on the quality and lipid degradation of cobia (Rachycentron canadum) fillets during frozen storage. Turk. J. Fish. Aquat. Sci. 2018, 18, 289–300. [Google Scholar]
- Maqsood, S.; Benjakul, S. Effect of bleeding on lipid oxidation and quality changes of Asian seabass (Lates calcarifer) muscle during iced storage. Food Chem. 2011, 124, 459–467. [Google Scholar] [CrossRef]
- Thiansilakul, Y.; Benjakul, S.; Richards, M.P. Changes in heme proteins and lipids associated with off-odour of seabass (Lates calcarifer) and red tilapia (Oreochromis mossambicus×O. niloticus) during iced storage. Food Chem. 2010, 121, 1109–1119. [Google Scholar] [CrossRef]
- Hardy, R.; McGill, A.S.; Gunstone, F.D. Lipid and autooxidative changes in cold stored cod (Gadus morhua L.). J. Sci. Food Agric. 1979, 30, 999–1006. [Google Scholar] [CrossRef]
- Shewfelt, R. Fish muscle lipolysis—A review. J. Food Biochem. 1981, 5, 79–100. [Google Scholar] [CrossRef]
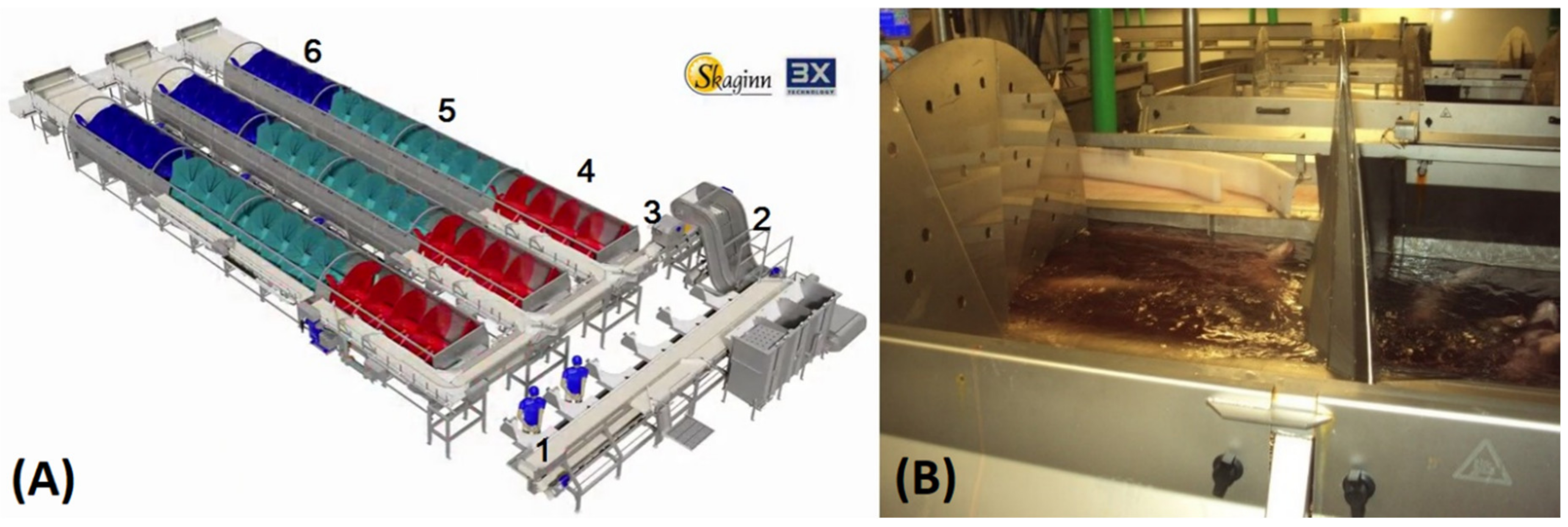
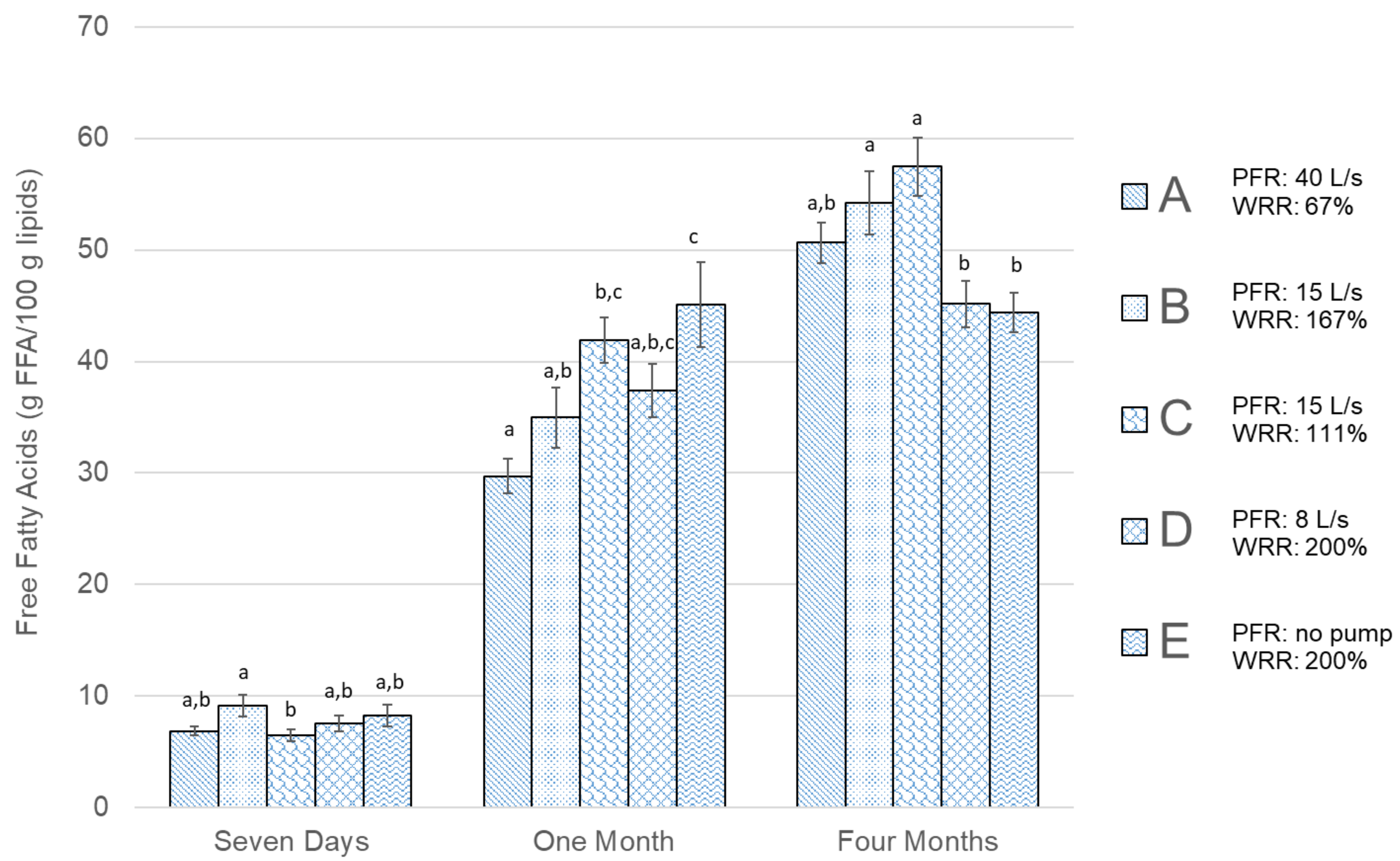

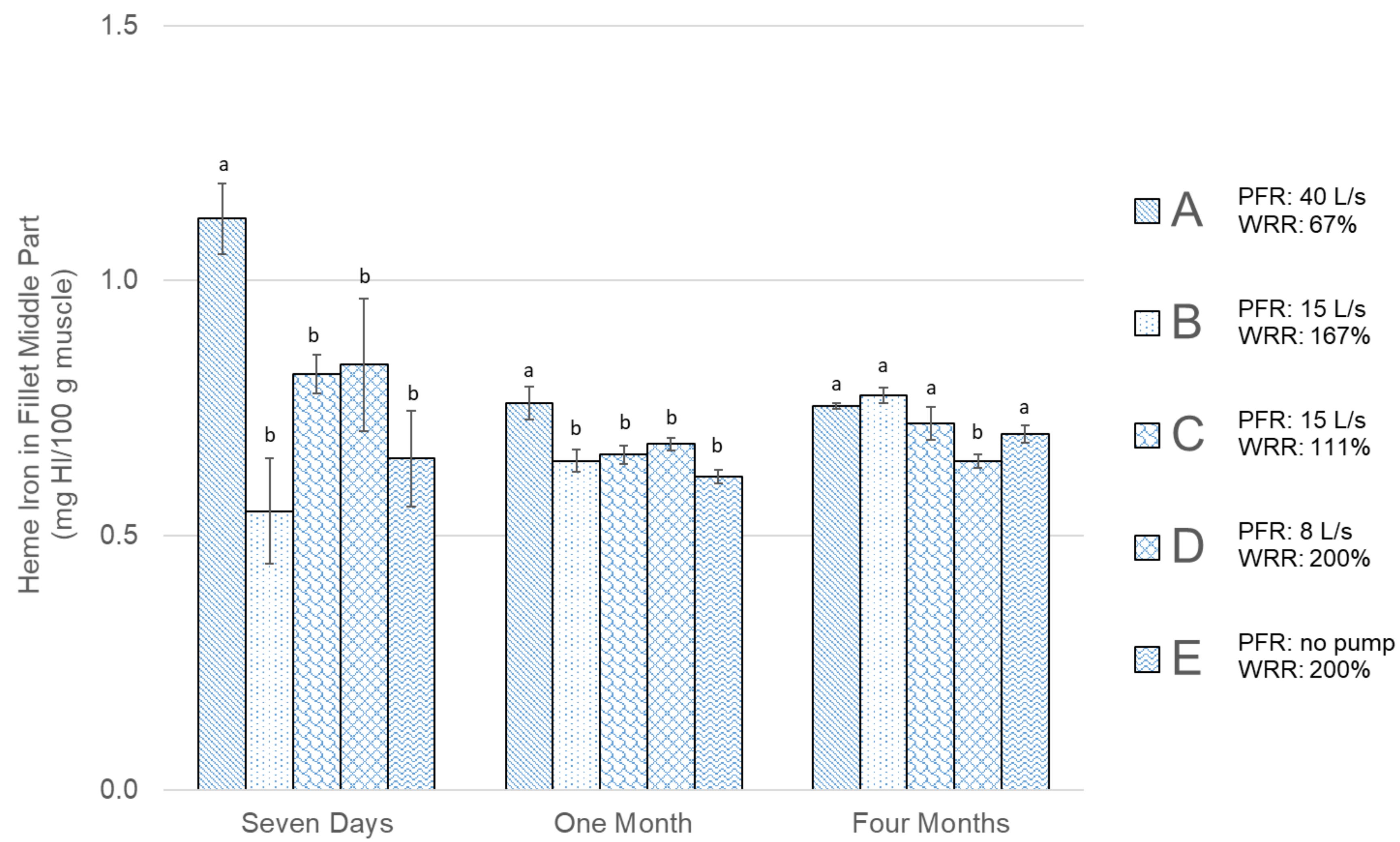
| Group | Bleeding Water to Fish Ratio | Pump Flow Recirculation (PFR) (Pump Power) | Water Replacement Frequency | Water Replacement Method | Water Replacement Ratio (WRR) * (Replacement Time) |
|---|---|---|---|---|---|
| A | 3:1 | 40 L/s (8 kW) | 235 L/min | 25 s injection intervals | 67% (30 min) |
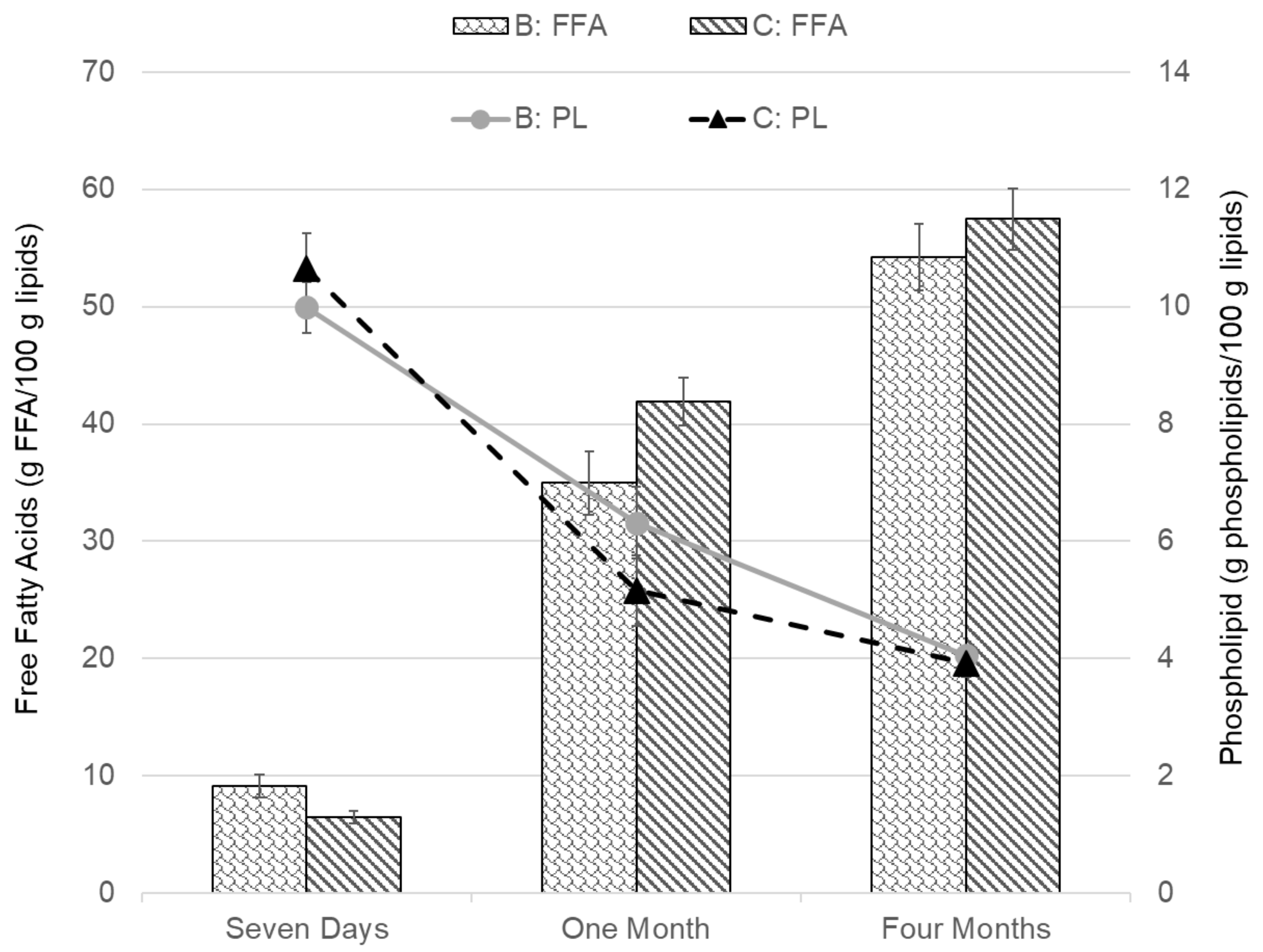
| B | 3:1 | 15 L/s (3 kW) | 585 L/min | 15 s injection intervals | 167% (12 min) |
| C | 3:1 | 15 L/s (3 kW) | 390 L/min | 10 s injection intervals | 111% (18 min) |
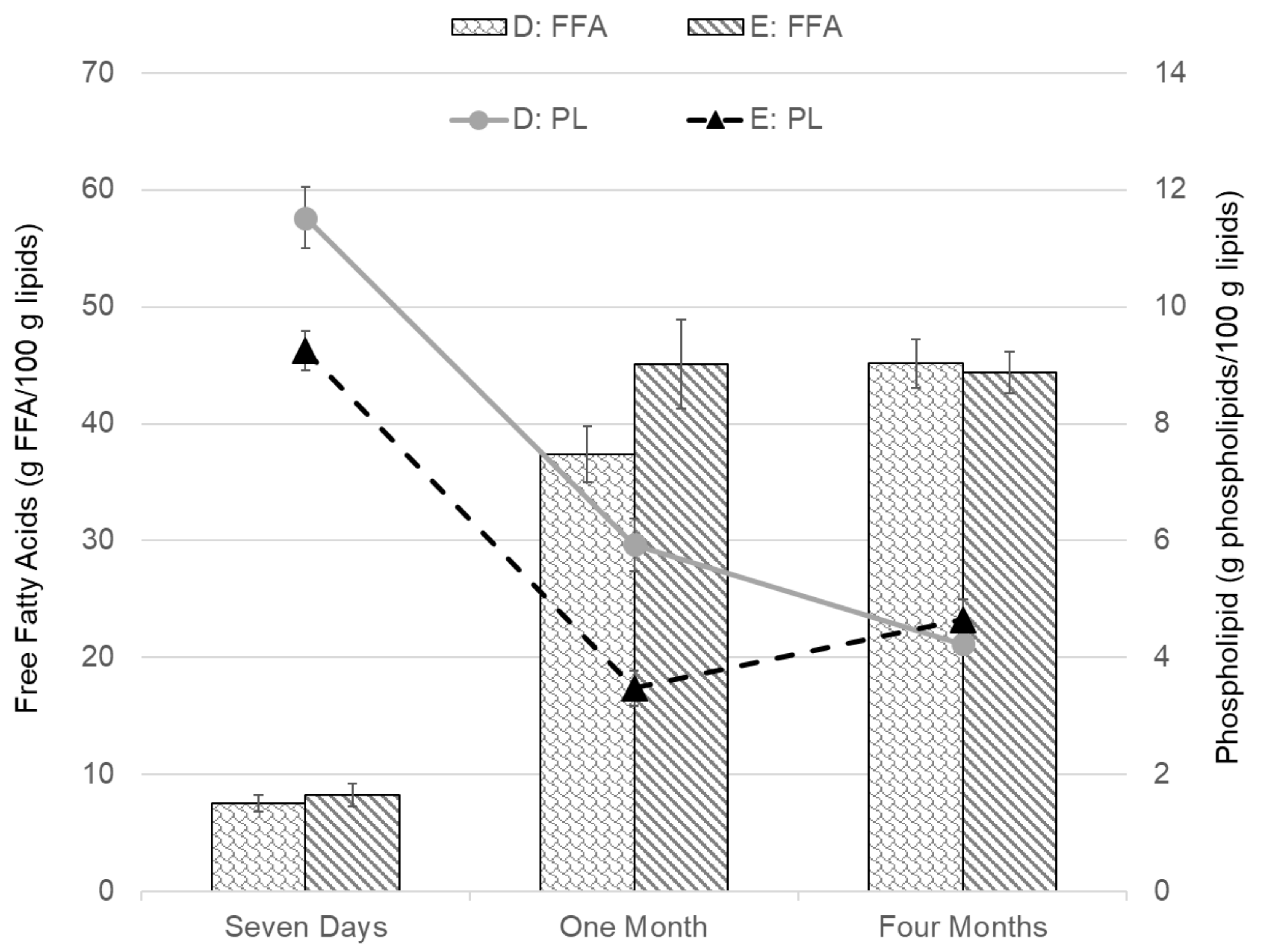
| D | 2:1 | 8 L/s (1.5 kW) | 20 L/min | batch replacement | 200% (10 min) |
| E | 2:1 | No pump | 20 L/min | batch replacement | 200% (10 min) |
| Dependent Variables | Parameter/Period | 7 Days | 1 Month | 4 Months |
|---|---|---|---|---|
| Free Fatty Acids (FFAs)—Equation (2) | b1 | 0.06 | −0.75 | −0.31 |
| (p-value) | (0.36) | (1 × 10−3) | (0.15) | |
| b2 | 2.50 | −11.47 | −13.04 | |
| (p-value) | (0.13) | (0.03) | (0.02) | |
| b0 | 3.03 | 66.60 | 74.67 | |
| (p-value) | (0.36) | 9 × 10−7 | (4 ×10−7) | |
| R2 | 0.12 | 0.45 | 0.24 | |
| Prob(F) | 0.17 | (3 × 10−4) | 0.02 | |
| Phospholipids (PLs)—Equation (3) | b1 | 0.14 | 0.16 | −0.01 |
| (p-value) | (3 × 10−3) | (3 × 10−4) | (0.70) | |
| b2 | 1.18 | 2.17 | 0.25 | |
| (p-value) | (0.29) | (0.04) | (0.62) | |
| b0 | 7.08 | −0.07 | 3.90 | |
| (p-value) | (4 × 10−3) | (0.97) | (8 × 10−4) | |
| R2 | 0.53 | 0.54 | 0.14 | |
| Prob(F) | 4 × 10−5 | 3 × 10−5 | 0.14 | |
| Heme Iron (HI)—Equation (4) | b1 | 0.01 | 0.002 | −0.002 |
| (p-value) | (0.05) | (0.40) | (0.60) | |
| b2 | 0.07 | −0.06 | −0.13 | |
| (p-value) | (0.69) | (0.33) | (0.16) | |
| b0 | 0.53 | 0.71 | 0.92 | |
| (p-value) | (0.13) | (5 × 10−6) | (4 × 10−5) | |
| R2 | 0.38 | 0.40 | 0.17 | |
| Prob(F) | 2 × 10−3 | 1 × 10−3 | 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eliasson, S.; Arason, S.; Margeirsson, B.; Palsson, O.P. Onboard Evaluation of Variable Water Flow and Recirculation Effects on Bleeding of Atlantic Cod (Gadus morhua). Foods 2020, 9, 1519. https://doi.org/10.3390/foods9111519
Eliasson S, Arason S, Margeirsson B, Palsson OP. Onboard Evaluation of Variable Water Flow and Recirculation Effects on Bleeding of Atlantic Cod (Gadus morhua). Foods. 2020; 9(11):1519. https://doi.org/10.3390/foods9111519
Chicago/Turabian StyleEliasson, Saemundur, Sigurjon Arason, Bjorn Margeirsson, and Olafur P. Palsson. 2020. "Onboard Evaluation of Variable Water Flow and Recirculation Effects on Bleeding of Atlantic Cod (Gadus morhua)" Foods 9, no. 11: 1519. https://doi.org/10.3390/foods9111519
APA StyleEliasson, S., Arason, S., Margeirsson, B., & Palsson, O. P. (2020). Onboard Evaluation of Variable Water Flow and Recirculation Effects on Bleeding of Atlantic Cod (Gadus morhua). Foods, 9(11), 1519. https://doi.org/10.3390/foods9111519







